Abstract
In this study, Delonix regia seed pods (DRSPs) as a locally available material were refluxed in 90% H2SO4 to yield a novel D. regia seed pods biochar-sulfur oxide (DRB-SO). FTIR, BET, BJH, SEM, EDX, XRD, DSC and TGA were applied to investigate the characterizations of the prepared DRB-SO. Various adsorption parameters like pH effect, dye concentration effect, adsorbent dose, reaction time isotherm and kinetic study were carried out to explain the process of adsorption of methyl orange (MO) and methyl red (MR) onto DRB-SO. Langmuir's adsorption model perfectly explained the adsorption process onto the surface of DRB-SO as a monolayer. The maximum adsorption efficiency of DRB-SO was (98%) and (99.6%) for MO and MR respectively which attained after 150 min with an adsorbent dose of 0.75 g/L. The pseudo-second-order kinetic model best explained the process of adsorption of MO and MR dyes by DRB-SO. The highest observed adsorption amount was as high as 144.9 mg/g for MO dye and 285.7 mg/g for MR dye, comparable with other reported materials based on activated carbon materials. All of the outcomes signposted a prodigious perspective of the fabricated biochar composite material in wastewater treatment. Using the regenerating DRB-SO through an acid–base regeneration process, six cycles of adsorption/desorption were examined. Over the course of the cycles, there was a minor decrease in the adsorption and desorption processes. Also, it was revealed what the most plausible mechanism was for DRB-SO to absorb the ions of the MO and MR dyes.
Similar content being viewed by others
Introduction
Synthetic dyes used in large quantities in textiles are physically and chemically most stable and biodegradable compounds, which are toxic to humans as well as to the environment. Thanks to the stability and water solubility, synthetic organic dyes accumulate in wastewater1,2,3. Numerous traditional techniques including aerobic, anaerobic, biological, adsorption, flocculation, flotation, precipitation, oxidation–reduction, photo-reduction and electrochemical were in practice for the decontamination of wastewater4,5,6,7. However, most of these techniques are not so efficient regarding dye removal performance and cost-effectiveness.
Methyl orange (MO) dye is a synthetic anionic mono azo dye, which is frequently and uninterruptedly used in many industries including textiles8,9,10 in laboratory experiments and other commercial products11. This dye is noxious to aquatic animals and plant life12. Acute exposure to MO dye increases the risk of quadriplegia, jaundice, cyanosis, heart rate, tissue necrosis, shock and vomiting in humans13,14. Hence, it is imperative to eliminate this pigment from polluted water. Similarly, methyl red (MR) dye is also used in many industrial products as coloring agent and ultimately reaches natural water bodies with industrial wastewater15,16. It causes ocular and skin sensitivity, irritation of the pharynx or digestive tract and is mutagenic, if inhaled or ingested17,18. Therefore the removal of these toxic azo dyes is very crucial for sustaining life on Earth.
Various techniques are in practice for the removal of these and other noxious pollutants from water including, photocatalytic decomposition photo-Fenton, combined electrochemical processes, membrane filtration and adsorption7,19,20,21,22. Adsorption is the best and most comprehensive technology for the purification of industrial wastewater before disposal to the environment23. There are several low-cost adsorbent materials reported for the treatment of wastewater like layered double hydroxides (LDH), silica, activated carbon (AC), chitosan, pomegranate peel, and ferrites22,24,25,26.
However, these low-cost adsorbents did not have satisfactory adsorption efficiency for MO and MR dyes. Consequently, more work is needed in this area to explore other types of adsorbents to eliminate these hazardous pollutants from industrial wastewater.
Delonix regia (Flame tree, Royal Poinciana or Flamboyant) is a large ornamental plant which is native to Madagascar27. This plant has much medical importance, as its leaves, barks, flowers and seeds have been broadly utilized for anti-appetent, anti-fertility, antifungal, antiulcer insecticidal, anti-inflammatory and cytotoxic activities28,29,30,31. Recently, D. regia was discovered as an adsorbent for the removal of contaminants from polluted water. Vargas et al.32 reported the acid dyes (acid red and acid yellow) adsorption with activated carbon (AC) obtained from D. regia. Saravanan and co-authors33 fabricated surface-modified AC from D. regia seed for the removal of reactive yellow dye from an aqueous medium. Utsev et al.34 reported methylene blue removal from water by using D. regia activated carbon. However, the adsorption results of these D. regia based AC were not so good. Therefore it is the need of the hour to do some modifications in AC obtained from D. regia to enhance its adsorption performance.
