Abstract
Cuttage propagation involves adventitious root formation induced by auxin. In our previous study, Larix kaempferi BABY BOOM 1 (LkBBM1), which is known to regulate adventitious root formation, was affected by auxin. However, the relationship between LkBBM1 and auxin remains unclear. Auxin response factors (ARFs) are a class of important transcription factors in the auxin signaling pathway and modulate the expression of early auxin-responsive genes by binding to auxin response elements. In the present study, we identified 14 L. kaempferi ARFs (LkARFs), and found LkARF7 and LkARF19 bound to LkBBM1 promoter and enhanced its transcription using yeast one-hybrid, ChIP-qPCR, and dual-luciferase assays. In addition, the treatment with naphthalene acetic acid promoted the expression of LkARF7 and LkARF19. We also found that overexpression of these two genes in poplar promoted adventitious root formation. Furthermore, LkARF19 interacted with the DEAD-box ATP-dependent RNA helicase 53-like protein to form a heterodimer to regulate adventitious root formation. Altogether, our results reveal an additional regulatory mechanism underlying the control of adventitious root formation by auxin.
Similar content being viewed by others
Introduction
Cutting propagation, which can rapidly form numerous adventitious roots (ARs), is an effective step in the asexual propagation of economically important woody species1. However, the capacity to form ARs on stem cuttings varies from species to species, and an insufficient rooting ability or poor quality of a newly established root system may cause economic losses2. Therefore, it is important to identify and elucidate the underlying molecular regulatory mechanisms of AR formation to improve the efficiency of vegetative reproduction of woody plants.
AR formation and development is a complex biological process involving cell dedifferentiation, differentiation, root primordia formation, and development. These processes are predominantly affected by endogenous hormones and environmental factors3,4,5. It has been reported that auxin is the major growth-promoting hormone for AR initiation6,7. A high Indole-3-acetic acid (IAA) concentration is required for spontaneous AR formation in the Arabidopsis thaliana hypocotyls during the early steps8. Auxin response factors (ARFs), a class of important transcription factors in the auxin signaling pathway, specifically bind to the auxin response elements (AuxREs, TGTCTC) in the promoters of the downstream auxin response genes9,10. A typical ARF protein contains a conserved N-terminal B3-type DNA binding domain and a variable middle region with activator or repressor activity11. Some ARF proteins also contain a dimerization domain in the C-terminal region12. To date, ARFs have been extensively characterized in numerous plant species. For example, there are 23, 25, 31, and 51 ARFs in Arabidopsis, Oryza sativa, Zea mays, and Glycine max, respectively13,14,15,16. ARFs also have been characterized in woody plants, e.g. there are 39 ARFs in Populus trichocarpa17 and 17 ARFs in Eucalyptus grandis18.
In the past few decades, some ARF genes have been found to be involved in AR formation. In Arabidopsis, three auxin-induced Gretchen Hagen3 family genes are regulated by AtARF6, AtARF8, and AtARF17 to promote the activation of the COI1 signaling pathway, affecting AR formation19,20,21. In addition, AtARF7/19 regulates AtLBD16 and AtLBD18 to positively control AR and lateral root (LR) formation in Arabidopsis22,23,24. In rice, OsARF16 activates CRL1/ARL1 expression for crown root primordia initiation and development25,26. In poplar, overexpression of PeARF17.1 and mPeARF17.2 (a peu-miR160a-resistant version of PeARF17.2) increased the number and length of ARs27. In apple, MdSIZ1-mediated SUMOylation of MdARF8 promotes AR and LR formation28. However, the underlying mechanisms and the target genes of ARF7/19 regulating AR development are largely unknown in woody species.
BABY BOOM (BBM) is a member of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) family, which works as a major regulator of plant cell totipotency and hence promotes cell proliferation and regeneration during embryogenesis29,30,31. For example, BBM functions in cell differentiation and growth along with PLETHORA1 (PLT1), PLT2, and PLT3 proteins in Arabidopsis32,33. BBM also regulates the transcription of LEAFY COTYLEDON1 (LEC1) and LEC2 to mediate somatic embryogenesis in Arabidopsis in a dose- and context-dependent manner31, indicating that BBM acts upstream of the other known embryogenesis transcription factors. Interestingly, BBM participates in root primordium formation in an auxin signaling pathway. MtBBM1 was enriched in the root tips of Medicago truncatula and responded strongly to auxin with an extreme increase in root-forming callus34. The expression of the LkBBM1 and LkBBM2 genes from L. kaempferi (larch) was enhanced after naphthalene acetic acid (NAA) treatment during adventitious rooting in larch. Overexpression of LkBBM1 or LkBBM2 in poplar increased the number of ARs35,36. However, the BBM genetic pathway has not been well characterized37, and it is unknown how this protein regulates AR formation. Since ARFs are known to be regulators of auxin response and BBM1 acts downstream of auxin induction during AR formation, the potential relationship between ARFs and BBM needs to be investigated.
DEAD-box RNA Helicases (DDXs or RHs) play important roles in auxin-related plant development. AuxREs are distributed in the promoter regions of various RHs in longan and soybean38,39. Knockdown of AtRH7/AtPRH75 caused an auxin-related developmental defect phenotype40. In addition, it has been demonstrated that RHs play roles in root development. For example, tissue culture of root initiation defective1-1 (rid1-1; an RH gene) mutant plants showed that these plants were temperature sensitive for callus, AR, and LR formation from hypocotyl explants in Arabidopsis41. Compared with Col-0, Atrh31 mutant seedlings had a shorter primary root length and lower fresh weight, while the Atrh31 mutant lines complemented with the AtRH31 gene rescued the Atrh31 mutant phenotype42. Loss-of function of the RH gene AteIF4A-1 reduced the proportion of mitotic cells in the root meristem and disrupted the relationship between cell size and cell cycle progression, thereby reducing Arabidopsis LR formation43. BnRH24 is a target of miR158 in Brassica napus and overexpression of BnRH24 in Arabidopsis reduced root length44. These results suggest that there may be interactions between auxin and RHs, which may affect AR development.
In this study, we identified the ARF genes in the larch genome and studied the roles of LkARF7/19 and LkBBM1 in promoting AR formation. We found that NAA increased the expression of LkARF7 and LkARF19, which in turn promoted AR formation by activating LkBBM1 transcription and forming heterodimers between LkARF19 and LkRH53. To the best of our knowledge, these findings enrich the understanding of AR formation.
