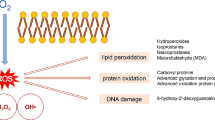
Abstract
The review addresses the role of blood–brain barrier (BBB) dysfunction in neurological pathology, and describes epigenetic and metabolic regulators of its functional activity and structural integrity. It also provides information on the neuroprotective effects of hypoxia and hypercapnia with an assessment of their impact on the functioning of the BBB. In addition, the review provides arguments for the co-administration of drugs, which modulate BBB metabolism and permeability, and hypercapnic hypoxia to achieve a higher neuroprotective efficacy. The provided data demonstrate that there is a significant number of drugs with a high potential to increase neuroprotective efficacy when combined with hypercapnic hypoxia. These drugs include antioxidants, endothelial cell and membrane protectors, JNK inhibitors, and energotropic drugs.

Similar content being viewed by others
REFERENCES
Tregub P, Kulikov V, Motin Y, Bespalov A, Osipov I (2015) Combined exposure to hypercapnia and hypoxia provides its maximum neuroprotective effect during focal ischemic injury in the brain. J Stroke Cerebrovasc Dis 24(2):381–387 https://doi.org/10.1016/jjstrokecerebrovasdis201409003
Obrenovitch TP (2008) Molecular Physiology of Preconditioning-Induced Brain Tolerance to Ischemia. Physiol Rev 88:211–247. https://doi.org/10.1152/physrev000392006
Kuvacheva NV, Morgun AV, Khilazheva ED, Boytsova EB, Ruzaeva VA, Shuvaev AN, Malinovskaja NA, Pozhilenkova EA, Salmina AB (2016) Features of proliferation of bloodbrain barrier cells upon suppression of HIF-1 activity in vitro. Sib Med Rev 98(2):51–56. (In Russ).
Berezhanskaja SB, Luk’yanova EA, Zhavoronkova TJe, Kaushanskaja EJa, Sozaeva DI (2017) Sovremennaya koncepciya strukturno-funkcional’noi organizatsii gematoentsefalicheskogo bar’era i osnovnye mehanizmy narusheniya ego rezistentnosti. Pediatrija Zhurn im GN Speranskogo 96(1):135–141. (In Russ).
Salmina AB, Komleva JuK, Malinovskaja NA, Morgun AV, Tepljashina EA, Lopatina OL, Gorina JaV, Haritonova EV, Hilazheva ED, Shuvaev AN (2021) Povrezhdenie gematojencefalicheskogo bar’era pri stresse i nejrodegeneracii: biohimicheskie mehanizmy i novye modeli dlja transljacionnyh issledovanij. Biochemistry 86(6):917–932. https://doi.org/10.31857/S0320972521060130
Ballabh P, Braun A, Nedergaard M (2014) The blood-brain barrier: an overview structure, regulation and clinical implications. Neurobiology of Disease 16:1–13. https://doi.org/10.1016/j.nbd.2003.12.016
Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY (2016) Endothelial β-catenin signaling is required for maintaining adult bloodbrain barrier integrity and central nervous system homeostasis. Circulation 133(2):177–186. https://doi.org/10.1161/CIRCULATIONAHA.115.015982
Liu JY, Thom M, Catarino CB, Martinian L, Figarella-Branger D, Bartolomei F, Koepp M, Sisodiya SM (2012) Neuropathology of the blood-brain barrier and pharmaco-resis tance in human epilepsy. Brain 135(10):3115–3133. https://doi.org/10.1093/brain/aws147
Gang L, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hammer HM (2011) Cytokines and epilepsy. Seizure-Eur J Epilep 20(3):249–256. https://doi.org/10.1016/j.seizure.2010.12.005
Morgun AV, Ovcharenko NV, Taranushenko TE, Ustinova SI, Okuneva OS, Antonova SK, Gilyazova DF, Uspenskaya OA, Salmina AB (2013) Markers of apoptosis and neurospecific proteins in the diagnosis of perinatal lesions of the central nervous system in newborns. Sib Med Rev 3(81):3–11. (In Russ).
