Abstract
Background
Adventitious root (AR) formation is a complex genetic trait, which is controlled by various endogenous and environmental cues. Auxin is known to play a central role in AR formation; however, the mechanisms underlying this role are not well understood.
Results
In this study, we showed that a previously identified auxin signaling module, AUXIN RESPONSE FACTOR(ARF)7/ARF19-LATERAL ORGAN BOUNDARIES DOMAIN(LBD)16/LBD18 via AUXIN1(AUX1)/LIKE-AUXIN3 (LAX3) auxin influx carriers, which plays important roles in lateral root formation, is involved in AR formation in Arabidopsis. In aux1, lax3, arf7, arf19, lbd16 and lbd18 single mutants, we observed reduced numbers of ARs than in the wild type. Double and triple mutants exhibited an additional decrease in AR numbers compared with the corresponding single or double mutants, respectively, and the aux1 lax3 lbd16 lbd18 quadruple mutant was devoid of ARs. Expression of LBD16 or LBD18 under their own promoters in lbd16 or lbd18 mutants rescued the reduced number of ARs to wild-type levels. LBD16 or LBD18 fused to a dominant SRDX repressor suppressed promoter activity of the cell cycle gene, Cyclin-Dependent Kinase(CDK)A1;1, to some extent. Expression of LBD16 or LBD18 was significantly reduced in arf7 and arf19 mutants during AR formation in a light-dependent manner, but not in arf6 and arf8. GUS expression analysis of promoter-GUS reporter transgenic lines revealed overlapping expression patterns for LBD16, LBD18, ARF7, ARF19 and LAX3 in AR primordia.
Conclusion
These results suggest that the ARF7/ARF19-LBD16/LBD18 transcriptional module via the AUX1/LAX3 auxin influx carriers plays an important role in AR formation in Arabidopsis.
Similar content being viewed by others
Background
Root architecture in higher plants, which is critical for anchorage in soil and the uptake of water and nutrients, is diverse at both the system and anatomical levels [1]. In general, dicotyledonous plants, such as Arabidopsis thaliana, have a primary root that branches to form lateral roots (LRs). In both monocot and dicotyledonous plants, the primary root can develop adventitious roots (ARs) that arise naturally from the aerial organs as an adaptive response to environmental changes, such as flooding and dark-light transitions, or artificially by wounding [2,3,4,5]. AR formation is critical for vegetative propagation of elite genotypes in agriculture and is also important for plant survival under a variety of biotic and abiotic stresses [4, 5]. Auxin plays a central role in both LR and AR formation [6]. Although signaling and molecular mechanisms of auxin-regulated primary and LR development are relatively well characterized, our understanding of how auxin regulates AR formation is rudimentary [1, 7,8,9].
Plant root development is regulated by establishing auxin maxima at the primordium tip through auxin transport [10,11,12,13]. During auxin transport, auxin travels acropetally or basipetally over long distances by the combined action of plasma membrane-localized auxin efflux and influx carriers and triggers various regulatory mechanisms along its path [14,15,16,17]. Auxin efflux carriers, including two major transmembrane proteins, i.e., PIN-FORMED (PIN) and ATP-binding cassette subfamily B (ABCB), were shown to be involved in both LR and AR formation in different plant species [10, 18,19,20,21,22]. Studies with the polar auxin transport inhibitor, N-1-naphthylphthalamic acid, showed that PIN1-mediated auxin transport is necessary for AR emergence in rice plants [23]. Auxin together with ethylene positively regulates AR initiation through DIAGEOTROPICA (DGT), which encodes a cyclophilin A-type protein (SlCYP) [24, 25]. SlCYP1 changes the abundance of PIN efflux carriers at the plasma membrane to modulate polar auxin transport during AR initiation [26,27,28,29].
Auxin signaling is regulated by two large protein families: the AUXIN RESPONSE FACTOR (ARF) proteins, which act as the DNA-binding transcriptional regulators of auxin responses, and the Aux/IAA proteins, negative regulators of ARFs [30]. Several ARFs have been identified as playing a role in AR formation in both Arabidopsis and rice. ARF6 and ARF8 have been shown to act as positive regulators of AR initiation in Arabidopsis hypocotyls, whereas ARF17 acts as a negative regulator [31, 32]. These ARFs regulate each other’s expression at the transcriptional and posttranscriptional levels by modulating the homeostasis of miR160, which targets ARF17 and miR167, subsequently targeting both ARF6 and ARF8 [32]. This complex network of transcription factors regulates the expression of three auxin-inducible GRETCHEN HAGEN3 (GH3) genes, encoding acyl-acid-amido synthetases, which are required for fine-tuning AR initiation in the Arabidopsis hypocotyls by modulating jasmonic acid homeostasis [33]. Some studies have indicated that auxin signaling modules involved in LR formation could play a role in AR formation as well. For instance, ARF7 and ARF19 control LR formation as well as AR formation in Arabidopsis [34,35,36,37,38]. OsARF16, which is a rice ortholog of ARF7 and ARF19, controls the initiation of adventitious crown root primordia in rice by activating the expression of CROWN ROOTLESS1/ADVENTITIOUS ROOTLESS1 (CRL1/ARL1), which encodes a LATERAL ORGAN BOUNDARIES DOMAIN (LBD) protein [39,40,41].