Results
Identification of LkARFs in larch
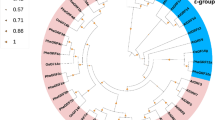
To identify the LkARF genes in larch, we performed a BLASTP search against the larch genome database using the protein sequences of the conserved domains of ARFs from Arabidopsis, P. trichocarpa, Picea abies, and Pseudotsuga menziesii. Ultimately, a total of 14 potential LkARF protein sequences were identified in the larch genome with high protein sequence similarity to the query sequences (Supplementary Data 1). To evaluate the evolutionary relationships among ARFs, a total of 89 full-length ARF protein sequences from Arabidopsis, P. trichocarpa, L. kaempferi, P. abies, and P. menziesii were used to generate an unrooted phylogenetic tree (Supplementary Fig. 1). The results showed that all the ARFs were grouped into six classes. Based on the phylogenetic tree, LkARFs showed high sequence homology with those of other gymnosperms.
A phylogenetic tree was also constructed for all the 14 LkARFs using protein sequences (Supplementary Fig. 2a), followed by motif analysis using MEME. Motif 1 was annotated as the B3 domain, motifs 2 and 3 were annotated as the Aux_resp domain, while motifs 5, 6, and 7 were annotated as the Aux/IAA domain (Supplementary Fig. 2b). All of the LkARF proteins contain motifs 1 and 2, whereas LkARF16-1, LkARF16-2, LkARF1-1, LkARF1-2, and LkARF2-2 lack motif 5 and motif 7. Notably, members with close phylogenetic relationships showed similar occurrences of the detected motifs.
The expressions of LkARF7, LkARF19, and LkBBM1 during adventitious rooting
We measured the relative expression levels of the 14 LkARF genes in the ARs, stems, stem tips, and leaves of the hybrid larch (L. kaempferi × L. olgensis) clone D9 using quantitative real-time PCR (qRT-PCR) (Fig. 1a). We found that LkARF6-3, LkARF7, and LkARF19 were strongly expressed in ARs, which might be related to AR formation in larch (Fig. 1a). Then, we analyzed the expression of LkARF6-3, LkARF7, and LkARF19 in the D9 clone during adventitious rooting. Even though the relative expression levels of LkARF6-3 varied from 0 to 40 DAC (days after cutting; Fig. 1b), that of LkARF7 and LkARF19 gradually increased following AR formation (Fig. 1c, d). Interestingly, we also found that the relative expression levels of LkBBM1 gradually increased starting from 8 DAC (Fig. 1e). The expression of LkARF7 and LkARF19 were highly correlated with that of LkBBM1 with Pearson coefficients of 0.94 and 0.97, respectively. Therefore, LkARF7, LkARF19, and LkBBM1 were chosen for further analysis.
a Heat map of the expression of 14 LkARF genes in different tissues based on qRT-PCR. AR, adventitious roots; S, stems; ST, stem tips; L, leaves. The heat map was made using the Omiscshare tools (http://omicshare.com/tools/) with parameters as default. b–d qRT-PCR analysis of LkARF6-3 (b), LkARF7 (c), LkARF19 (d), and LkBBM1 (e) in the basal stem sections (0.5 cm) during in vitro propagation. DAC days after cutting. The LaEF1A gene was used as the internal control gene. The error bars represent standard deviations from three biological replicates; *P < 0.05 and **P < 0.01, t test.
The localization of LkARF7 and LkARF19 in the nucleus
To identify the subcellular localizations of LkARF7 and LkARF19, the 35S::LkARF7-GFP, 35S::LkARF19-GFP, and the control vector (35S::GFP) were transiently transformed into tobacco leaf cells individually. The GFP fluorescence signals were detected exclusively in the nucleus when the LkARF7-GFP and LkARF19-GFP fusion vectors were transformed individually, while that of the control vector were expressed in the nucleus and cytoplasm (Fig. 2). Thus, LkARF7 and LkARF19 are localized in the nucleus.
The roles of LkARF7 and LkARF19 in regulating AR formation
To investigate the functions of LkARF7 and LkARF19 in the promotion of AR formation, we constructed the pBI121-LkARF7 and pBI121-LkARF19 vectors and transformed them into 84 K poplar individually. PCR confirmed that each transgene was present in the genomic DNA of each transgenic line, while qRT-PCR confirmed their overexpression in each transgenic line (Supplementary Fig. 3). We counted the AR numbers in 3-week-old wild-type, the control plants (transformed with empty vector), and the transgenic seedlings (Fig. 3a, b). There was no difference in the AR numbers between the wild-type and control plants. When compared to the control plants, the ARs numbers in LkARF7-OE lines (LkARF7-OE3, LkARF7-OE4, and LkARF7-OE5) and LkARF19-OE lines (LkARF19-OE4, LkARF19-OE14, and LkARF19-OE16) increased by at least 46% and 61%, respectively (Fig. 3c). Meanwhile, the total AR length in LkARF7-OE lines and LkARF19-OE lines increased by at least 26% and 46%, respectively, when compared to the control plants (Fig. 3d). We observed that the ARs in LkARF7-OE and LkARF19-OE lines appeared one day earlier than that in the control plants (Fig. 3e). In addition, we found that the roots of the 8-week-old LkARF7-OE and LkARF19-OE lines showed increased fresh (110.0% and 119.1%, respectively) and dry (84.5% and 98.3%, respectively) weight (Fig. 4a–c) when compared to the control plants. The plant height, internode numbers, base internode diameters, and fresh and dry weight of the aerial parts of LkARF7-OE and LkARF19-OE lines were significantly higher than those of the control plants (Supplementary Fig. 4). Thus, overexpression of LkARF7 and LkARF19 significantly promoted AR formation in transgenic poplar.
a, b AR phenotypes on 3-week-old control plants (Ctrl, transformed with empty vector), wild-type (WT), LkARF7-OE, and LkARF19-OE lines. Scale bars, 5 cm. c, d Average AR number (c) and total AR length (d) in the control plants and overexpression lines of 3-week-old seedlings. e Rooting rate of the control plants, WT, and overexpression lines within 11 days following cutting (n = 30 per line). DAC, days after cutting. Error bars represent standard deviations from three biological replicates; *P < 0.05 and **P < 0.01, t test.
a AR phenotypes on 8-week-old control plants (Ctrl), wild-type (WT), LkARF7-OE, and LkARF19-OE lines. Scale bars, 10 cm. b Fresh and c dry weight of roots in the control plants, WT, and overexpression lines. Error bars represent standard deviations from three biological replicates; *P < 0.05 and **P < 0.01 t test.