The effects of the physiochemical properties of Delonix regia Biochar-SO (DRB-SO) made from D. regia seed pods (DRSPs) on the elimination of MO and MR dyes from wastewater are not well studied at this time. Therefore, this study examines how well biochar is generated from D. regia seed pods by dehydrating the material with 90% sulfuric acid (H2SO4) at boiling (~ 280 °C). The purpose of this is to investigate how well the prepared DRB-SO absorbs MO and MR dyes as a highly cost-effective agricultural waste product. The study looks at the effectiveness of DRB-SO absorption of MO and MR dyes in a batch technique under various working circumstances (pH, dosage of DRB-SO, starting concentration of MO and MR dyes, and contact time). The DRB-SO was characterized using XRD, FTIR, SEM, EDX, BET, BJH, DTA, DSC and TGA studies. Additionally, when designing the adsorption process, the kinetic and equilibrium isotherm models for the absorption of MO and MR dyes by DRB-SO were specifically taken into account.
Materials and methods
Instrument and materials
Delonix regia seed pods (DRSPs) were collected from a local area in Alexandrian and utilized as the raw material to create DRB-SO, an adsorbent substance. The supplier of sulfuric acid (H2SO4, Purity 98%) was obtained from the Sigma Aldrich, USA. The MO and MR dyes (obtained from Aldrich, USA) concentrations were measured using an analytical Jena digital spectrophotometer (SPEKOL1300 UV/Visible spectrophotometer) in conjunction with 1 cm optical path glass cells, a shaker (JSOS-500) for mixing procedures, and a pH metre (JENCO 6173) for pH surveys. The adsorption–desorption isotherm of DRB-SO was measured in the N2 environment. Using an instrument (BELSORP – Mini II, BEL Japan, Inc.), the surface area, pore size and pore distribution of DRB-SO were determined35,36. Monolayer volume (Vm) (cm3 (STP), surface area (SBET) (m2/g), average pore diameter (MPD) (nm), total pore volume (p0/p0) (cm3/g) and energy constant (C) values of DRB-S were obtained by modeling of the adsorption–desorption graph. The microporous surface area (Smi), mesoporous surface area (Smes), mesoporous volume (Vmes), and microporous volume (Vmi) of DRB-S were calculated by the Barrett–Joyner–Halenda (BJH) model. The calculations were carried out with the software of the BELSORP analysis programme. Using the BJH approach, the pore size dispersion was also ascertained from the desorption isotherm37. An investigation of the form of the biochar surface was conducted using a scanning electron microscope (SEM; QUALITY 250). Fourier Transform Infrared (FTIR) spectroscopy (VERTEX70) and the ATR unit model V-100 were used to investigate the functional groups on the surface of DRB-S. IR-observable functional groups on the DRB-S surface were identified in the 400–4000 cm–1 wavenumber region using FTIR spectroscopy in combination with the platinum ATR unit. Employing the SDT650-Simultaneous Thermal Analyzer apparatus, thermal analyses were conducted at a ramping temperature of 10 °C/min throughout a temperature range of 50–1000 °C.
DRB-SO preparation
DPSPs were extensively cleansed with tap water many times to remove any dust, and they were thereafter dried in a furnace at 120 °C for twenty-four hours before being ground and pulverized. A total of 140 g of powdered DPSPs was heated at 285 °C in 750 mL of 90% H2SO4 solution for 10 h, then diluted with distilled water, filtered and then washed with distilled water until pH 7. The DRB-SO was then cleaned with EtOH and dried at 150 °C in a furnace. Biochar with the designation DRB-SO was produced as a consequence of this reaction.
Batch adsorption experiment
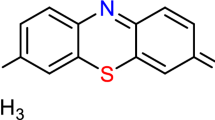
A batch adsorption experiment was used to assess the sorption capacity, thermodynamic, and kinetic properties of DRB-SO. A reasonable quantity of flasks with 100 mL of MO (methyl orange, C14H14N3NaO3S) and MR (methyl red, C15H15N3O2) dyes (Fig. 1) solutions at various starting concentrations and DRB-SO at various weights were shaken for a predetermined amount of time at 200 rpm. Solution pHs were raised or lowered to the appropriate levels with 0.1 M NaOH or HCl. Furthermore, during the adsorption equilibrium investigations, the pH of the solution was maintained at the intended level. Taking a sample (0.1 mL) from the solution at regular intervals (removed from the adsorbent) allowed for the determination of the MO and MR dye concentration using a spectrophotometer set at λmax = 505 and 525 nm, respectively. The qt of DRB-SO was calculated using Eq. (1).
where C0 (mg/L) is the MO and MR dyes initial concentration; Ct (mg/L) is the remaining MO and MR dyes concentration at the end of time t; qt (mg/g) is the adsorption capacity of DRB-SO at time t; W (g) is the mass of the DRB-SO and V (L) is the volume of the MO and MR dyes solutions.