Kuvacheva NV, Salmina AB, Komleva YuK, Malinovskaia NA, Morgun AV, Pozhilenkova EA, Zamaĭ GS, Iauzina NA, Petrova MM (2013) Permeability of the hematoencephalic barrier in normalcy, brain development pathology and neurodegeneration. Zh Nevrol Psikhiatr im SS Korsakova 113(4):80–85. (In Russ).
Chekhonin VP, Lebedev SV, BIinov DV, Gurina OI, Semenova AV, Lazarenko IP, Petrov SV, Ryabukhin LA, Rogatkin SO, Volodin NN (2004) Pathogenetic role of impaired permeability of the blood-brain barrier for neurospecific proteins in perinatal hypoxic-ischemic lesions of the central nervous system in newborns. Vopr Ginеcol Akush Perinat 3(2):50–61. (In Russ).
Chehonin VP, Lebedev SV, Dmitrieva TB, Blinov DV, Gurina OI, Semenova AV, Volodin NN (2003) Enzyme immunoassay of NSE and GFAP as a criterion for dynamic assessment of the permeability of the blood-brain barrier of rats with perinatal hypoxic-ischemic CNS lesion. Bull Exp Biol Med 136 (9):299–303. (In Russ).
Abbott NJ, Ronnback L, Hansson E (2017) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. https://doi.org/10.1038/nrn1824
Chalbot S, Zetterberg H, Blennow K, Fladby T, Grundke-Iqbal I, Iqbal K (2010) Cerebrospinal Fluid Secretory Ca2+ dependent phospholipase A2 activity: a biomarker of blood-cerebrospinal fluid barrier permeability. Neurosci Lett 478(3):179–183. https://doi.org/10.1016/j.neulet.2010.05.012
Vanyushin BF (2006) DNA methylation and epigenetics. Genetics 42(9):1186–1199. (In Russ).
Netrebenko OK, Scheplyagina LA, Gribakin SG (2020) Metabolic programming and epigenetics in pediatrics. Treatment and Prevention 10(1):29–35. (In Russ).
Meng Q, Yang G, Yang Y, Ding F, Hu F (2020) Protective effects of histone deacetylase inhibition by Scriptaid on brain injury in neonatal rat models of cerebral ischemia and hypoxia. Int J Clin Exp Pathol 13(2):179–191.
Jambhekar A, Dhall A, Shi Y (2019) Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol 20(10):625–641. https://doi.org/10.1038/s41580-019-0151-1
Yao B, Jin P (2014) Unlocking epigenetic codes in neurogenesis. Genes Dev 28(12):1253–1271. https://doi.org/10.1101/gad.241547.114
Churilova AV, Gluschenko TS, Rybnikova EA, Samoilov MO (2018) The effect of histone deacetylase inhibitor on the expression level of glucococrticoid receptor in rat forebrain under hypoxia. Cytology 60(12):1016–1021. (In Russ).
Pierre WC, Legault LM, Londono I, McGraw S, Lodygensky GA (2020) Alteration of the brain methylation landscape following postnatal inflammatory injury in rat pups. FASEB J 34(1):432–445. https://doi.org/10.1096/fj.201901461R
Deniz BF, Confortim HD, Miguel PM, Bronauth L, Fernandes IR, Muotri AR, Pereira LO (2021) High gestational folic acid supplementation prevents hypoxia-ischemia-induced caspase-3 augmenting without changing synapsin and H3 methylation levels in the rat hippocampus. Int J Dev Neurosci 81(6):510–519. https://doi.org/10.1002/jdn.10132
Tornabene E, Helms HCC, Pedersen SF, Brodin B (2019) Effects of oxygen-glucose deprivation (OGD) on barrier properties and mRNA transcript levels of selected marker proteins in brain endothelial cells/astrocyte co-cultures. PLoS One 14:e0221103. https://doi.org/10.1371/journal.pone.0221103
Rink C, Khanna S (2017) Micro RNA in ischemic stroke etiology and pathology. Physiol Genomics 43(10):521–528. https://doi.org/10.1152/physiolgenomics.00158.2017
Shen G, Ma Q (2020) MicroRNAs in the Blood-Brain Barrier in Hypoxic-Ischemic Brain Injury. Curr Neuropharmacol 18(12):1180-1186. https://doi.org/10.2174/1570159X18666200429004242
Ek CJ, D'Angelo B, Baburamani AA, Lehner C, Leverin AL, Smith PL, Nilsson H, Svedin P, Hagberg H, Mallard C (2015) Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. J Cereb Blood Flow Metab 35(5):818– 827. https://doi.org/10.1038/jcbfm.2014.255