The effect of auxin treatment on LkARF7 and LkARF19 expression during AR formation
To assess whether auxin plays an important role in regulating LkARF7 and LkARF19 expression in AR formation, we measured the time-course relative expression levels of LkARF7 and LkARF19 in the basal stem sections (0.5 cm in length) of the D9 clone after 0.01 mM NAA treatment. Compared with 0 hours post treatment (hpt), the relative expression levels of LkARF7 and LkARF19 at 2, 6, 12, 16, and 24 hpt showed significant increases with an average of 3–8 folds (Supplementary Fig. 5). As a result, auxin treatment significantly increased the relative expression of both genes during AR formation.
Induction of LkBBM1 expression by LkARF7 and LkARF19 via promoter binding
To examine whether LkARF7 or LkARF19 can induce LkBBM1 expression, the 35S::LkARF7-flag, 35S::LkARF19-flag, and the control vector (35S::flag) were transiently transformed into the basal stem sections (0.5 cm in length) of the D9 clone individually. Using qRT-PCR, we found that LkARF7 and LkARF19 induced LkBBM1 expression by 7 and 10 times, respectively, which were significantly higher than that in the mock treatment (Fig. 5a).
a Activation of LkBBM1 expression by transient overexpression of LkARF7 and LkARF19, respectively. The value from the empty vector (CK) was set to 1.0. b Yeast one-hybrid analysis of interactions between LkARF7/19 and the LkBBM1 promoter. P, Positive control (p53HIS2 + pGAD53T); N, Negative control (pHIS2-LkBBM1 promoter + pGADT7); LkARF7 (pHIS2-LkBBM1 promoter + pGADT7-LkARF7); LkARF19 (pHIS2-LkBBM1 promoter + pGADT7-LkARF19). DDO, SD/-Trp-Leu; TDO, SD/-Trp-Leu-His. c Diagram of the LkBBM1 promoter showing the relative positions of three auxin response elements (AuxRE) and one abscisic acid response element (ABRE). d Binding of LkARF7 and LkARF19 with the LkBBM1 promoter using ChIP-qPCR assay. The relative abundance of LkBBM1 promoter fragments in chromatin was determined by isolation from the 35S::LkARF7-flag, 35S::LkARF19-flag, and 35S::flag lines. e Activation of the LUC expression driven by the LkBBM1 promoter reporter when co-transformed with 35S::LkARF7 and 35S::LkARF19, respectively. 62SK + Luc-LkBBM1 promoter was used as the negative control (Ctrl), and its value was set to 1. f Representative images of the LUC expression driven by the LkBBM1 promoter reporter when co-transformed with 35S::LkARF7 and 35S::LkARF19, respectively. Red and white signals indicate strong binding capacity between effectors and reporters. Scale bars, 4 cm. Error bars represent standard deviations from four biological replicates; *P < 0.05 and **P < 0.01, t test.
We cloned the 1,727-bp-long promoter fragment of LkBBM1. Promoter motif analysis identified several important cis-acting elements such as ARR1AT and ABRE (associated with hormonal response), DRE (associated with stress response), and MYBCORE (associated with protein binding) in the LkBBM1 promoter (Supplementary Fig. 6; Supplementary Data 2). Moreover, three AuxREs (AuxRE-1, AuxRE-2, and AuxRE-3) were identified in the LkBBM1 promoter (Fig. 5b), suggesting that ARF genes may be involved in regulating the transcription of LkBBM1.
To investigate whether LkARF7 and LkARF19 can bind to the LkBBM1 promoter, we carried out yeast one-hybrid (Y1H) assays. We found that 75 mM 3-amino-1,2,4-triazole (3-AT) was the appropriate concentration to inhibit the self-activation (Fig. 5c). The Y187 strain co-expressing pGADT7-LkARF7 and pHIS2-LkBBM1 and the strain co-expressing pGADT7-LkARF19 and pHIS2-LkBBM1 grew normally on TDO medium containing 75 mM 3-AT, while the negative control strain did not (Fig. 5c). These data indicate that LkARF7 and LkARF19 bind directly to the LkBBM1 promoter in yeast cells.
To confirm that LkBBM1 is the direct target gene of LkARF7 and LkARF19, we conducted chromatin immunoprecipitation (ChIP) assays to detect the interactions of LkARF7 and LkARF19 proteins with the LkBBM1 promoter. Our ChIP-qPCR results showed that the DNA fragments containing each of the three AuxREs in the LkBBM1 promoter were enriched by overexpressing LkARF7 and LkARF19 individually, while the DNA fragment containing the ABRE element was not (Fig. 5d). These results demonstrate that LkARF7 and LkARF19 directly bind to the AuxREs in the LkBBM1 promoter in vitro.
To further verify the direct interactions between LkARF7/19 and the LkBBM1 promoter in planta, we conducted dual-luciferase assays using tobacco leaf agroinfiltration. When compared to the negative controls, the relative LUC/REN activities driven by the LkBBM1 promoter reporter were increased by ~2.33 and 2.62-folds when co-transformed with 35S::LkARF7 and 35S::LkARF19, respectively (Fig. 5e). Furthermore, strong fluorescent signals were detected in the tobacco leaves infiltrated with the LkBBM1 promoter-driven reporter gene and each of 35S::LkARF7 and 35S::LkARF19 (Fig. 5f). These results indicate that both LkARF7 and LkARF19 directly bind to the LkBBM1 promoter and activate LkBBM1 expression in plant cells.
The interaction of LkARF19 with LkRH53 in the nucleus
We conducted yeast two-hybrid (Y2H) assays by using the 2-week-old AR’s cDNA library constructed in Wang et al.36 to screen for the LkARF19-interacting proteins. As shown in Fig. 6a, we found that the AH109 strain containing pGBKT7-LkARF19 and pGADT7-LkRH53 and the positive control strain grew normally on QDO medium (SD/-Trp-Leu-His-Ade) and turned blue in QDO/X medium (SD/-Trp-Leu-His-Ade + X-α-gal), while the negative control strain did not. Thus, LkARF19 directly interacts with LkRH53. To further confirm the interaction between LkARF19 and LkRH53, we conducted the bimolecular fluorescence complementation (BiFC) assays by transforming LkARF19-cYFP and LkRH53-nYFP into tobacco leaves. The detected YFP fluorescence signals in the nucleus demonstrated that LkARF19 directly interacted with LkRH53 in the nucleus (Fig. 6b).
a Yeast two-hybrid analysis of interaction between LkARF19 and LkRH53. N, negative control (pGBKT7-LamC + pGADT7-LargeT); P, positive control (pGBKT7-p53 + pGADT7-LargeT); LkRH53 (pGBKT7-LkARF19 + pGADT7-LkRH53). DDO, SD/-Trp-Leu; QDO, SD/-Trp-Leu-His-Ade; QDO/X, SD/-Trp-Leu-His-Ade + X-α-gal. b Bimolecular fluorescence complementation assay analysis of interaction between LkARF19 and LkRH53. LkARF19-cYFP + nYFP and cYFP + LkRH53-nYFP were used as negative controls. Scale bars, 30 μm. c qRT-PCR analysis of LkRH53 in the basal stem sections (0.5 cm) during cutting propagation. DAC, days after cutting. The value on DAC 0 was set to 1. d Overexpression of LkRH53 promoted AR formation in 84 K poplar. Scale bars, 3 cm. e Average AR number and total AR length of the control plants, WT, and overexpression lines in 2-week-old seedlings. Error bars represent standard deviations from three biological replicates; *P < 0.05 and **P < 0.01, t test.