To examine the impact of pH on the adsorption of MO and MR ions by DRB-SO, studies were achieved at different pH values (1.01 to 13.23) and (0.98 to 13.36), respectively, by adding 0.1 g DRB-SO to 100 mL of solutions containing 100 ppm of MO dye and MR. The mixtures were agitated for 150 min. at 200 rpm when the mixtures were at room temperature.
MO dye and MR dye solutions with varying initial concentrations (50–150 ppm) were made, and isotherm measurements and the effect of DRB-SO dose on the adsorption of MO dye and MR dye ions were investigated. Intervals between 0.75 and 1.75 g/L of DRB-SO doses and MO dye and MR dye solutions with diverse starting concentrations were used to measure the MO dye and MR dye concentrations. The mixtures were agitated at 200 rpm and 25 °C. Every adsorption investigation was carried out in triplicate, and the results are presented as an average.
Author statement for the use of plants
In this study, Experimental research and field studies on plant material (Orange peels), including the collection of plant waste material, complies with relevant institutional, national, and international guidelines and legislation.
Results and discussion
DRB-SO characterization
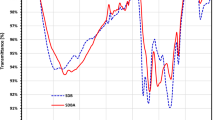
Using FT-IR spectroscopy, the functional groups present on the surface of the resulting DRB-SO adsorbent were identified. The FTIR graph of the raw DPSPs and the FTIR graph of the DRB-SO were compared, as shown in Fig. 2. The FT-IR spectra of the materials show changes in their functional groups. The stretching oscillation of the O–H present in the DPSPs and DRB-SO is shown by the band between 3582.15 and 3347.90 cm–1 (Fig. 2). The presence of –CH2 stretching groups in DPSPs is suggested by the high absorption peaks between 2922.10 cm–1 (Fig. 2). These groups were enlarged in DRB-SO and appeared at 2922.56 cm–1 (Fig. 2). The C=O stretching of the ester groups in the DPSPs is responsible for the high absorption band at 1733.88 cm–1 (Fig. 2). This band was later transformed into a carboxyl group in DRB-SO at 1703.48 cm–1 (Fig. 2). Nevertheless, the strength at 1703.48 cm–1 increased when DRB-SO was compared to raw DPSPs, indicating that sulphoric acid treatment may increase the carbonyl (C=O) group. The bands at 1627.27 cm–1 suggest that the β-ketone's C=O stretching oscillation was nearly existent in the DPSPs. This oscillation shifted to 1599.89 cm–1 in DRB-SO with high intensity, and it might also be a stretching vibration of –C=C– in DRB-SO (Fig. 2). The DPSPs' C-O functional group is shown by the peaks at 1513.00–1251.19 cm–1. This group was replaced by the band at 1405.58 and 1358.18 cm–1 in DRB–SO, which displayed the sulfonyl group (SO) stretching vibration (Fig. 2). Additionally, the development of peaks at 1185.98 and 1041.63 cm–1 was facilitated by the dehydration process with H2SO4. These peaks resulted from the production of -SO3H and SO groups in DRB-SO. These bands show that the DPSPs treatment with H2SO4 results in the creation of the DRB-SO. The DPSPs showed a more noticeable rise in the -C–O–C- asymmetric stretching functional group at 1159.21, 1109.16 and 1052.29 cm–1 (Fig. 2), compared to DRB-SO, which showed a very weak band at 1041.78 cm–1 and two other weak bands at 774.82 and 643.60 cm–138,39,40,41.
To find out how H2SO4 affected the DRB-SO's surface characteristics, the N2 adsorption–desorption isotherm of the DRB-SO was studied. The specific surface area and mesopore area were calculated using the BET and BJH techniques, respectively. Figure 3 shows the textural properties of DRB-SO, including BET-specific surface area, mass of mesopores, mesopore area, total volume of pores, mesopore distribution peak, average pore diameter, and monolayer volume. The DRB-SO has a relatively tiny BET-specific surface area of 15.39 m2/g. DRB-SO had a monolayer volume value of 3.5243 cm3 (STP) g–1. DRB-SO has a total volume value of 0.1534 cm3/g. DRB-SO had mean pore diameters of 4.9481 nm and a total pore volume of 1.8975 × 10–2 cm–1. The values of 14.823 m2/g, 2.1873 × 10–2 cm3/g, and 1.22 nm were found to be the mesopore volume, meso surface area, and mesopore distribution peak values of DRB-S analysis of adsorption, respectively. The values of 8.3569 m2/g, 1.5902 × 10–2 cm3/g, and 1.66 nm were found to be the mesopore volume, meso surface area, and mesopore distribution peak values of DRB-S analysis of adsorption, respectively.
The DRB-S is shown in SEM pictures in Fig. 4, where it is clear that it is clean and free of impurities. The DPSPs' pore structure remained unharmed by the intense sulfuric acid treatment. The particle size distribution shows that the particle sizes were within the range of 2108–7397 nm and the determined average particle size distribution of the DPKB-SO was 5811 ± 1.65 nm.
