Abstract
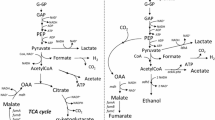
The genes maeA and maeB, encoding NADH- and NADPH-dependent malic enzymes, have been deleted in a recombinant Escherichia coli strain with inactivated mixed-acid fermentation pathways and a modified system of glucose transport and phosphorylation upon the heterological expression of the pyruvate carboxylase gene. During anaerobic glucose utilization, the parental strain synthesized malic, fumaric, and succinic acids as the main fermentation end products, while pyruvic acid was accumulated as the main by-product resulting from the functioning of the pyruvate–oxaloacetate–malate–pyruvate futile cycle. Upon individual deletions of the maeA and maeB genes, the mutant strains converted glucose into four-carbon dicarboxylic acids with increased efficiency still secreting notable amounts of pyruvic acid. The combined inactivation of both malic enzymes in the constructed strain significantly elevated the portion of malic, fumaric, and succinic acids among the fermentation end products with a concomitant decrease in the secretion of pyruvic acid and other by-products due to the abolishment of the action of the futile cycle competing with the target biosynthetic processes.

Similar content being viewed by others
REFERENCES
Werpy, T., Petersen, G., Aden, A., Bozell, J., Holladay, J., White, J., Manheim, A., Eliot, D., Lasure, L., and Jones, S., Top Value Added Chemicals from Biomass, vol. 1: Results of Screening for Potential Candidates from Sugars and Synthesis Gas, Washington DC, USA: Pacific Northwest National Laboratory, National Renewable Energy Laboratory and Department of Energy, 2004.
West, T.P., Malic acid production from thin stillage by Aspergillus species, Biotechnol. Lett., 2011, vol. 33, no. 12, pp. 2463– 2467. doi 10.1007/s10529-011-0720-7
Roa, EngelC.A., Straathof, A.J., Zijlmans, T.W., et al., Fumaric acid production by fermentation, Appl. Microbiol. Biotechnol., 2008, vol. 78, no. 3, pp. 379–389. doi 10.1007/s00253-007-1341-x
Guettler, M.V., Rumler, D., and Jain, M.K., Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen, Int. J. Syst. Bacteriol., 1999, vol. 49, no. 1, pp. 207–216. doi 10.1099/00207713-49-1-207
Nghiem, N.P., Davison, B.H., Suttle, B.E., and Richardson, G.R., Production of succinic acid by Anaerobiospirillum succiniciproducens, Appl. Biochem. Biotechnol., 1997, vol. 63-65, pp. 565–576. doi 10.1007/bf02920454
Lee, P.C., Lee, S.Y., Hong, S.H., and Chang, H.N., Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBEL55E, from bovine rumen, Appl. Microbiol. Biotechnol., 2002, vol. 58, no. 5, pp. 663–668. doi 10.1007/s00253-002-0935-6
Yin, X., Li, J., Shin, H.D., et al., Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: advances and prospects, Biotechnol. Adv., 2015, vol. 33, no. 6, pp. 830–841. doi 10.1016/j.biotechadv.2015.04.006
Liu, J., Li, J., Shin, H.D., et al., Protein and metabolic engineering for the production of organic acids, Bioresour. Technol., 2017, vol. 239, pp. 412–421. doi 10.1016/j.biortech.2017.04.052
Vemuri, G.N., Eiteman, M.A., and Altman, E., Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli, Appl. Environ. Microbiol., 2002, vol. 68, no. 4, pp. 1715–1727. doi 10.1128/AEM.68.4.1715-1727.2002
Sanchez, A.M., Bennett, G.N., and San, K.Y., Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity, Metab. Eng., 2005, vol. 7, no. 3, pp. 229–239. doi 10.1016/j.ymben.2005.03.001
Skorokhodova, A.Y., Morzhakova, A.A., Gulevich, A.Y., and Debabov, V.G., Manipulating pyruvate to acetyl-CoA conversion in Escherichia coli for anaerobic succinate biosynthesis from glucose with the yield close to the stoichiometric maximum, J. Biotechnol., 2015, vol. 214, pp. 33–42. doi 10.1016/j.jbiotec.2015.09.003
Cao, Y., Cao, Y., and Lin, X., Metabolically engineered Escherichia coli for biotechnological production of four-carbon 1,4-dicarboxylic acids, J. Ind. Microbiol. Biotechnol., 2011, vol. 38, no. 6, pp. 649–656. doi 10.1007/s10295-010-0913-4
Thakker, C., Martinez, I., Li, W., et al., Metabolic engineering of carbon and redox flow in the production of small organic acids, J. Ind. Microbiol. Biotechnol., 2015, vol. 42, no. 3, pp. 403–422. doi 10.1007/s10295-014-1560-y
Sauer, U. and Eikmanns, B.J., The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria, FEMS Microbiol. Rev., 2005, vol. 29, no. 4, pp. 765–794. doi 10.1016/j.femsre.2004.11.002
Gokarn, R.R., Evans, J.D., Walker, J.R., et al., The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli, Appl. Microbiol. Biotechnol., 2001, vol. 56, nos. 1–2, pp. 188–195. doi 10.1007/s002530100661
Skorokhodova, A.Yu., Stasenko, A.A., Gulevich, A.Yu., and Debabov, V.G., Effect of anaplerotic pathways activation on CO2-dependent anaerobic glucose utilization by Escherichia coli strains deficient in the main pathways of mixed acid fermentation, Appl. Biochem. Microbiol., 2018, vol. 54, no. 2, pp. 141–148.