The role of LkRH53 in regulating AR formation
We used qRT-PCR to measure the relative expression levels of LkRH53 during cutting propagation of the D9 clone (Fig. 6c). Compared with that at 0 DAC, the relative expression levels of LkRH53 increased in the basal stem sections (0.5 cm) during cutting propagation by 1.84–2.86 times at 2–40 DAC with the highest expression level at 40 DAC.
We also stably transformed the pBI121-LkRH53 vector into 84 K poplar (Fig. 6d). We used PCR to confirm that the transgene was present in the genomic DNA of each transgenic line and qRT-PCR to confirm its overexpression in the transgenic lines (Supplementary Fig. 3c, f). We found that the average AR numbers and total AR length in 2-week-old transgenic seedlings were significantly higher than the control plants (Fig. 6e). Thus, overexpression of LkRH53 promoted AR formation in transgenic poplar.
Discussion
Plant-specific ARF transcription factors regulate plant growth and development such as embryo morphogenesis, vascular tissue formation, cotyledon development, fiber cell initiation, fruit development, leaf senescence, and floral organ abscission45,46,47,48. In addition, AtARF7/19 play roles in leaf expansion, AR and LR formation49,50. SmARF20, which is homologous to AtARF19, regulates LR formation in Salvia miltiorrhiza51. However, the underlying mechanism of ARF7/19 regulating AR development remains largely unknown. In the present study, we identified 14 LkARF genes in larch (Supplementary Fig. 1) and found the expression LkARF7 and LkARF19 was induced by auxin during the early stage of AR formation (Supplementary Fig. 5), while overexpression of LkARF7 and LkARF19 promoted AR formation by directly activating the expression of their target gene LkBBM1 (Figs. 3–5). We also found that LkARF19 could interact with LkRH53, and overexpression of LkRH53 in transgenic poplar-induced AR formation (Fig. 6e). All of these suggest an additional mechanism regulating AR formation via auxin signaling, LkARF7/19 transcriptionally activation of LkBBM1, and interaction between LkARF19 and LkRH53.
In Arabidopsis, the loss-of-function mutant of AtARF7 or AtARF19 displays altered leaf expansion, AR and LR formation, and hypocotyl phototropism52,53. We found that LkARF7 and LkARF19 were strongly expressed in ARs (Fig. 1a) and the relative expression levels of both genes gradually increased following AR formation (Fig. 1c, d). In this study, we chose to overexpress LkARF7 and LkARF19 individually in transgenic poplar, a model species for woody plants. We found that overexpression of each gene significantly increased AR formation (Fig. 3). These findings indicate that LkARF7 and LkARF19 play important roles in AR formation.
It is essential to conduct the knockdown and overexpression experiment in larch to provide a more comprehensive understanding of LkARF7/19 function54,55,56. However, it remains challenging to obtain transgenic larch plants, as a rapid, efficient, and stable transgenic system is still lacking. Model plants such as Arabidopsis and poplar have been used to study AR formation and the function of genes from other plants due to the well-established genetic transformation system in them57,58,59,60. Here, it was found that the sequence similarity of ARF7/19 between larch and poplar was greater than that of Arabidopsis, so poplar was used to study the function of LkARF7/19 in AR formation. Now, transformed larch embryonic cells61,62 have been obtained and gene editing has been performed in larch protoplasts63, giving us more opportunities to study the function of LkARF7/19 in larch.
It was reported that auxin content could affect the expression of ARFs64. After 0.5 mM NAA treatment, the expression of PpARF2B, PpARF4, PpARF7, and PpARF10A increased rapidly during fruit maturation of peach65. In Arabidopsis, the expression of ARF7/19 in hypocotyls is affected by the asymmetric distribution of auxin, which affects the geotropism and phototropism of hypocotyls66,67. Previous studies also showed that an auxin signal is required in the induction stage of AR and LR in Arabidopsis, Petunia hybrida, and E. globulus68,69,70. In the present work, we found that the expression levels of LkARF7 and LkARF19 significantly increased after the treatment of exogenous NAA for 2–24 h (Supplementary Fig. 5). These data suggest that the participation of LkARF7 and LkARF19 in AR formation is promoted by auxin.
Recent research on BBM started to focus on root development34. It was reported that expression of LkBBM1 was enriched in ARs and overexpression of LkBBM1 increased the AR number35,36. Our results showed that the expression of LkBBM1 gradually increased in the basal stem sections during in vitro propagation (Fig. 1e), which was highly correlated with that of LkARF7 and LkARF19 (Fig. 1c, d). We also found three AuxREs in the LkBBM1 promoter (Supplementary Fig. 6; Supplementary Data 2), which LkARF7 and LkARF19 can directly bind, leading to enhanced LkBBM1 expression (Fig. 5). Overall, these data suggest that LkARF7/19 promote AR formation by directly activating the transcription of LkBBM1.
While our data suggest that LkARF7/19 promote AR formation by activating transcription of LkBBM1, this hypothesis would require further validation by methods like electrophoretic mobility shift assays to evaluate the relationship between LkARF7/19 proteins and the LkBBM1 promoter. Likewise, while our Y2H and BiFC data suggest an interaction between LkARF19 and a larch DEAD-box RNA helicase, LkRH53, this interaction should be further verified through other GST pull-down or co-immunoprecipitation assays.
Previous studies have reported that DEAD-box RNA helicases are involved in root development and stress responses42,71. In Arabidopsis, AtRH53 showed strong promoter activities in root tips in 6-, 9-, and 16-day-old seedlings and a wounding-induced expression in detached leaves72. In this study, we identified a larch DEAD-box RNA helicase LkRH53 that interacted with LkARF19 by Y2H and BiFC assays. (Fig. 6a; 6b). Furthermore, we found a high expression level of LkRH53 during AR formation in larch (Fig. 6c) and the overexpression of LkRH53 in transgenic poplar promoted AR formation (Fig. 6e). These data suggest that wounding stimulates the expression of LkRH53, which may directly interact with LkARF19 to regulate AR formation.