In Arabidopsis, several LBD genes, such as LBD16, − 18, − 29 and − 33, have been demonstrated to play critical and distinct roles in auxin-regulated LR development [37, 42,43,44,45,46,47,48,49]. It has been shown that the auxin influx carriers AUXIN1 (AUX1) and LIKE-AUX1 3 (LAX3) are required for auxin-responsive expression of LBD16 and LBD18 to control various stages of LR development in Arabidopsis [45, 50, 51]. In the present study, we show that the AUX1/LAX3-ARF7/ARF19-LBD16/LBD18 signaling module is also important for AR formation in Arabidopsis, providing evidence of a common regulatory mechanism being utilized for LR and AR formation during auxin signaling.
Results
Analysis of GUS expression patterns of Pro ARF7 :GUS, Pro ARF19 :GUS, Pro LAX3 :GUS, Pro LBD16 :GUS and Pro LBD18 :GUS during AR development
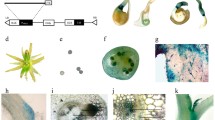
To gain insights into the function of the LAX3-ARF7/ARF19-LBD16/LBD18 signaling module during AR development, we analyzed GUS expression in ProLBD16:GUS, ProLBD18:GUS, ProARF7:GUS, ProARF19:GUS and ProLAX3:GUS transgenic plants during the early stages of AR formation (Fig. 1). GUS expression was detected in the cotyledon and lower part of the hypocotyl of 3-d-old dark-grown ProLBD16:GUS seedlings at time T0 (Fig. 1a). After transferring these seedlings to the light for 72 h, GUS expression was clearly detected in the early AR primordium in the hypocotyl (Fig. 1b). After 6 d in the light, GUS expression generally increased in both the hypocotyl and root and was detected in the hypocotyl stele tissue near the emerged AR (Fig. 1c). Regarding ProLBD18:GUS, GUS expression was detected only in the hypocotyl of 3-d-old dark-grown seedlings (Fig. 1d): after transferring to the light for 72 h, strong GUS expression was detected in the AR primordium in the hypocotyl (Fig. 1e). In ProARF7:GUS, ProARF19:GUS and ProLAX3:GUS seedlings, GUS expression was detected in both the hypocotyl stele tissue and AR primordium after transferring 3-d-old dark-grown seedlings to the light for 72 h (Fig. 1g–o). These overlapping and distinctive GUS expression patterns in the hypocotyl stele tissue and AR primordium of the GUS reporter transgenic lines indicated that LBD16 and LBD18 may play an overlapping role in early AR primordium development and LBD18 may play a distinctive role in the AR primordium in later developmental stages downstream of ARF7/ARF19-LAX3 during AR development.
GUS expression in hypocotyls of ProLBD16:GUS, ProLBD18:GUS, ProARF7:GUS, ProARF19:GUS and ProLAX3:GUS transgenic plants. a-c GUS staining for the expression of ProLBD16:GUS, d-f ProLBD18:GUS, g-i ProARF7:GUS, j-l ProARF19:GUS and m-o ProLAX3:GUS in seedlings grown in the dark for 3 d (a, d, g, j and m) and then in the light for 72 h (b, e, h, k and n) or 6 d (c, f, i, l and o). Magnified images of the regions boxed in b, c, e, h, i, k and n are shown in b1, b2, c1, e1, e2, h1, h2, i1, k1, k2, n1 and n2. Arrows point to ARs or primordia. Bars = 1 cm in a-o and 50 μm in b1, b2, c1, e1, e2, h1, h2, i1, k1, k2, n1 and n2
AUX1, LAX3, LBD16 and LBD18 are involved in AR formation in Arabidopsis hypocotyls
To determine the roles of auxin influx carriers, AUX1 and LAX3, and two critical LBD transcription factors, LBD16 and LBD18, in AR formation, we measured AR numbers on hypocotyls from single and multiple mutants derived from aux1, lax3, lbd16 and lbd18 (Fig. 2). To induce AR formation in hypocotyls, seeds germinated were etiolated vertically for 3 d and grown in the light for 7 d, and the ARs were counted [32]. The aux1, lax3, lbd16 and lbd18 single mutants showed a significant decrease in AR numbers compared with that of the wild type after transferring 3-d-old dark-grown seedlings to the light for 7 d (49.01, 41.17, 23.52 and 41.17% for aux1, lax3, lbd16 and lbd18, respectively) (Fig. 2). The number of ARs in double mutants, lbd16 lbd18, aux1 lbd16, aux1 lbd18, lax3 lbd16, lax3 lbd18 and aux1 lax3, was further reduced compared with that of their corresponding single mutants (Fig. 2). AR numbers in triple mutants, aux1 lbd16 lbd18 and lax3 lbd16 lbd18, were further reduced to ~ 8% compared to that of the wild type, whereas the aux1 