Skorokhodova, A.Yu., Gulevich, A.Yu., and Debabov, V.G., Effect of extra- and intracellular sources of CO2 on anaerobic utilization of glucose by Escherichia coli strains deficient in carboxylation-independent fermentation pathways, Appl. Biochem. Microbiol., 2017, vol. 53, no. 3, pp. 304–309. doi 10.7868/S0555109917030151
Sambrook, J., Fritsch, E., and Maniatis, T., Molecular Cloning: A Laboratory Manual, New York, USA: Cold Spring Harbor Laboratory Press, 1989, 2nd ed.
Katashkina, Zh.I., Skorokhodova, A.Yu., Zimenkov, D.V., et al., Tuning the expression level of a gene located on a bacterial chromosome, Mol. Biol. (Moscow), 2005, vol. 39, no. 5, pp. 719–726.
Datsenko, K.A. and Wanner, B.L., One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, no. 12, pp. 6640–6645. doi 10.1073/pnas.120163297
Gulevich, A.Yu, Skorokhodova, A.Yu., Ermishev, V.Yu., et al., A New method for the construction of translationally coupled operons in a bacterial chromosome, Mol. Biol. (Moscow), 2009, vol. 43, no. 3, pp. 505–514.
Skorokhodova, A.Yu., Gulevich, A.Yu., Morzhakova, A.A., et al., Anaerobic Synthesis of succinic acid by recombinant Escherichia coli strains with activated NAD+-reducing pyruvate dehydrogenase complex, Appl. Biochem. Microbiol., 2011, vol. 47, no. 4, pp. 373–380.
Bologna, F.P., Andreo, C.S., and Drincovich, M.F., Escherichia coli malic enzymes: two isoforms with substantial differences in kinetic properties, metabolic regulation, and structure, J. Bacteriol., 2007, vol. 189, no. 16, pp. 5937–5946. doi 10.1128/JB.00428-07
Kwon, Y.D., Kwon, O.H., Lee, H.S., and Kim, P., The effect of NADP-dependent malic enzyme expression and anaerobic C4 metabolism in Escherichia coli compared with other anaplerotic enzymes, J. Appl. Microbiol., 2007, vol. 103, no. 6, pp. 2340–2345. doi 10.1111/j.1365-2672.2007.03485.x
Hoefel, T., Faust, G., Reinecke, L., et al., Comparative reaction engineering studies for succinic acid production from sucrose by metabolically engineered Escherichia coli in fed-batch-operated stirred tank bioreactors, Biotechnol. J., 2012, vol. 7, no. 10, pp. 1277–1287. doi 10.1002/biot.201200046
Jantama, K., Zhang, X., Moore, J.C., et al., Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C, Biotechnol. Bioeng., 2008, vol. 101, no. 5, pp. 881–893. doi 10.1002/bit.22005
Zhang, X., Wang, X., Shanmugam, K.T., and Ingram, L.O., L-malate production by metabolically engineered Escherichia coli, Appl. Environ. Microbiol., 2011, vol. 77, no. 2, pp. 427–434. doi 10.1128/AEM.01971-10
ACKNOWLEDGMENTS
The work was supported by a grant from the Russian Science Foundation (project 16-14-10389).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by P. Kuchina
Rights and permissions
About this article
Cite this article
Skorokhodova, A.Y., Gulevich, A.Y. & Debabov, V.G. Inactivation of Malic Enzymes Improves the Anaerobic Production of Four-Carbon Dicarboxylic Acids by Recombinant Escherichia coli Strains Expressing Pyruvate Carboxylase. Appl Biochem Microbiol 54, 849–854 (2018). https://doi.org/10.1134/S0003683818090065
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683818090065