In conclusion, we propose that auxin-induced expression of transcription factors LkARF7 and LkARF19 promote AR formation by forming an LkARF19- LkRH53 heterodimer and transcriptionally activating LkBBM1 (Fig. 7). These findings provide further insights into the molecular mechanisms of AR formation, which could benefit the molecular breeding of larch.
The expression of LkARF7 and LkARF19 increases after the semi-lignified stem is cutoff. Auxin also promotes the expression of LkARF7 and LkARF19. LkARF7 and LkARF19 bind to AuxREs and promote LkBBM1 transcription to regulate AR formation. In addition, LkARF19 interacts with LkRH53 to form heterodimers to regulate AR formation.
Methods
Plant materials and growth conditions
D9, a hybrid larch clone (L. kaempferi × L. olgensis), was grown in a climate-controlled greenhouse with 22 °C, 16-h/light, and 17 °C, 8-h/dark. Semi-lignified tender stems were propagated by in vitro culture as previously described35. Specifically, the newly grown stem tips of 6–8 cm were selected from 2-year-old seedlings, and cultured in medium (L9) with 0.01 mM NAA for 2 weeks. Then, the materials were transferred into NAA-free L9 medium for further culture. The basal stem sections (0.5 cm) on 0, 2, 4, 8, 12, 16, 24, and 40 days, and different tissues, roots, leaves, stems, and stem tips from two-month-old seedlings were collected and then immediately frozen in liquid nitrogen for RNA extraction. In addition, 0.01 mM NAA was added to the L9 medium for 0, 2, 6, 12, 16, and 24 h, and the cultured basal stems were collected and prepared for RNA extraction.
84 K, a hybrid poplar (P. alba × P. glandulosa) clone was grown at 23–25 °C under a 16-h/8-h light/dark cycle. Agrobacterium-mediated poplar transformation was conducted with leaf discs being used as the explants73. Specifically, 2–3 leaves were taken from the tips of 4-week-old 84 K poplar, and 4–5 wounds were cut on the leaves. The leaves were immersed in A. tumefaciens solution for 10 min, and co-cultured for 3 days at 25 °C in the darkness on differentiation medium (1/2 Murashige and Skoog (MS) + 0.5 mg/L 6-benzylaminopurine + 0.05 mg/L NAA). After 3 days, the leaves were transferred to differentiation medium with 200 mg/L timentin and 50 mg/L kanamycin for culture. After adventitious buds regenerated, they were cutoff and transferred to rooting medium (1/2 MS + 0.02 mg/L NAA + 0.05 mg/L indole-3-butyric acid) to grow roots.
Nicotiana benthamiana was sown onto 1/2 MS solid medium, and 1-week-old seedlings were transplanted into the flowerpot filled with peat and perlite, and grown in an artificial climate room with 22 °C and a 16-h/8-h light/dark cycle. Then, 5-week-old seedlings were used for dual-luciferase assays.
Promoter cloning and bioinformatics analysis of LkBBM1
Larch genomic DNA was extracted from leaves using the TaKaRa MiniBEST Plant Genomic DNA Extraction Kit (Takara, Japan). The cis-regulatory element of the LkBBM1 promoter was analyzed using the PLACE tool (http://www.dna.affrc.go.jp/htdocs/PLACE/).
Identification of LkARFs in larch
The hidden Markov model profiles of the ARF family, including the B3 DNA binding domain (DBD, Pfam02362), AUX_RESP domain (MR, Pfam06507), and AUX/IAA domain (PB1, Pfam02309) were applied to identify ARFs from the L. kaempferi genome74. Furthermore, 23 Arabidopsis (https://phytozome-next.jgi.doe.gov/), 36 P. trichocarpa (https://phytozome-next.jgi.doe.gov/), 8 P. abies (http://planttfdb.gao-lab.org/index.php) and 8 P. menziesii (http://planttfdb.gao-lab.org/index.php) ARF proteins were used as the queries to perform a BLASTP search against the protein sequences of the L. kaempferi genome with a cutoff e value of 1 × 10−5. All identified LkARFs were used for a conserved domain search using CDD (https://www.ncbi.nlm.nih.gov/cdd/) and SMART (http://smart.embl-heidelberg.de/) to confirm the presence of both the intact B3 and Auxin_resp domains. The sequence length, molecular weight, and theoretical isoelectric point of LkARF proteins were calculated using the online tool ExPASy (http://web.expasy.org/protparam/). The subcellular localizations were predicted according to the integration of prediction results of Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) and CELLO v2.5 (http://cello.life.nctu.edu.tw/).
Phylogenetic analysis, conserved motif analysis, and multiple sequence alignment
For phylogenetic reconstruction of the ARFs family, a total of 89 ARF protein sequences from L. kaempferi, Arabidopsis, P. trichocarpa, P. abies, and P. menziesii were aligned using ClustalW with default options. Phylogenetic trees were constructed by the neighbor-joining method using MEGA 7.0 software with 1,000 bootstrap replicates75. The Multiple Em for Motif Elicitation (MEME) v4.10.2 program (http://meme-suite.org/tools/meme) was applied to identify conserved motifs in the candidate ARF protein sequences. The number of motifs was set to ten. Multiple sequence alignment of ARF protein sequences was analyzed using DNAMAN 9.0 (Lynnon Corp., Canada).
Promoter cloning and bioinformatics analysis of LkBBM1
Larch genomic DNA was extracted from leaves using the TaKaRa MiniBEST Plant Genomic DNA Extraction Kit (Takara, Japan). The cis-regulatory element of the LkBBM1 promoter was analyzed using the PLACE tool (http://www.dna.affrc.go.jp/htdocs/PLACE/).
Isolation of RNA and qRT-PCR analysis
Total RNA was extracted from different tissues using RNAprep Pure Plant Plus Kit (TianGen, China). Total cDNA was synthesized using PrimeScript™ RT reagent Kit (TaKaRa, Japan). qRT-PCR was applied using SYBR Premix Ex Taq II (TaKaRa, Japan) with LightCycler 480 system (Roche, Switzerland). Gene-specific primers for qRT-PCR analysis were designed using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/primer3/input.htm) and listed in Supplementary Data 3. LaEF1A176 and PtoActin77 were used as the internal control genes. Three biological replicates were performed in each sample and relative gene expression levels were analyzed using the 2−△△Ct method78,79.