Cerutti C, Ridley AJ (2017) Endothelial cell-cell adhesion and signaling. Exp Cell Res 358(1):31–38. https://doi.org/10.1016/j.yexcr.2017.06.003
Shi Y, Jiang X, Zhang L, Pu H, Hu X, Zhang W, Cai W, Gao Y, Leak RK, Keep RF, Bennett MV, Chen J (2017) Endothelium-targeted overexpression of heat shock protein 27 ameliorates blood-brain barrier disruption after ischemic brain injury. Proc Natl Acad Sci U S A 114(7):1243–1252. https://doi.org/10.1073/pnas.1621174114
Fan F, Yang J, Xu Y, Guan S (2018) MiR-539 targets MMP-9 to regulate the permeability of blood-brain barrier in ischemia/reperfusion injury of brain. Neurochem Res 43(12):2260–2267. https://doi.org/10.1007/s11064-018-2646-0
Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H (2018) Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci 38(1):32–50. https://doi.org/10.1523/JNEUROSCI.1348-17.2017
Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW, Yue ZY, Hu B (2018) MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J 32(2):935–944. https://doi.org/10.1096/fj.201700139RRR
Huang L, Ma Q, Li Y, Li B, Zhang L (2018) Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol 300:41–50. https://doi.org/10.1016/j.expneurol.2017.10.024
Pan J, Qu M, Li Y, Wang L, Zhang L, Wang Y, Tang Y, Tian HL, Zhang Z, Yang GY (2020) MicroRNA-126-3p/-5p overexpression attenuates blood-brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke 51(2):619–627. https://doi.org/10.1161/STROKEAHA.119.027531
Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, Rom S (2020) miR-98 reduces endothelial dysfunction by protecting blood-brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab 40(10):1953–1965. https://doi.org/10.1177/0271678X19882264
Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X (2016) MiR-34a regulates bloodbrain barrier permeability and mitochondrial function by targeting cytochrome c. J Cereb Blood Flow Metab 36(2):387–392. https://doi.org/10.1177/0271678X15606147
Hu H, Hone EA, Provencher EAP, Sprowls SA, Farooqi I, Corbin DR, Sarkar SN, Hollander JM, Lockman PR, Simpkins JW, Ren X (2020) MiR-34a interacts with cytochrome c and shapes stroke outcomes. Sci Rep 10(1):3233. https://doi.org/10.1038/s41598-020-59997-y
Li Z, Li J, Tang N (2017) Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience 354:1–10. https://doi.org/10.1016/j.neuroscience.2017.04.017
Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE (2018) Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci 30(18):6398–6408. https://doi.org/10.1523/JNEUROSCI.0780-10.2018
Wan Y, Jin HJ, Zhu YY, Fang Z, Mao L, He Q, Xia YP, Li M, Li Y, Chen X, Hu B (2018) MicroRNA-149-5p regulates bloodbrain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes. FASEB J 32(6):3133–3148. https://doi.org/10.1096/fj.201701121R
Chan YC, Banerjee J, Choi SY, Sen CK (2012) miR-210: the master hypoxamir. Microcirculation 19(3):215–223. https://doi.org/10.1111/j.1549- 8719.2011.00154.x
Ma Q, Dasgupta C, Li Y, Huang L, Zhang L (2017) MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int J Mol Sci 18(7):E1356. https://doi.org/10.3390/ijms18071356
Zaccagnini G, Greco S, Voellenkle C, Gaetano C, Martelli F (2021) miR-210 hypoxamiR in Angiogenesis and Diabetes. Antioxid Redox Signal 36:685–706. https://doi.org/10.1089/ars.2021.0200
Ma Q, Zhang L, Pearce WJ (2019) MicroRNAs in brain development and cerebrovascular pathophysiology. Am J Physiol Cell Physiol 317(1):3–19. https://doi.org/10.1152/ajpcell.00022.2019
Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C (2015) miR-210 mediates vagus nerve stimulationinduced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem 134(1):173–181. https://doi.org/10.1111/jnc.13097
Meng ZY, Kang HL, Duan W, Zheng J, Li QN, Zhou ZJ (2018) MicroRNA-210 promotes accumulation of neural precursor cells around ischemic foci after cerebral ischemia by regulating the SOCS1-STAT3-VEGF-C pathway. J Am Heart Assoc 7(5):e005052. https://doi.org/10.1161/JAHA.116.005052