The DRB-SO adsorbent chemical composition was studied using scattered X-ray spectrometry (EDX). The percent of each element is presented in Table 1, which indicates that, in addition to carbon mass %, which makes up 58.02% of the sample, there are around 39.63 and 0.66% of oxygen and sulfur, respectively.
The impact of structural variations on the operating temperature and degradation behavior of the DRB-SO samples and raw DRSPs were assessed using thermal gravimetric analysis (TGA). Every sample was cooked from 50 to 1000 °C in a N2 atmosphere. Figure 5 displays the TGA, Differential Scanning Calorimetry (DSC) and Differential Thermal Analysis (DTA) analytical curves for DPSPs and DRB-SO adsorbent. The first weight reduction was caused by the evaporation of water in the raw DPSPs and DRB-SO, and it peaked before 140 °C. Raw DPSPs and DRB-SO lost weight as a result of the breakdown of many acidic oxygen functional groups that occurred as the temperature rose beyond 140 °C. Moreover, acidic groups break down at different temperatures. For example, phenol breaks down at a greater temperature than lactones, anhydrides, and carboxylic groups. Raw DPSPs exhibit a high weight loss at temperatures up to 330 °C and the final weight loss occurred between 330 and 460 °C. DRB-SO shows three weight losses at temperatures between 25–140, 140–390 and 390–950 °C, which explains the higher stability of DRB-SO compared to the raw DPSPs. TGA curve of DRB-SO converged at temperatures > 410 °C due to carbon breakdown in biomass. At the finishing temperature, various weight loss percentages of 77.03 and 49.06% were obtained for raw DPSPs and DRB-SO, respectively, indicating the greater stability of DRB-SO (Fig. 5a).
DTA graph of DRB-SO and raw DPSPs is illustrated in Fig. 5b. The DTA curve of the raw DPSPs peaked at two points at temperature (Tf, 59.10 and 330.30 °C), while the curve of DRB-SO peaked at three points at temperature (Tf, 86.40, 352.06 and 456.06 °C) (Fig. 5b). The DTA curve demonstrating the production of DRB-SO adsorbents from raw DPSPs indicates that the dehydration of raw DPSPs yielded two distinct degradation bands. The degradation bands of raw DPSPs decreased from three to two at higher temperatures after treatment with 90% H2SO4, demonstrating that the degradation degree was intensely affected by treatment with H2SO4.
Thermal transitions can be employed by DSC to compare materials. Figure 5b depicts the DSC graph of DRB-SO and raw DPSPs. The crystallisation temperatures (TC) of DPSPs are 73.66 °C, while DRB-SO displays TC values was 82.11 °C. When the temperature rises, DRB-SO melts at 587.95 °C, while DPSPs melts at 565.13 °C. A lower Tm was shown by DPSPs, whereas the highest Tm was shown by DRB-SO. The grains became more crystalline due to the higher transitional temperatures, which improved both their structural stability and resistance to gelatin disintegration.
The DRB-SO XRD is shown in Fig. 6 and shows an amorphous carbon structure with arbitrarily oriented aromatic sheets. A tiny peak is located around 2Ɵ = 43.54, and a wide peak is indexed as the C (002) diffraction peak in the area of 2Ɵ ~ 24.5. A tiny peak is located around 2Ɵ ~ 16.4 (101) prominent cellulosic peaks. This might point to a variety of inorganic materials, primarily Quartz and Albite (plagioclase feldspar mineral)42,43.
Effect of pH on dye solution
The pH of the dye solution plays a crucial role in the adsorption process by altering the surface charge of the adsorbent molecule and, eventually, the behaviour of the functional groups that are bound to the adsorbent molecule44. The pH effect of MO dye has been examined at various pH values ranging from 1 to 13 with a dye concentration of 100 mg/L (Fig. 7a). The results explored that Delonix regia Biochar-Sulfur Oxide (DRB-SO) have maximum adsorption performance in the acidic medium at pH 1.01 (85.6% removal of MO dye) and least performance in the basic medium at pH 13.23 (1.1%). Similarly, the MR dye removal efficiency reached 94% in an acidic medium (pH = 0.98) as compared to a basic medium (11.8% at pH = 13.36). This may be because both MO and MR dyes are basic in nature (Fig. 7a). Because of the strong columbic force that exists between the dye molecules and the positively charged surface of DRB-SO, their adsorptions are highly effective in acidic environments. The adsorption of MO and MR dyes was poor in alkaline media; this might be because the negatively charged surface of DRB-SO and the dye molecules resisted each other45,46,47.