lax3 double mutant and aux1 lax3 lbd16 and aux1 lax3 lbd18 triple mutants exhibited 3.5–4% that of the wild-type AR number (Fig. 2). In the quadruple mutant, aux1 lax3 lbd16 lbd18, AR formation could not be detected. These results indicate that auxin influx carriers, such as AUX1 and LAX3, are critical for AR formation and that AUX1, LAX3, LBD16 and LBD18 genes are essential for AR formation. To further confirm the role of LBD16 and LBD18 in AR formation, we generated transgenic Arabidopsis plants expressing LBD16:MYC (ProLBD16:LBD16:Myc/lbd16) or LBD18:HA (ProLBD18:LBD18:HA/lbd18) in lbd16 or lbd18 mutant background under the control of their own promoter, respectively, (Fig. 3a, b), and analyzed AR numbers of these complementation lines. The three different complementation lines selected for AR analysis rescued AR development defects caused by lbd16 or lbd18 mutations to varying degrees (Fig. 3c). ProLBD16:LBD16:Myc/lbd16 (#2–1) and ProLBD18:LBD18:HA/lbd18 (#2–1) transgenic mutant lines showed wild-type levels of AR numbers (Fig. 3c). These results demonstrated that LBD16 and LBD18 play significant roles in AR development.
Number of ARs in the hypocotyls of wild-type (Col-0), lbd16, lbd18, aux1 and lax3 single and multiple mutants. ARs were measured in seedlings that were grown on vertical plates for 3 d in darkness, until their hypocotyls were 6 mm long, and then transferred to the light for 7 d. Data are presented as means ± SE, determined from at least 90 seedlings. Different letters indicate a significant difference determined by one-way ANOVA followed by Duncan’s post hoc test (P < 0.05)
Complementation analysis of AR formation of lbd16 and lbd18. a Expression analysis of LBD16 in Col-0, lbd16 and ProLBD16:LBD16:Myc/lbd16 plants. b Expression analysis of LBD18 in Col-0, lbd18 and ProLBD18:LBD18:3XHA/lbd18 plants. Seven-d-old seedlings were used for real-time RT-PCR analysis (A and B). The relative fold changes were plotted after normalization to ACTIN7. The relative fold change represents the ratio in the mutant or transgenic plants relative to the transcript level in Col-0 plants. Mean ± SE values were determined from three technical replicates. c Number of ARs of ProLBD16:LBD16:myc/lbd16 and ProLBD18:LBD18:HA/lbd18 plants. Numbers above the lines indicate the line numbers of transgenic plants. The number of adventitious roots was analyzed as described in Fig. 2. Data are presented as means ± SE, determined from 100 seedlings. Different letters indicate a significant difference determined by one-way ANOVA followed by Duncan’s post hoc test (P < 0.05)
Both LBD16:SRDX and LBD18:SRDX suppress cell cycle gene promoter activities during induction of AR formation
Previous studies have shown that LBD16 and LBD18 regulate expression of some cell cycle genes during LR initiation [45, 48]. To examine if LBD16 and LBD18 might be involved in the expression of cell cycle genes during AR formation, we used transgenic Arabidopsis expressing LBD16:SUPERMAN REPRESSIVE DOMAIN X (SRDX) or LBD18:SRDX under the control of their own promoters and harboring Cyclin-Dependent Kinase (CDK)A1;1 or CDKB1:1 in Col-0 or in lbd18 mutants [45]. We found that LBD16:SRDX suppressed GUS expression in the vasculature of the hypocotyl in ProCDKB1;1:GUS transgenic plants, whereas LBD18:SRDX suppressed GUS expression in the primordium and vasculature in the hypocotyl of ProCDKA1;1:GUS transgenic plants (Fig. 4). Suppression of GUS expression in the hypocotyl vasculature of ProCDKB1;1:GUS by LBD16:SRDX or in the AR of ProCDKA1;1:GUS by LBD18:SRDX correlates with preferential expression of LBD16 or LBD18 in the hypocotyl stele tissue or only in the AR primordium, respectively. We also noted that LBD16:SRDX completely blocked AR formation in the hypocotyl in the light, whereas LBD18:SRDX did not block AR formation (Fig. 4). These results indicated that LBD16 and LBD18 might be involved in the expression of some cell cycle genes during AR development at distinctive stages. However, further quantitative and functional analyses are necessary to clarify the direct involvement of LBD16 and LBD18 in regulating cell cycle gene expression during AR development.