Garberg HT, Huun MU, Baumbusch LO, Åsegg-Atneosen M, Solberg R, Saugstad OD (2017) Tempora profile of circulating microRNAs after global hypoxia-ischemi in newborn piglets. Neonatology 111(2):133–139. https://doi.org/10.1159/000449032
Whitehead CL, Teh WT, Walker SP, Leung C, Larmour L, Tong S (2018) Circulating MicroRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One 8(11):e78487. https://doi.org/10.1371/journal. pone.0078487
Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J (2015) MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res 1603:150–157. https://doi.org/10.1016/j.brainres.2015.01.009
Yao X, Wang Y, Zhang D (2018) microRNA-21 confers neuroprotection agains cerebral ischemia-reperfusion injury and alleviates blood-brain barrier disruption in rats via the MAPK signaling pathway. J Mol Neurosci 65(1):43–53. https://doi.org/10.1007/s12031-018-1067-5
Salmina AB, Morgun AV, Kuvacheva NV, Pozhilenkova EA, Solonchuk JuR, Lopatina OL, Komleva JuK, Taranushenko TE (2014) Endothelial Progenitor Cells in the Development and Recovery of the Cerebral Endothelium (Review). Sovrem Technol Med 6(4):213–222. (In Russ)].
Siegenthaler JA, Sohet F, Daneman R (2013) ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol 23(6):1057–1064. https://doi.org/10.1016/j.conb.2013.06.006
Masada T, Hua Y, Xi G, Ennis SR, Keep RF (2013) Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cereb Blood Flow Metab 21:22–33. https://doi.org/10.1097/00004647-200101000-00004
Zhang FY, Chen XC, Ren HM, Bao WM (2006) Effects of ischemic preconditioning on blood-brain barrier permeability and MMP-9 expression of ischemic brain. Neurol Res 28:21–24. https://doi.org/10.1179/016164106X91825
Ikeda T, Xia XY, Xia YX, Ikenoue T (1999) Hyperthermic preconditioning prevents blood-brain barrier disruption produced by hypoxia-ischemia in newborn rat. Brain Res 117:53–58. https://doi.org/10.1016/s0165-3806(99)00097-8
Maruoka N, Murata T, Omata N, Fujibayashi Y, Yonekura Y, Wada Y (2012) Hypoxic tolerance induction in rat brain slices following 3-nitropropionic acid pretreatment as revealed by dynamic changes in glucose metabolism. Neurosci Lett 319:83–86. https://doi.org/10.1016/s0304-3940(01)02542-3
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2017) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709. https://doi.org/10.1038/sj.jcbfm.9600375
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12:441–445. https://doi.org/10.1038/nm1387
Tohidpour A, Morgun AV, Boitsova EB, Malinovskaya NA, Martynova GP, Khilazheva ED, Kopylevich NV, Gertsog GE, Salmina AB (2017) Neuroinflammation and infection: molecular mechanisms associated with dysfunction of neurovascular unit. Front Cell Infect Microbiol 7:276. https://doi.org/10.3389/fcimb.2017.00276
Frijns CJ, Kappelle LJ (2012) Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 33:2115–2122. https://doi.org/10.1161/01.str.0000021902.33129.69
Zhang W, Smith C, Shapiro A, Monette R, Hutchison J, Stanimirovic D (1999) Increased expression of bioactive chemokines in human cerebromicrovascular endothelial cells and astrocytes subjected to simulated ischemia in vitro. J Neuroimmunol 101:148–160. https://doi.org/10.1016/s0165-5728(99)00137-x
Zahler S, Kupatt C, Becker BF (2000) Endothelial preconditioning by transient oxidative stress reduces inflammatory responses of cultured endothelial cells to TNF-α. FASEB J 14:555–564. https://doi.org/10.1096/fasebj.14.3.555
Zhou SG, Lei XY, Liao DF (2013) Effects of hypoxic preconditioning on the adhesion of neutrophils to vascular endothelial cells induced by hypoxia/reoxygenation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xu 15:159–162.