The influence of dye solution pH on adsorbent adsorption efficiency may also be clarified using pH zero point charge (pHZPC). Depending on the functional groups that are connected to the molecule, an amphoteric adsorbent molecule can have both positive and negative charges. The pH of the environment these molecules are in has an impact on their net surface charge. They can become more positively or negatively charged by either receiving or losing protons (H+). In the absence of particular sorption, the pH value at which the net charge on a solid surface becomes zero is referred to as the zero point charge48,49,50. In Fig. 7b, the plot of pH versus ΔpH is shown. From the figure pHZPC of DRB-SO was found to be 12.4. The surface of the adsorbent becomes more positively charged by losing protons when the solution pH is less than pHZPC (12.5), which promotes the absorption of anionic dyes due to an enhanced electrostatic force of attraction. Adsorption of MO and MR dyes onto DRB-SO was therefore preferred at solution pH values below 12.5 (pHZPC). The equilibrium uptake was low because the adsorbent surfaces resist anionic dye molecules at higher pH values because they become more negatively charged (Fig. 7b).
Effect of contact time
The adsorption of MO and MR dyes onto Delonix regia biochar-sulfur (DRB-SO) was investigated for different reaction times (Fig. 8a,b). For the first half hour, the adsorption rate was greater; as contact time grew, it then decreased and crushed off. The rate of adsorption was high in the beginning because there were more active adsorption sites and space available to transport dye molecules, and the active adsorption sites were empty at that time51,52,53. However, as time goes on, the adsorbate molecules occupy fewer adsorption active sites, which causes the rate of adsorption to gradually decline. From Fig. 8a, it is clear that the maximum adsorption efficiency of Delonix regia biochar-sulfur (DRB-SO) was achieved at 150 min (98%) with an adsorbent dose of 0.75 g/L for MO dye. Similarly, for MR dye the maximum adsorption efficiency of Delonix regia biochar-sulfur (DRB-SO) was achieved at 150 min (99.6%) with DRB-SO dose 0.75 g/L (Fig. 8b).
Adsorption kinetics
The kinetic study of the adsorption of MO and MR dyes with DRB-SO was carried out by applying pseudo-first-order, pseudo-second-order, intrapaticle diffusion and film diffusion kinetic models on the experimental data (Figs. 9, 10). Table S1–S4 (Supporting data) displayed the kinetic parameters that were derived from these models. For the adsorption of MO dye with DRB-SO, the linear fitting of pseudo first order and pseudo-second-order, intrapaticle diffusion and film diffusion kinetic models, respectively, is shown in Fig. 9a–d54,55,56,57.
When compared to other kinetic models, the pseudo-second-order has better R2 values, indicating that it fits the experimental data the best (Fig. 4a–d). Also, the qe Cal. (52.63 mg/g) has much closeness to that of qe Exp. (64.94 mg/g) with MO dye concentration 50 mg/L and DRB-SO dose 0.75 g/L. similar results were obtained for other concentrations of MO dye and DRB-SO as presented in Table S1. This suggests that the rate of adsorption depends on the adsorption capacity and is not affected by the adsorbate concentration58,59,60. Similarly, the adsorption of MR dye also follows pseudo-second-order kinetic models with relatively higher R2 value and closed qe Cal. (66.23 mg/g) and qe Exp. (64.94 mg/g) with MR dye concentration of 50 mg/L and DRB-SO dose 0.75 g/L, and qe Cal. (217.39 mg/g) and qe Exp. (191.81 mg/g) with MR dye concentration 150 mg/L and DRB-SO dose 0.75 g/L (Table S3).
Adsorption isotherm study
For the real process of adsorbate adsorption onto adsorbent, the adsorption isotherms offer reliable validation (Figure S1)61,62,63. Figure 11 shows the analysis of the adsorption process using the Langmuir and Freundlich isotherm model applied to experimental data. These models' linearized graphs can be used to assess how well these adsorption isotherm models fit. To evaluate each adsorption isotherm model's fitness, the regression coefficient (R2) is employed as a criteria. Reports were made on the linear form of the Freundlich and Langmuir isotherm model57,64,65.
The various parameters related to Langmuir and Freundlich isotherm model are described in Table 2. Rendering to the regression coefficients (R2 = 0.995 for DRB-SO dose = 0.75 g/L ), the Langmuir isotherm model best pronounced the experimental data, which revealed the adsorption process as a monolayer with uniform adsorption energies may occur in the DRB-SO system for the adsorption of MO dye. Similarly, the adsorption of MR dye onto the surface of DRB-SO also follows the Langmuir isotherm model66,67,68. The maximum adsorption capacity calculated with the Langmuir isotherm model was 144.9 and 285.7 mg/g for MO and MR dyes, respectively (Table 2).