LBD16 and LBD18 act downstream of ARF7 and ARF19 to regulate AR development
During LR development, LBD16 and LBD18 are transcriptionally regulated downstream of ARF7 and ARF19 during auxin signaling [34, 37, 52]. We thus investigated whether the same transcriptional module functions during AR formation in a light-dependent manner. To this end, we analyzed expression of LBD16 and LBD18 in hypocotyls of arf7 and arf19 single mutants and an arf7 arf19 double mutant at different time points during the early stages of AR formation by RT-qPCR analysis (Fig. 5). At T0, which is the etiolated stage of seedlings (just before transferring to light), the expression of LBD16 and LBD18 was unchanged in all three mutants compared with that of the wild type (Fig. 5). After transferring to the light for 72 h (T72L), the expression of LBD16 and LBD18 was enhanced in the wild type as well as in all mutant plants. However, light-induced expression of LBD16 and LBD18 was significantly reduced in arf7 (by 48.5 and 45.6%, respectively) and arf19 mutants (20.9 and 33.3%, respectively) compared with that of the wild type (Fig. 5a). LBD16 and LBD18 expression were further reduced in arf7 arf19 double mutants (by 66.6 and 55.9%, respectively), compared with that of the arf7 and arf19 single mutants (Fig. 5b), indicating that ARF7 and ARF19 regulate LBD16 and LBD18 expression during AR development after the dark-light transition.
Expression analyses of LBD16 and LBD18 in hypocotyls of Col-0, arf7, arf19 and arf7 arf19 mutants. Expression of LBD16 (a) and LBD18 (b) transcripts in hypocotyls of Col-0, arf7, arf19 and arf7 arf19. Seedlings were etiolated in the dark until their hypocotyls were 6 mm long (T0) and then transferred to the light for 72 h (T72L). The seedlings were then harvested for RT-qPCR analysis. The relative fold change represents the ratio of the transcript level in the mutants relative to the transcript level in Col-0 at T0. Mean ± SE values were determined from three biological replicates (each biological replicate was estimated as the average of two technical RT-qPCR replicates). Different letters indicate a significant difference determined by one-way ANOVA followed by Duncan’s post hoc test (P < 0.05)
In a previous study, overexpression of LBD16 or LBD18 in arf7 arf19 mutant under the control of the Cauliflower mosaic virus (CaMV 35S) promoter resulted in induction of notable LR formation within 12 d, demonstrating that LBD16 and LBD18 can independently function downstream of ARF7 and ARF19 to control LR development [37, 42]. As reported in previous studies [37, 42], the LR phenotype of the arf7 arf19 mutant was rescued by LBD16 or LBD18 overexpression to some extent (Fig. 6b, top panel). We next examined whether overexpression of LBD16 or LBD18 in arf7 arf19 mutants could cause ectopic initiation of ARs, but could not detect any AR formation even after 2 weeks of plant growth in the light (Fig. 6b, bottom panel). This result suggested that unlike LR initiation, LBD16 and LBD18 require additional factors produced by ARF7 and ARF19 to induce AR initiation.
Analysis of LR and AR formation in arf7 arf19 mutants expressing LBD16:GR or LBD18:GR. a Expression analysis of LBD16 and LBD16:GR in Col-0, arf7 arf19 and Pro35S:LBD16:GR/arf7arf19 (left panels) and LBD18 and LBD18:GR in Col-0, arf7 arf19 and Pro35S:LBD18:GR/arf7arf19 plants (right panels). Seven-d-old seedlings were used for real-time RT-PCR analysis. In the left panels, the relative fold changes were plotted after normalization to ACTIN7. The relative fold change represents the ratio of the transcript level in the mutant relative to the transcript level in Col-0 plants. In the right panels, copies of the transcripts were plotted per ng of total RNA after normalization to ACTIN7 RNA. Mean ± SE values were determined from three technical replicates. b Lateral root densities and number of ARs in the hypocotyls of Col-0, arf7 arf19, Pro35S:LBD16:GR/arf7arf19 and Pro35S:LBD18:GR/arf7arf19 plants. The LR densities were plotted by LR numbers (#) per unit primary root length (cm) measured from plants grown vertically for 2 weeks and shown in the top panel. Data are presented as means ± SE determined from 20 seedlings. Different letters indicate a significant difference determined by one-way ANOVA followed by Duncan’s post hoc test (P < 0.05). ARs were measured in seedlings that were grown on vertical plates for 3 d in darkness and then transferred to the 16-h photoperiod for 2 weeks with or without DEX and plotted in the bottom panel. Data are presented as means ± SE determined from 100 seedlings
Previous studies have shown that ARF6 and ARF8 act as positive regulators of AR formation [31, 32]. Thus, we tested if ARF6 and ARF8 could control the expression of LBD16 and LBD18 for AR formation. The time-course response of LBD16 and LBD18 expression in arf6 and arf8 mutants after treatment with auxin indole-3-acetic acid was analyzed using RT-qPCR, but no alteration in the expression of LBD16 or LBD18 in arf6 and arf8 mutant backgrounds was observed compared with that of the wild type (Additional file 1: Figure S1), suggesting that ARF6 and ARF8 regulate AR formation through a distinct pathway, independent of LBD16 and LBD18, during auxin signaling. Taken together, these results indicated that LBD16 and LBD18 expression is regulated downstream of ARF7 and ARF19, but not of ARF6 and ARF8, for AR development.