Hilazheva ED, Pisareva NV, Morgun AV, Bojcova EB, Taranushenko TE, Frolova OV, Salmina AB (2017) Aktivatsiya laktatnyh retseptorov GPR81 stimuliruet mitohondrial’nyi biogenez v kletkah endoteliya cerebral’nyh mikrososudov. Ann Сlin Exp Neurol 11(1):34–39. (In Russ).
Verdegem D, Moens S, Stapor P, Carmeliet P (2014) Endothelial cell metabolism: parallels and divergences with cancer cell metabolism. Cancer Metab 2:19. https://doi.org/10.1186/2049-3002-2-19
Pavlides S, Whitaker-Menezes D, Castello-Cros R (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8:3984–4001. https://doi.org/10.4161/cc.8.23.10238
Bergersen LH, Gjedde A (2012) Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenergetics 4:5. https://doi.org/10.3389/fnene.2012.00005
Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594(3):509-525. https://doi.org/10.1113/JP271301
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. https://doi.org/10.1042/bse0470069
Lin XW, Tang L, Yang J, Xu WH (2016) HIF-1 regulates insect lifespan extension by inhibiting c-Myc-TFAM signaling and mitochondrial biogenesis. Biochim Biophys Acta 1863:2594–2603. https://doi.org/10.1016/j.bbamcr.2016.07.007
Ruzaeva VA, Morgun AV, Khilazheva ED, Kuvacheva NV, Pozhilenkova EA, Boitsova EB, Martynova GP, Taranushenko TE, Salmina AB (2016) Development of blood-brain barrier under the modulation of HIF activity in astroglialand neuronal cells in vitro. Biomed Khim 62:664–669. https://doi.org/10.18097/pbmc20166206664
Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE (2010) Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrialderived reactive oxygen species. Mol Biol Cell 21(18):3247–3257. https://doi.org/10.1091/mbc.E10-01-0025
Kulikov VP, Tregub PP, Kovzelev PD, Dorohov EA, Belousov AA (2015) Giperkapniya—al’ternativnyi gipoksii signal’nyi stimul dlya povysheniya HIF-1a i eritropoetina v golovnom mozge. Patol Fiziol Exp Ter 59(3):34–37. (In Russ)].
Tregub PP, Malinovskaya NA, Morgun AV, Osipova ED, Kulikov VP, Kuzovkov DA, Kovzelev PD (2020) Hypercapnia potentiates HIF-1α activation in the brain of rats exposed to intermittent hypoxia. Respir Physiol Neurobiol 278:103442. https://doi.org/10.1016/j.resp.2020.103442
Tregub PP, Malinovskaya NA, Kulikov VP, Kuzovkov DA (2021) Hypercapnia and its combination with hypoxia reduce the permeability of the blood-brain barrier in rats. Patol Fiziol Exp Ter 65(2):30–36. (In Russ).
Yang W, Wang Q, Chi L, Wang Y-Z, Cao H-L, Li W-Z (2019) Therapeutic hypercapnia reduces blood–brain barrier damage possibly via protein kinase Cε in rats with lateral fluid percussion injury. J Neuroinflamm 16:36.