Comparison of results with reported literature
A selection of earlier research on the removal of MO and MR ions from aquatic medium is shown in Table 3. According to the maximum adsorption capacity (Qm) reported in this Table, at room temperature, the DRB-SO adsorbent has the highest Qm for MR and is equivalent to MO among the literature cited61,69,70,71,72,73,74,75,76,77. For the elimination of MR and MO ions at a given concentration of 1.0 g L–1 of DRB-SO adsorbent, these values were 285.71 and 144.93 mg g–1, respectively. It is clear from this comparison that the DRB-SO made from DRSPs was a highly effective adsorbent for taking out MR and MO dyes from aqueous solutions.
Regeneration study
To investigate the feasibility and adsorbent reusability for the absorption of MO and MR dyes, desorption tests of the MO and MR dyes from the DRB-SO adsorbent were done using 0.1 M NaOH as an elution desorption media and its concentrations were measured, and then 0.1 M HCl was used for reactivation of the DRB-SO. In this investigation, when the regeneration cycles increased, the proportion of dye desorption decreased (Fig. 12). Using the regenerated DRB-SO, six cycles of adsorption/desorption have been investigated. Throughout the cycles, the changes in adsorption and desorption were mostly almost constant78,79,80. However, after six cycles, it decreased by about 12.4% for MO dye and 9.6% for MR dye. It suggests DRB-SO could be used as a durable MO and MR dye removal from water (Fig. 12).
Adsorption mechanism of MB Dye by DRB-S
Figure 13 explains the likely mechanism via which DRB-SO absorbed the MO dye and MR dye ions. Following the 90% H2SO4 dehydration of the DPSPs (D. regia seed pods raw material). According to FTIR analysis, a variety of functional groups, including C=O, COOH, C–O–C, hydroxyl O–H, C-S, SH, SO3H and SO groups, developed on the surface of the adsorbent (DRB-SO). Because of the electrostatic interaction between the sulfur and oxygen lone pair on the DRB-SO surface and the negative charge on the MO dye and MR dye, the adsorption mechanism of the MO dye and MR dye ions in an acidic medium (pH 1.0) can be accomplished through physical interaction. The acidic pH of the acidic medium attracted ions after the surface charge became positive.
In an acidic environment, the surface of DRB-SO picks up a positive charge, which attracts negatively charged dye molecules81,82,83. Furthermore, there is interaction between the functional groups of the positive ions on the DRB-SO's surface and the negative ions in the solution. Furthermore, dye molecules are more soluble at an acidic pH, which makes it easier for them to adhere to the adsorption sites and diffuse through the DRB-SO's pores. Since the acidic pH of DRB-SO is necessary to promote the adsorption of MO and MR dye molecules onto the material, it is a great way to remove colour from industrial effluent. The most important mechanism is the electrostatic interaction-mediated adsorption of ionizable organic molecules to the positively charged surface of the biochar83. An aqueous solution's pH and ionic strength determine how well it draws or repels contaminants.83,84.
Furthermore, the capacity of organic contaminants in industrial effluent to adsorb is influenced by the pH of the solution85. Parshetti et al.'s study86 examined the use of food waste-derived biochar in the adsorption of textile colours in wastewater. They found that an alkaline pH enhanced the adsorption of dyes. It was explained by the strong interaction between the positively charged dyes and the negatively charged sites on the biochar surface86. It was less successful in adsorbing organic dye, though, because there was an excess of H+ at pH 1.5, which competed with the positive charges of the dye86. Tsai and Chen87 and Xu et al.88 have noted that pH has an impact on biochar's capacity to absorb materials. The capacity of organic and inorganic pollutants from industrial effluent to adsorb on biochar is hence influenced by the pH of the solution, which also affects the charged sites.
Conclusion
Various adsorption parameters like the effect of dye pH, reaction time, dye concentration and adsorbent dose were analysed to evaluate the performance of DRB-SO for the adsorption removal of MO and MR dyes. Results indicate that DRB-SO performed best in the acidic medium as compared to the basic or neutral medium for the removal of MO and MR dyes. Also with 150 min of contact time, the removal percentage was 97.95% for MO dye and 99.60% for MR dye using DRB-SO (initial dye concentration = 50 mg/L, and DRB-S dose = 1.75 g/L). Various kinetic models were applied to the experimental data and the outcomes showed that the pseudo-second-order kinetic model best explains the process of adsorption of dyes by DRB-SO. In other words, over the complete adsorption range, chemical sorption, also known as chemisorption, is the rate-limiting process. Moreover, an analysis of the adsorption isotherm showed that the adsorption data and the Langmuir equation suited each other rather well. For MO and MR dyes, the highest adsorption capacities determined using Langmuir isotherm models were 144.9 and 285.7 mg/g, respectively. According to the study's findings, DRB-SO could be a suitable option for the adsorption of anionic dye found in wastewater.