Subcellular localization of LkARF7 and LkARF19
The coding sequences (CDS) of LkARF7 and LkARF19 (without the stop codons) were amplified and sequenced using the primers listed in Supplementary Data 3. The verified fragments were inserted into the 35S::GFP vector (modified from the PHB vector). The recombinant LkARF7-GFP and LkARF19-GFP fusion and the 35S::GFP control vector were transformed individually into A. tumefaciens (GV3101), which were used in the leaves of 5-week-old tobacco seedlings for tobacco leaf agroinfiltration. GFP fluorescence signals in the leaves were visualized under a confocal laser scanning microscope (LSM710, Karl Zeiss, Germany) after 48 h of infection. All transient expression assays were repeated at least three times.
Transient transformation assay of larch
The full-length CDS of LkARF7 and LkARF19 were cloned into the pBI121 vector containing the flag reporter gene to obtain the fusion constructs 35S::LkARF7-flag and 35S::LkARF19-flag. The resulting vectors were transformed into the A. tumefaciens (GV3101) individually and then used for transient transformation of the freshly-grown seedlings of D9 clone80. Transient transgenic seedlings were cultured on 1/2 MS solid medium under 22 °C, 16-h/light and 17 °C, 8-h/dark for 48 h and then collected for detecting the expression of LkARF7, LkARF19, and LkBBM1. The primers are listed in Supplementary Data 3. Three biological replicates were used for each vector.
Y1H assays
The full-length CDS of LkARF7 and LkARF19 were cloned into the prey vector pGADT7 individually, and the LkBBM1 promoter was cloned into the bait vector pHIS2. The combinations of the prey vectors and bait vectors were co-transformed into the Y187 yeast strain, which was grown on SD/-Trp-Leu medium for 3 days at 30 °C and then transferred onto SD/-Trp-Leu-His + 75 mM 3-AT for further incubation at 30 °C. p53HIS2 + pGAD53T were used as the positive control, while pHIS2-LkBBM1 promoter + pGADT7 were used as the negative control.
ChIP assays
Transient transgenic larch plants were used for ChIP experiments, which were performed as previously reported81. The plants were crosslinked with 1% formaldehyde, pulverized in liquid nitrogen, and then 1% SDS cracking buffer was added for ultrasonication. The sonicated chromatin was immunoprecipitated with anti-flag antibody (1:100 dilution, CWBIO) or without the anti-flag antibody. Each DNA sample was analyzed by ChIP-qPCR to determine the enrichment of promoter fragments. LaEF1A was used as the internal control76. The primers used are listed in Supplementary Data 3.
Dual-luciferase assays
The transactivation roles of LkARF7 and LkARF19 on the target LkBBM1 promoter were assessed by dual-luciferase assays as previously described82. The full-length CDS of LkARF7 and LkARF19 were inserted into pGreen II 62SK vector individually, and the promoter fragment of LkBBM1 was cloned into pGreen II 0800‐LUC vector. Each resulting construct was transformed into the A. tumefaciens (GV3101-psoup) and transiently expressed in 5-week-old N. benthamiana leaves. The Dual-Luciferase® Reporter Assay System (Promega, USA) was used for detection of LUC and REN activities with a dual-luciferase reporter assay system (Promega, GloMax 20/20 Luminometer, Wisconsin, USA). Dual-luciferase assays were performed with at least four biological replicates. The luciferase complementation imaging assay was applied with the Newton 7.0 Bio Plant Imaging System (Vilber Lourmat, France).
Y2H assays
An Y2H library from the AR cDNA of the D9 clone was constructed in Wang et al.36. The CDS of LkARF19 was cloned into the bait vector pGBKT7 and transformed into the yeast strain AH109 together with the prey vector pGADT7 to evaluate self-activation. Then protein-protein interactions were screened following the manufacturer’s instructions (Clontech, USA). The cDNA library was screened on SD/-Trp-Leu, SD/-Trp-Leu-His, and SD/-Trp-Leu-His-Ade/X-α-Gal medium to identify LkARF19-interacting proteins. The positive clones interacting with LkARF19 were inserted into the prey vector pGADT7 and co-transformed into AH109 with bait vector pGBKT7-LkARF19 to confirm the interactions. The bait vectors pGBKT7-p53 and pGBKT7-LaminC were co-transformed into AH109 with the prey vector pGADT7-LargeT as positive and negative controls, respectively.
BiFC assays
The CDS of LkARF19 (without the stop codon) was cloned into the 35S::SPYCE vector to obtain the fusion construct LkARF19-cYFP. The CDS of LkRH53 (without the stop codon) was inserted into the 35S::SPYNE vector to obtain the fusion constructs LkRH53-nYFP. The combination of LkARF19-cYFP + LkRH53-nYFP was transformed into the A. tumefaciens (GV3101) and transiently expressed in tobacco leaves. The YFP fluorescence was detected by confocal laser scanning microscopy (LSM710; Karl Zeiss, Jena, Germany). The primers used are listed in Supplementary Data 3.
Overexpression of LkARF7, LkARF19, and LkRH53 in 84 K poplar and phenotypic analyses
The full-length CDS of LkARF7, LkARF19, and LkRH53 were amplified and cloned individually into the binary vector pBI121 with the help of XbaI/SmaI to generate the 35S::LkARF7, 35S::LkARF19, and 35S::LkRH53 vectors. The constructs were then transformed into A. tumefaciens (GV3101) and used to transform 84 K poplar83. After screening on a selection medium with 50 mg/L kanamycin, transgenic poplar lines were identified through PCR and qRT-PCR. The primers are listed in Supplementary Data 3. Three independent lines were used for each construct for further study. Phenotype analysis of AR number and length in the 3-week-old control (Ctrl, transformed with empty vector), WT, and transgenic poplar were conducted. The plant height, stem diameter, and root fresh and dry weight were measured and the internode numbers were counted in the 8-week-old plants. Significant differences relative to control plants were determined by Student’s t test.
Statistics and reproducibility
Statistics, which n = 3 biologically independent samples were conducted with multiple t test using GraphPad Prism v6.02 (GraphPad, America), and asterisks and double asterisks indicate P < 0.05 and P < 0.01, respectively. The heat map and bar charts were generated using the Omiscshare tools (http://omicshare.com/tools/) and GraphPad Prism v6.02 with the default parameters, respectively. For the dual-luciferase assays, the detection of relative LUC/REN activities was carried out with four biological replicates to ensure the reliability of results. The phenotypic assessment of each poplar overexpression line was independently repeated at least three times. All the qRT-PCR assays were performed with three biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequences of LkARF7, LkARF19, and LkRH53 were deposited in NCBI with GenBank accession numbers ON529550, ON529549, and ON529551, respectively. The uncropped gels of LkARF7-, LkARF19- and LkRH53-overexpression transgenic poplar lines are shown in Supplementary Fig. 7. The source data behind the graphs in this study are listed in Supplementary Data 4.