Tregub PP, Morgun AV, Osipova ED, Kulikov VP, Malinovskaya NA, Kuzovkov DA (2020) Hypercapnia and Hypoxia Stimulate Proliferation of Astrocytes and Neurons In Vitro. Bull Exp Biol Med 169(6):755–758. https://doi.org/10.1007/s10517-020-04972-w
Yang W, Zhang X, Wang N, Tan J, Fang X, Wang Q, Tao T, Li W (2016) Effects of acute systemic hypoxia and hypercapnia on brain damage in a rat model of hypoxia-ischemia. PLoS One 11:e0167359. https://doi.org/10.1371/journal.pone.0167359
Kangwantas K, Pinteaux E, Penny J (2016) The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J Neuroinflammation 13:25. https://doi.org/10.1186/s12974-016-0495-9
Wacker BK, Freie AB, Perfater JL, Gidday JM (2017) Junctional protein regulation by sphingosine kinase 2 contributes to blood-brain barrier protection in hypoxic preconditioning-induced cerebral ischemic tolerance. J Cereb Blood Flow Metab 32(6):1014–1023. https://doi.org/10.1038/jcbfm.2012.3
Chi OZ, Mellender SJ, Barsoum S, Liu X, Weiss HR (2017) Hypoxic Preconditioning Increases Blood-Brain Barrier Disruption in the Early Stages of Cerebral Ischemia. Curr Neurovasc Res 14(1):26–31. https://doi.org/10.2174/1567202614666161104114821
Tregub P, Kulikov V, Bespalov A (2013) Tolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in rats. Pathophysiology 3:165–170. https://doi.org/10.1016/j.pathophys.2013.09.001
Kulikov VP, Kalanova LA, Tregub PP (2021) Potencirovanie zashhitnogo jeffekta giperkapnicheskoj gipoksii pri kombinacii s farmakologicheskimi nejroprotektorami. Patol Fiziol Exp Ter 3:21–25. (In Russ).
Shahmardanova SA, Gulevskaya ON, Seletskaya VV, Zelenskaja AV, Hananashvili JaA, Nefedov DA, Galenko-Jaroshevskii PA (2016) Antioxidants: classification, pharmacotherapeutic properties, use in practical medicine. J Fund Med Biol 3:4-15. (In Russ).
Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC (2015) Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr 113(8):1182–1194. https://doi.org/10.1017/S0007114515000227
Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, Langguth P, Alteheld B, Fimmers R, Stehle P, Egert S (2017) Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: a randomized, double-blind, placebo-controlled, crossover trial. Eur J Nutr 56(3):1347–1357. https://doi.org/10.1007/s00394-016-1185-1
Smirnova KV, Gil’dikov DI (2021) Comparative efficacy of antioxidant drugs in an in vitro experiment. Ros Veterin J 2:37–40 (In Russ).
Moskalenko SV, Shahmatov II, Bondarchuk JuA, Alekseeva OV, Ulitina OM (2018) The reaction of the hemostasis system in hypercapnic hypoxia after a course of mexidol using the method of thromboelastography. Kaz Med J 99(6):936–941. (In Russ).
Khlebodarov FE, Mikhin VP, Tyurikov PYu (2009) Vascular endothelial dysfunction and its correction by cytoprotectors in patients with stable angina pectoris and arterial hypertension. Rus Cardiol J 6:34–39. (In Russ).
Semenenkov II, Pristrom MS (2019) Influence of the combined use of normobaric hypoxia and omega-3 polyunsaturated fatty acids on changes in the fatty acid composition of blood plasma and indicators of systemic immune inflammation in patients with coronary heart disease associated with chronic obstructive pulmonary disease. Cardiovasc Ter Prof 18(S1):136–137. (In Russ).
Shvedova MV, Anfinogenova JaD, Popov SV, Shhepetkin IA, Atochin DN (2016) C-Jun n-terminal kinases and their modulators in ischemia-reperfusion myocardial injury (literature review). Sib J Clin Exp Med 31(3):7–15. (In Russ).
Plotnikov MB, Chernysheva GA, Smolyakova VI, Aliev OI, Trofimova ES, Sherstoboev EY, Osipenko AN, Khlebnikov AI, Anfinogenova YJ, Schepetkin IA, Atochin DN (2020) Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia. Cells 9(8):1860. https://doi.org/10.3390/cells9081860
Zhou Q, Lam PY, Han D, Cadenas E (2008) c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem 104(2):325–335. https://doi.org/10.1111/j.1471-4159.2007.04957.x
Funding
The writing of this review was state budget funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The author declares that he has no conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 5, pp. 579–593https://doi.org/10.31857/S0869813922050120.
Rights and permissions
About this article
Cite this article
Tregub, P.P. Effect of Hypercapnia and Hypoxia on the Physiology and Metabolism of the Cerebral Endothelium under Ischemic Conditions. J Evol Biochem Phys 58, 769–780 (2022). https://doi.org/10.1134/S0022093022030127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022030127