Data availability
The datasets used in this investigation are accessible for review upon request from the paper's corresponding author.
References
Vallejo, W. et al. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 10(5), 528 (2020).
Lellis, B. et al. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 3(2), 275–290 (2019).
Shahzad, K. et al. Synthesis of nanoadsorbent entailed mesoporous organosilica for decontamination of methylene blue and methyl orange from water. Int. J. Environ. Anal. Chem. 103, 1–14 (2021).
Ling, X. et al. The novel application of polyoxometalates for achieving sludge deep dewatering using low-temperature thermal hydrolysis pretreatment. J. Cleaner Prod. 444, 141125. https://doi.org/10.1016/j.jclepro.2024.141125 (2024).
Djehaf, K. et al. Textile wastewater in Tlemcen (Western Algeria): impact, treatment by combined process. Chem. Int. 3(4), 414–419 (2017).
Jafarinejad, S. Activated sludge combined with powdered activated carbon (PACT process) for the petroleum industry wastewater treatment: a review. Chem. Int 3(4), 368 (2017).
Kumar, O. P. et al. Photo-Fenton activated C3N4x/AgOy@Co1-xBi0.1-yO7 dual s-scheme heterojunction towards degradation of organic pollutants. Opt. Mater. 126, 112199 (2022).
Bhatnagar, A. & Jain, A. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Collid Interface Sci. 281(1), 49–55 (2005).
Mahmoodian, H. et al. Enhanced removal of methyl orange from aqueous solutions by poly HEMA–chitosan-MWCNT nano-composite. J. Mol. Liq. 202, 189–198 (2015).
Shahzad, K. et al. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorganic Chem. Commun. 123, 108357 (2021).
Chaukura, N., Murimba, E. C. & Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 7, 2175–2186 (2017).
Chung, K.-T., Fulk, G. & Andrews, A. Mutagenicity testing of some commonly used dyes. Appl. Environ. Microbiol. 42(4), 641–648 (1981).
Azami, M. et al. Central composite design for the optimization of removal of the azo dye, methyl orange, from waste water using fenton reaction. J. Serb. Chem. Soc. 77(2), 235–246 (2012).
Nazir, M. A. et al. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorganic Chem. Commun. 145, 110008 (2022).
Gul, S. et al. Efficient removal of methyl red dye by using bark of hopbush. Water 14(18), 2831 (2022).
Takkar, S. et al. Biodegradation of methyl red dye by a novel actinobacterium Zhihengliuella sp. ISTPL4: Kinetic studies, isotherm and biodegradation pathway. Environ. Technol. Innov. 26, 102348 (2022).
Annadurai, G., Juang, R.-S. & Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 92(3), 263–274 (2002).
Ahmad, M. A., Ahmad, N. & Bello, O. S. Modified durian seed as adsorbent for the removal of methyl red dye from aqueous solutions. Appl. Water Sci. 5(4), 407–423 (2015).
Xue, Y., Liu, X., Zhang, N., Shao, Y. & Xu, C. C. Enhanced photocatalytic performance of iron oxides@HTCC fabricated from zinc extraction tailings for methylene blue degradation: Investigation of the photocatalytic mechanism. Int. J. Miner. Metall. Mater. 30(12), 2364–2374. https://doi.org/10.1007/s12613-023-2723-5 (2023).
Nazir, M. A. et al. Kinetics, isothermal and mechanistic insight into the adsorption of eosin yellow and malachite green from water via tri-metallic layered double hydroxide nanosheets. Korean J. Chem. Eng. 39(1), 216–226 (2022).
Khan, N. A. et al. Efficient removal of norfloxacin by MOF@GO composite: Isothermal, kinetic, statistical, and mechanistic study. Toxin Rev. 40(4), 915–927 (2021).
Jamshaid, M. et al. A novel bentonite–cobalt doped bismuth ferrite nanoparticles with boosted visible light induced photodegradation of methyl orange: synthesis, characterization and analysis of physiochemical changes. Int. J. Environ. Anal. Chem. 104, 1–16 (2022).
Liu, Y., Zheng, Y. & Wang, A. Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 22(4), 486–493 (2010).
Gündüz, F. & Bayrak, B. Biosorption of malachite green from an aqueous solution using pomegranate peel: Equilibrium modelling, kinetic and thermodynamic studies. J. Mol. Liq. 243, 790–798 (2017).
Wang, Z., Fernández-Blanco, C., Chen, J., Veiga, M. C. & Kennes, C. Effect of electron acceptors on product selectivity and carbon flux in carbon chain elongation with Megasphaera hexanoica. Sci. Total Environ. 912, 169509. https://doi.org/10.1016/j.scitotenv.2023.169509 (2024).
Gong, R. et al. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 52(39), 14297–14303 (2013).