References
Pijut, P., Woeste, K. & Michler, C. Promotion of adventitious root formation of difficult‐to‐root hardwood tree species. Hortic. Rev. 38, 213–251 (2011).
de Klerk, G. J., van der Krieken, W. & de Jong, J. C. The formation of adventitious roots: new concepts, new possibilities. Vitr. Cell. Dev. Biol. 35, 189–199 (1999).
Guan, L. et al. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 34, 506–521 (2015).
Ikeuchi, M. et al. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 70, 377–406 (2019).
Steffens, B. & Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 170, 603–617 (2016).
Li, S. W., Xue, L., Xu, S., Feng, H. & An, L. Mediators, genes and signaling in adventitious rooting. Bot. Rev. 75, 230–247 (2009).
Bellini, C., Pacurar, D. I. & Perrone, I. Adventitious roots and lateral roots: similarities and differences. Annu. Rev. Plant Biol. 65, 639–666 (2014).
Boerjan, W. et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7, 1405–1419 (1995).
Hagen, G. & Guilfoyle, T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49, 373–385 (2002).
Ulmasov, T., Hagen, G. & Guilfoyle, T. J. Activation and repression of transcription by auxin-response factors. Proc. Natl Acad. Sci. USA 96, 5844–5849 (1999).
Wang, R. & Estelle, M. Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 21, 51–58 (2014).
Guilfoyle, T. J. & Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460 (2007).
Van Ha, C. et al. The auxin response factor transcription factor family in soybean: genome-wide identification and expression analyses during development and water stress. DNA Res. 20, 511–524 (2013).
Okushima, Y. et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463 (2005).
Wang, D. et al. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 394, 13–24 (2007).
Xing, H. et al. Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genomics 12, 178 (2011).
Kalluri, U. C., Difazio, S. P., Brunner, A. M. & Tuskan, G. A. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 7, 59–72 (2007).
Yu, H. et al. Genome-wide characterization and expression profiling of the AUXIN RESPONSE FACTOR (ARF) gene family in Eucalyptus grandis. PLoS ONE 9, e108906 (2014).
Wang, J. W. et al. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17, 2204–2216 (2005).
Gutierrez, L. et al. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21, 3119–3132 (2009).
Gutierrez, L. et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24, 2515–2527 (2012).
Okushima, Y., Fukaki, H., Onoda, M., Theologis, A. & Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–130 (2007).
Lee, H. W., Kim, N. Y., Lee, D. J. & Kim, J. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151, 1377–1389 (2009).
Lee, H. W. et al. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 19, 46–56 (2019).
Inukai, Y. et al. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17, 1387–1396 (2005).
Liu, H. et al. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 43, 47–56 (2005).
Liu, S. et al. The peu-miR160a-PeARF17.1/PeARF17.2 module participates in the adventitious root development of poplar. Plant Biotechnol. J. 18, 457–469 (2020).
Zhang, C. L. et al. Apple SUMO E3 ligase MdSIZ1 facilitates SUMOylation of MdARF8 to regulate lateral root formation. N. Phytol. 229, 2206–2222 (2021).
Boutilier, K. et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14, 1737–1749 (2002).
Jha, P. & Kumar, V. BABY BOOM (BBM): a candidate transcription factor gene in plant biotechnology. Biotechnol. Lett. 40, 1467–1475 (2018).
Horstman, A. et al. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol. 175, 848–857 (2017).
Aida, M. et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120 (2004).
Galinha, C. et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449, 1053–1057 (2007).
Imin, N., Nizamidin, M., Wu, T. & Rolfe, B. G. Factors involved in root formation in Medicago truncatula. J. Exp. Bot. 58, 439–451 (2007).
Li, K. P., Sun, X. M., Han, H. & Zhang, S. G. Isolation, characterization and expression analysis of the BABY BOOM (BBM) gene from Larix kaempferi × L. olgensis during adventitious rooting. Gene 551, 111–118 (2014).
Wang, H. et al. Isolation and characterization of larch BABY BOOM2 and its regulation of adventitious root development. Gene 690, 90–98 (2019).
Horstman, A., Willemsen, V., Boutilier, K. & Heidstra, R. AINTEGUMENTA-LIKE proteins: hubs in a plethora of networks. Trends Plant Sci. 19, 146–157 (2014).
Xu, X. et al. Genome-wide identification and characterization of DEAD-box helicase family associated with early somatic embryogenesis in Dimocarpus longan Lour. J. Plant Physiol. 258–259, 153364 (2021).
Wang, Y. et al. Genome-wide identification and characterization of the soybean DEAD-box gene family and expression response to rhizobia. Int. J. Mol. Sci. 23, 1120–1135 (2022).
Huang, C. K. et al. The DEAD-box RNA helicase AtRH7/PRH75 participates in pre-rRNA processing, plant development and cold tolerance in Arabidopsis. Plant Cell Physiol. 57, 174–191 (2016).
Ohtani, M., Demura, T. & Sugiyama, M. Arabidopsis root initiation defective1, a DEAH-box RNA helicase involved in pre-mRNA splicing, is essential for plant development. Plant Cell 25, 2056–2069 (2013).
Liu, Y. et al. The TSN1 binding protein RH31 is a component of stress granules and participates in regulation of salt-stress tolerance in Arabidopsis. Front. Plant Sci. 12, 804356 (2021).
Bush, M. S., Crowe, N., Zheng, T. & Doonan, J. H. The RNA helicase, eIF4A-1, is required for ovule development and cell size homeostasis in Arabidopsis. Plant J. 84, 989–1004 (2015).
Zhang, X. D., Sun, J. Y., You, Y. Y., Song, J. B. & Yang, Z. M. Identification of Cd-responsive RNA helicase genes and expression of a putative BnRH24 mediated by miR158 in canola (Brassica napus). Ecotoxicol. Environ. Saf. 157, 159–168 (2018).
Zhao, Z. et al. Hormonal control of the shoot stem-cell niche. Nature 465, 1089–1092 (2010).
de Jong, M. et al. Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J. Exp. Bot. 66, 3405–3416 (2015).
Chandler, J. W. Auxin response factors. Plant. Cell Environ. 39, 1014–1028 (2016).
Xiao, G. et al. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 69, 4323–4337 (2018).
Wilmoth, J. C. et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43, 118–130 (2005).