Discussion
Studies on genetic aspects and hormonal responses of LR and AR formation suggested that LRs and ARs share key elements of genetic and hormonal regulatory networks but with different regulatory mechanisms [1]. While genetic components and molecular signaling pathways during LR development in Arabidopsis have been well characterized [9], those involved in AR development are largely unknown. In this work, we showed that the auxin-responsive ARF7/ARF19-LBD16/LBD18 transcriptional module, via AUX1/LAX3 transporters, plays an important role in AR formation in the Arabidopsis hypocotyl.
In Arabidopsis, the developmental processes of LRs are well defined, and consists of the priming of pericycle cells in the basal meristem of the primary root, the first anticlinal asymmetric cell division (initiation), ordered anticlinal and periclinal cell divisions to form a dome-shaped LR primordium, and the emergence of an LR primordium from the primary root [53, 54]. Although the developmental stages of AR formation are not well described in Arabidopsis, AR formation in apple cuttings have been similarly divided into four successive phases: cell dedifferentiation, induction as the beginning of cell division, the outgrowth of a dome-shaped primordium, and the emergence of the AR [55]. In Arabidopsis, ARs initiate from the pericycle cells adjacent to the xylem pole in the hypocotyl, similar to how LRs initiate [56]. ARF proteins, which are involved in LR initiation, were found to regulate AR initiation in Arabidopsis [32, 33], indicating that although AR and LR originate from different organs, similar molecular mechanisms may regulate AR initiation. In rice, CRL1/ARL1, which promotes crown root initiation, positively regulates 277 genes, and among those, it positively regulates many genes homologous to Arabidopsis genes involved in LR formation [57].
In the present study, we showed that the signaling module of AUX1/LAX3-ARF7/ARF19-LBD16/LBD18, which has been shown to regulate LR formation [37, 42, 45, 48], is involved in AR formation (Fig. 7), indicating conservation of developmental processes for AR and LR formation. However, we noted some differences in the roles of each signaling component between LR and AR development. We had previously found that quadruple mutations in lbd16 lbd18 aux1 lax3 nearly abolished the formation of emerged LRs, but barely affected LR primordium density at early developmental stages from I to III [45]. However, we could not detect any AR primordium in this quadruple mutant (Fig. 2). This observation indicates that the LBD16/LBD18 transcriptional module downstream of AUX1/LAX3 is essential for both AR initiation and development, whereas the same signaling module functions redundantly during LR initiation possibly in conjunction with other LBD genes. Moreover, we found that overexpression of LBD16 or LBD18 in the arf7 arf19 mutant could not rescue AR defects, whereas the same approach significantly stimulated LR formation in the arf7 arf19 mutant (Fig. 6) [34, 37], indicating that LBD16 and LBD18 require additional components produced by ARF7 and ARF19 to regulate AR formation. In addition, LBD29 plays an important role in LR formation downstream of ARF7 by directly activating LAX3 expression in response to auxin [47, 49]. LBD18 expression is regulated downstream of LAX3 [45] and LAX3 is involved in AR formation (Fig. 1). Thus, it is most likely that LBD29 also plays a role in AR formation.
We noticed that the complementation lines of the lbd16 or lbd18 mutant generated by expressing LBD16 or LBD18 under the control of their own promoter exhibited much higher expression levels of each transgene compared with that of the wild-type LBD16 or LBD18, and yet the AR numbers in the complementation lines are comparable to that of the wild-type Col-0 [Fig. 3]. This result indicates that overexpression of a single LBD transcription factor gene regulated downstream of ARF7 is not sufficient to overproduce ARs in transgenic plants.
It has been previously reported that the apical part of argonaute1 (ago1) mutants displays a defect in AR formation, but not in LR development, in response to auxin [31]. AGO1 is one of the components that plays a critical role in the regulation of posttranscriptional gene silencing [58]. ARF17, which is upregulated in ago1 mutants, negatively regulates AR formation by repressing GH3 genes and thus perturbing auxin homeostasis in a light-dependent manner [31]. Together, these results suggest that AR development has both unique and shared components with LR development in Arabidopsis. Identification of unique components, which play critical roles in AR development, aid in the discovery of the distinctive developmental processes between AR and LR development.
Conclusions
The ARF7/ARF19-LBD16/LBD18 transcriptional module via the AUX1/LAX3 auxin influx carriers plays an important role in AR formation in the Arabidopsis hypocotyl, suggesting that a common regulatory mechanism is utilized for LR and AR formation during auxin signaling.
Methods
Plant growth and tissue treatment
Arabidopsis thaliana seedlings were grown and treated as described previously [59]. For treatment with auxin IAA, seedlings were grown in a 16-h photoperiod on a 3 MM Whatman filter paper on top of agar plates at 23°C. The filter paper with seedlings was then transferred to a plate containing IAA at 20 μM and incubated for a given period of time with gentle shaking in the light at 23°C. The light intensity was approximately 120 μmol m− 2 s− 1 and was provided by three daylight wavelength color fluorescent bulbs (Kumho Electric Co.).
Plant materials
The Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. We used the homozygous T-DNA insertion mutant lines lbd16, lbd18, lax3, aux1–21, lbd16 lbd18, aux1 lax3, lax3 lbd16, lax3 lbd18, lax3 lbd16 lbd18, aux1 lbd16, aux1 lbd18, aux1 lbd16 lbd18, aux1 lax3 lbd16, aux1 lax3 lbd18, and aux1 lax3 lbd16 lbd18, arf7, arf19, and arf7 arf19, which were developed in previous studies [37, 45, 51, 52]. We identified arf6–1 (CS24606) and arf8–2 (CS24608) knockout T-DNA insertion mutants from the Arabidopsis Biological Resource Center (ABRC). The ProLBD16:GUS and ProLBD18:GUS transgenic plants were obtained from a previous study [37]. ProLAX3:GUS transgenic seeds were generously provided by Dr. Malcolm Bennett [51]. ProARF7:GUS (CS24633) and ProARF19:GUS (CS24634) transgenic seeds were obtained from the ABRC. To generate ProLBD16:LBD16:4xMyc in the lbd16 mutant background, the promoter region of LBD16, encompassing − 1309 to − 21 bp relative to the AUG codon, was amplified by PCR using the pfu DNA polymerase with primers harboring the NotI (N-terminus) and AscI (C-terminus) sites, and was cloned into the pENTR™/SD/D-TOPO (Invitrogen) vector to yield pENTR™/SD/D-TOPO:ProLBD16. The LBD16 DNA fragment was inserted into the pENTR™/SD/D-TOPO:ProLBD16 plasmid with the AscI restriction site in both the N- and C-terminus to yield a pENTR™/SD/D-TOPO:ProLBD16:LBD16 plasmid. This construct was subcloned into the destination vector pGWB516 (Nakagawa, Shimane University, Japan) by an LR recombination reaction, and was then transformed into the lbd16 mutant by Agrobacterium-mediated transformation, generating ProLBD16:LBD16:4xMyc/lbd16 Arabidopsis. ProLBD18:LBD18:3xHA/lbd18 Arabidopsis generated from a previous study was used [60]. Pro35S:LBD16:GR/arf7 arf19 and Pro35S:LBD18:GR/arf7 arf19 transgenic mutants were generated by crossing arf7–1 arf19–1 (female) with Pro35S:LBD16:GR (male) or Pro35S:LBD18:GR (male) [37]. Homozygous lines were isolated according to genotype, the lack of lateral root phenotype for arf7–1 arf19–1 and by PCR detection of genomic DNA for the LBD16:GR or LBD18:GR transgenes. All mutants and transgenic plants were confirmed via genotyping prior to usage. The primer sequences used in this study are shown in Additional file 2: Table S1.
AR analysis
Induction of ARs in the hypocotyl was performed as previously described [32]. After seed sterilization, the seeds were sown on plates, incubated at 4°C for 2 d for stratification, and transferred to the light for several hours to induce germination. Plates were then placed vertically in the dark for 3 d, until the hypocotyls reached approximately 6 mm length, and were then transferred to the light for 7 d before counting the emerged ARs.