Kalve, S. et al. Osmotic stress inhibits leaf growth of Arabidopsis thaliana by enhancing ARF-mediated auxin responses. N. Phytol. 226, 1766–1780 (2020).
Xu, Z., Ji, A., Song, J. & Chen, S. Genome-wide analysis of auxin response factor gene family members in medicinal model plant Salvia miltiorrhiza. Biol. Open 5, 848–857 (2016).
Harper, R. M. et al. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12, 757–770 (2000).
Li, Q. Q. et al. Phytochrome B inhibits darkness-induced hypocotyl adventitious root formation by stabilizing IAA14 and suppressing ARF7 and ARF19. Plant J. 105, 1689–1702 (2021).
Peng, J. Gene redundancy and gene compensation: an updated view. J. Genet. Genomics 46, 329–333 (2019).
Lange, M., Yellina, A. L., Orashakova, S. & Becker, A. Virus-induced gene silencing (VIGS) in plants: an overview of target species and the virus-derived vector systems. Methods Mol. Biol. 975, 1–14 (2013).
Han, H. RNA interference to knock down gene expression. Methods Mol. Biol. 1706, 293–302 (2018).
Zhu, L. et al. Chrysanthemum transcription factor CmLBD1 direct lateral root formation in Arabidopsis thaliana. Sci. Rep. 6, 1–10 (2016).
Chang, Y. et al. Characterization of walnut JrWOX11 and its overexpression provide insights into adventitious root formation and development and abiotic stress tolerance. Front. Plant Sci. 13, 951737 (2022).
Wang, H. et al. Transcription factor LkWOX4 is involved in adventitious root development in Larix kaempferi. Gene 758, 144942 (2020).
Zhang, Y. et al. The bZIP53-IAA4 module inhibits adventitious root development in Populus. J. Exp. Bot. 71, 3485–3498 (2020).
Fan, Y. et al. Examination of the transcriptional response to LaMIR166a overexpression in Larix kaempferi (Lamb.) Carr. Biol. (Basel). 10, 576–592 (2021).
Kang, Y., Li, W., Zhang, L. & Qi, L. Over-expression of the cell-cycle gene LaCDKB1;2 promotes cell proliferation and the formation of normal cotyledonary embryos during Larix kaempferi somatic embryogenesis. Genes (Basel) 12, 1435–1448 (2021).
Ren, Q. et al. PAM-less plant genome editing using a CRISPR–SpRY toolbox. Nat. Plants 7, 25–33 (2021).
Szemenyei, H., Hannon, M. & Long, J. A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384–1386 (2008).
Diao, D. et al. Genome-wide identification of the ARF (auxin response factor) gene family in peach and their expression analysis. Mol. Biol. Rep. 47, 4331–4344 (2020).
Sun, J., Qi, L., Li, Y., Zhai, Q. & Li, C. PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell 25, 2102–2114 (2013).
Yin, H. et al. SAUR15 promotes lateral and adventitious root development via activating H1-ATPases and auxin biosynthesis. Plant Physiol. 184, 837–851 (2020).
Negishi, N., Oishi, M. & Kawaoka, A. Chemical screening for promotion of adventitious root formation in Eucalyptus globulus. BMC Proc. 5, P139 (2011).
Ahkami, A. H. et al. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238, 499–517 (2013).
Bustillo-Avendaño, E. et al. Regulation of hormonal control, cell reprogramming, and patterning during de novo root organogenesis. Plant Physiol. 176, 1709–1727 (2018).
Gu, L., Xu, T., Lee, K., Lee, K. H. & Kang, H. A chloroplast-localized DEAD-box RNA helicase AtRH3 is essential for intron splicing and plays an important role in the growth and stress response in Arabidopsis thaliana. Plant Physiol. Biochem. 82, 309–318 (2014).
Matthes, A. et al. Two DEAD-box proteins may be part of RNA-dependent high-molecular-mass protein complexes in Arabidopsis mitochondria. Plant Physiol. 145, 1637–1646 (2007).
Liu, B. et al. WUSCHEL-related homeobox genes in Populus tomentosa: diversified expression patterns and a functional similarity in adventitious root formation. BMC Genomics 15, 1–14 (2014).
Sun, C. et al. The Larix kaempferi genome reveals new insights into wood properties. J. Integr. Plant Biol. 64, 1364–1373 (2022).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Li, W. et al. Regulation of LaMYB33 by miR159 during maintenance of embryogenic potential and somatic embryo maturation in Larix kaempferi (Lamb.) Carr. Plant Cell Tissue Organ Cult. 113, 131–136 (2013).
Li, J. et al. The WUSCHEL-related homeobox 5a (PtoWOX5a) is involved in adventitious root development in poplar. Tree Physiol. 38, 139–153 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Zhao, F. et al. An optimized protocol for stepwise optimization of real-time RT-PCR analysis. Hortic. Res. 8, 179–199 (2021).
Ji, X. et al. A transient transformation system for the functional characterization of genes involved in stress response. Plant Mol. Biol. 32, 732–739 (2014).
Wang, L. Q. et al. WUSCHEL-related homeobox gene PagWOX11/12a responds to drought stress by enhancing root elongation and biomass growth in poplar. J. Exp. Bot. 71, 1503–1513 (2020).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13–26 (2005).
Nilsson, O. et al. Spatial pattern of cauliflower mosaic virus 35S promoter-luciferase expression in transgenic hybrid aspen trees monitored by enzymatic assay and non-destructive imaging. Transgenic Res. 1, 209–220 (1992).
Acknowledgements
This work were supported by the Forestry Industry Research Special Funds for Public Welfare Projects (201504104) and the Special Funds of Research Institute of Forestry (LYSZX202002).
Author information
Authors and Affiliations
Contributions
G.-Y.T., Y-H.X., and X.-M.S. conceived and designed the research. G.-Y.T. performed the experiments and wrote the manuscript. G.-Y.T., Y.-H.X., W.-F.L., K.-P.L., H.-M.W., C.S., and X.-M.S. analyzed the data. W.-F.L. helped write the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Inge Verstraeten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Inglis. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tao, GY., Xie, YH., Li, WF. et al. LkARF7 and LkARF19 overexpression promote adventitious root formation in a heterologous poplar model by positively regulating LkBBM1. Commun Biol 6, 372 (2023). https://doi.org/10.1038/s42003-023-04731-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04731-3
- Springer Nature Limited
This article is cited by
-
BpWOX11 promotes adventitious root formation in Betula pendula
BMC Plant Biology (2024)
-
Genome-wide systematic survey and analysis of the RNA helicase gene family and their response to abiotic stress in sweetpotato
BMC Plant Biology (2024)