RNA isolation and RT-qPCR analysis
Following treatment, Arabidopsis plants were immediately frozen in liquid nitrogen and stored at − 80°C. For the reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis, total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen), and real-time RT-PCR was carried out using a QuantiTect SYBR Green RT-PCR kit (Qiagen) in a CFX96™ Real-time system using a C1000™ Thermal cycler (Bio-Rad) as described previously [61]. All RT-qPCR was conducted in triplicate biological replications and subjected to statistical analysis. Analysis of relative gene expression data for determining fold changes was conducted as described previously [60, 62]. Data analysis for determining the copy number of the transcripts and for determination of reaction specificities was performed as described previously [61]. RT-qPCR conditions and primer sequences are shown in Additional file 2: Table S1. The experimental conditions used for RT-qPCR followed MIQE (minimum information for publication of quantitative real time PCR experiments) requirements as described in Additional file 3: Table S2.
Microscopy and histochemical GUS assays
Whole-mount visualization of the seedlings and histochemical assays for GUS activity were conducted as described previously [63].
Statistical analysis
Quantitative data were subjected to statistical analysis for every pair-wise comparison using software for Student’s t-Test (Predictive Analytics Software for Windows version 20.0).
Abbreviations
- AR:
-
Adventitious root
- ARF:
-
Auxin response factor
- Aux/IAA:
-
Auxin/indole acetic acid protein
- GUS:
-
ß-glucuronidase
- LBD/ASL:
-
Lateral organ boundaries domain/asymmetric leaves2-like
- RT-qPCR:
-
Quantitative reverse transcription-PCR
References
Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65:639–66.
Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann Bot. 2004;93:359–68.
Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annu Rev Plant Biol. 2007;58:93–113.
Geiss G, Gutierrez L, Bellini C. Adventitious root formation: new insights and perspectives. In: Annual plant reviews volume 37: root development; 2009.
Li S-W, Xue L, Xu S, Feng H, An L. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot. 2009;65:63–71.
Lavenus J, Goh T, Roberts I, Guyomarc'h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–8.
Salehin M, Bagchi R, Estelle M. SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell. 2015;27:9–19.
Weijers D, Wagner D. Transcriptional responses to the auxin hormone. Annu Rev Plant Biol. 2016;67:539–74.
Du Y, Scheres B. Lateral root formation and the multiple roles of auxin. J Exp Bot. 2018;69:155–67.
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602.
Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jurgens G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development. 2004;131:389–400.
Weijers D, Benkova E, Jager KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jurgens G. Developmental specificity of auxin response by pairs of ARF and aux/IAA transcriptional regulators. EMBO J. 2005;24:1874–85.
Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011;7:508.
Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–3.
Petrasek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–88.
Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–16.
Geisler M, Wang B, Zhu J. Auxin transport during root gravitropism: transporters and techniques. Plant Biol (Stuttg). 2014;16(Suppl 1):50–7.
Laskowski M, Grieneisen VA, Hofhuis H, Hove CA, Hogeweg P, Maree AF, Scheres B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6:e307.
Marhavy P, Vanstraelen M, De Rybel B, Zhaojun D, Bennett MJ, Beeckman T, Benkova E. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 2013;32:149–58.
Peret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Wells DM, Porco S, Mellor N, Band LR, et al. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol. 2013;9:699.
da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci. 2013;4:133.
Sukumar P, Maloney GS, Muday GK. Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiol. 2013;162:1392–405.
Xu M, Zhu L, Shou H, Wu P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005;46:1674–81.
Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010;63:551–62.
Lombardi-Crestana S, da Silva Azevedo M, e Silva GF, Pino LE, Appezzato-da-Gloria B, Figueira A, Nogueira FT, Peres LE. The tomato (Solanum lycopersicum cv. Micro-tom) natural genetic variation Rg1 and the DELLA mutant procera control the competence necessary to form adventitious roots and shoots. J Exp Bot. 2012;63:5689–703.
Oh K, Ivanchenko MG, White TJ, Lomax TL. The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signaling. Planta. 2006;224:133–44.
Ivanchenko MG, Zhu J, Wang B, Medvecka E, Du Y, Azzarello E, Mancuso S, Megraw M, Filichkin S, Dubrovsky JG, et al. The cyclophilin a DIAGEOTROPICA gene affects auxin transport in both root and shoot to control lateral root formation. Development. 2015;142:712–21.
Retzer K, Luschnig C. DIAGEOTROPICA: news from the auxin swamp. Trends Plant Sci. 2015;20:328–9.
Spiegelman Z, Ham BK, Zhang Z, Toal TW, Brady SM, Zheng Y, Fei Z, Lucas WJ. Wolf S. A tomato phloem-mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. Plant J. 2015;83:853–63.
Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol. 2008;24:55–80.
Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, et al. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005;17:1343–59.
Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell. 2009;21:3119–32.
Gutierrez L, Mongelard G, Flokova K, Pacurar DI, Novak O, Staswick P, Kowalczyk M, Pacurar M, Demailly H, Geiss G, et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012;24:2515–27.
Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–63.
Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell. 2005;17:3035–50.
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005;43:118–30.
Lee HW, Kim NY, Lee DJ, Kim J. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 2009;151:1377–89.
De Smet I, Lau S, Voss U, Vanneste S, Benjamins R, Rademacher EH, Schlereth A, De Rybel B, Vassileva V, Grunewald W, et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc Natl Acad Sci U S A. 2010;107:2705–10.
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell. 2005;17:1387–96.
Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005;43:47–56.
Wang D, Pei K, Fu Y, Sun Z, Li S, Liu H, Tang K, Han B, Tao Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene. 2007;394:13–24.
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–30.
Lee HW, Kim MJ, Kim NY, Lee SH, Kim J. LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 2013;73:212–24.
Lee HW, Kim MJ, Park MY, Han KH, Kim J. The conserved proline residue in the LOB domain of LBD18 is critical for DNA-binding and biological function. Mol Plant. 2013;6:1722–5.
Lee HW, Cho C, Kim J. Lateral organ boundaries Domain16 and 18 act downstream of the AUXIN1 and LIKE-AUXIN3 AUXIN influx carriers to control lateral root development in Arabidopsis. Plant Physiol. 2015;168:1792–806.
Berckmans B, Vassileva V, Schmid SP, Maes S, Parizot B, Naramoto S, Magyar Z, Alvim Kamei CL, Koncz C, Bogre L, et al. Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell. 2011;23:3671–83.
Feng Z, Sun X, Wang G, Liu H, Zhu J. LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot. 2012;110:1–10.
Goh T, Joi S, Mimura T, Fukaki H. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development. 2012;139:883–93.
Porco S, Larrieu A, Du Y, Gaudinier A, Goh T, Swarup K, Swarup R, Kuempers B, Bishopp A, Lavenus J, et al. Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development. 2016;143:3340–9.
Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–97.
Swarup K, Benkova E, Swarup R, Casimiro I, Peret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946–54.
Lee DJ, Park JW, Lee HW, Kim J. Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot. 2009;60:3935–57.
Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44.
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003;8:165–71.
de Klerk G-J, van der Krieken W, de Jong JC. Review the formation of adventitious roots. New concepts, new possibilities. In Vitro Cell Dev Biol - Plant. 1999;35:189–99.
Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–19.
Coudert Y, Le VA, Adam H, Bes M, Vignols F, Jouannic S, Guiderdoni E, Gantet P. Identification of CROWN ROOTLESS1-regulated genes in rice reveals specific and conserved elements of postembryonic root formation. New Phytol. 2015;206:243–54.
Vaucheret H, Vazquez F, Crete P, Bartel DP. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004;18:1187–97.
Park JY, Kim HJ, Kim J. Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of aux/IAA genes in steroid regulator-inducible system. Plant J. 2002;32:669–83.
Pandey SK, Lee HW, Kim MJ, Cho C, Oh E, Kim J. LBD18 uses a dual mode of a positive feedback loop to regulate ARF expression and transcriptional activity in Arabidopsis. Plant J. 2018;95:233–51.
Jeon J, Kim NY, Kim S, Kang NY, Novak O, Ku SJ, Cho C, Lee DJ, Lee EJ, Strnad M, et al. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem. 2010;285:23371–86.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8.
Jeon J, Cho C, Lee MR, Van Binh N, Kim J. CYTOKININ RESPONSE FACTOR2 (CRF2) and CRF3 regulate lateral root development in response to cold stress in Arabidopsis. Plant Cell. 2016;28:1828–43.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Next-Generation BioGreen 21 Program (PJ013220), Rural Development Administration (RDA), Republic of Korea and the Mid-career Researcher Program (2016R1A2B4015201) and Basic Research Laboratory (2017R1A4A1015620) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology of Korea to J. Kim. RDA and NRF did not participate in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Availability of data and materials
All the data supporting our findings is contained within the manuscript. Constructs and seeds are available upon request from JK.
Author information
Authors and Affiliations
Contributions
HWL, CC, SKP, YP, and MJK designed and conducted the experiments and analyzed the data. SKP prepared the manuscript draft. JK conceived the project, designed the experiments, analyzed the data, and wrote the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Time-course expression of LBD16 and LBD18 in response to auxin in Col-0, arf6 and arf8 mutants. (PDF 110 kb)
Additional file 2:
Table S1. Primer sequences and PCR conditions. (XLSX 12 kb)
Additional file 3:
Table S2. Experimental conditions used in RT-qPCR based on MIQE requirements. (XLSX 10 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, H.W., Cho, C., Pandey, S.K. et al. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol 19, 46 (2019). https://doi.org/10.1186/s12870-019-1659-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-019-1659-4