Vargas, A. M. et al. NaOH-activated carbon from flamboyant (Delonix regia) pods: optimization of preparation conditions using central composite rotatable design. Chem. Eng. J. 162(1), 43–50 (2010).
Deepa, B. & Remadevi, O. Larvicidal activity of the flowers of Delonix regia (Bojer Ex Hook.) Rafin.(Fabales: Fabaceae) against the teak defoliator, Hyblaea puera Cramer. Curr. Biot. 5(2), 237–240 (2011).
De Groot, H. Reactive oxygen species in tissue injury. Hepato-gastroenterology 41(4), 328–332 (1994).
Sethuraman, M. & Sulochana, N. The anti-inflammatory activity of Delonix elata Gamble. Curr. Sci. 55(7), 343–343 (1986).
Prakash, M. et al. Isolation and antibacterial activity of oleananoic acid acetate from Delonix regia leaves. J. Pharmacy Res. 6(4), 423–425 (2013).
Vargas, A. M., Martins, A. C. & Almeida, V. C. Ternary adsorption of acid dyes onto activated carbon from flamboyant pods (Delonix regia): analysis by derivative spectrophotometry and response surface methodology. Chem. Eng. J. 195, 173–179 (2012).
Saravanan, A. et al. Optimization and modeling of reactive yellow adsorption by surface modified Delonix regia seed: Study of nonlinear isotherm and kinetic parameters. Surfaces Interfaces 20, 100520 (2020).
Utsev, J., Iwar, R. & Ifyalem, K. Adsorption of methylene blue from aqueous solution onto Delonix regia pod activated carbon: batch equilibrium isotherm, kinetic and thermodynamic studies. Agric. waste 4(5), 18 (2020).
Gregg, S. J. & Sing, K. S. W. Adsorption Surface Area and Porosity 2nd edn. (Academic Press Inc, 1982).
Rouquerol, F., Rouquerol, J. & Sing, K. S. W. Adsorption by Powders and Porous Solids (Academic Press Inc., 1999).
Barrett, E. P., Joyner, L. G. & Halenda, P. P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 73(1), 373–380 (1951).
El-Nemr, M. A., Yılmaz, M., Ragab, S. & El Nemr, A. Biochar-SO prepared from pea peels by dehydration with sulfuric acid improves the adsorption of Cr6+ from water. Biomass Convers. Biorefinery 20, 01. https://doi.org/10.1007/s13399-022-02378-4 (2022).
El-Nemr, M. A., Yılmaz, M., Ragab, S. & El Nemr, A. Watermelon peels biochar-S for adsorption of Cu2+ from water. Desalin. Water Treat. 261, 195–213. https://doi.org/10.5004/dwt.2022.28506 (2022).
Hassaan, M. A. et al. Synthesis, characterization, optimization and application of Pisum sativum peels S and N-doping biochars in the production of biogas from Ulva lactuca. Renew. Energy. 221, 119747. https://doi.org/10.1016/j.renene.2023.119747 (2024).
Eldeeb, T. M. et al. Adsorption of methylene blue dye on sawdust ozone, purified sawdust, and sonicated sawdust biochars. Biomass Conversion and Biorefinery 12, 07. https://doi.org/10.1007/s13399-022-03015-w (2022).
Eleryan, A. et al. Copper (II) ion removal by chemically and physically modified sawdust biochar. Biomass Convers. Bioref. https://doi.org/10.1007/s13399-022-02918-y (2022).
Hassaan, M. A. et al. Synthesis, characterization, and synergistic effects of modified biochar in combination with α-Fe2O3 NPs on biogas production from red algae pterocladia capillacea. Sustainability 13, 9275 (2021).
Nazir, M. A. et al. Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects. Microchem. J. 164, 105973 (2021).
Angelina Thanga Ajisha, M. & Rajagopal, K. Fluoride removal study using pyrolyzed Delonix regia pod, an unconventional adsorbent. Int. J. Environ. Sci. Technol. 12(1), 223–236 (2015).
El-Nemr, M. A., Yılmaz, M., Ragab, S. & El Nemr, A. Biochar-SO prepared from pea peels by dehydration with sulfuric acid improves the adsorption of Cr6+ from water. Biomass Convers. Biorefinery 14, 2601–2619. https://doi.org/10.1007/s13399-022-02378-4 (2024).
Eldeeb, T. M. et al. Adsorption of methylene blue dye on sawdust ozone, purified sawdust, and sonicated sawdust biochars. Biomass Convers. Biorefinery 12(07), 2022. https://doi.org/10.1007/s13399-022-03015-w (2022).
Ponnusami, V., Gunasekar, V. & Srivastava, S. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: multivariate regression analysis. J. Hazard. Mater. 169(1–3), 119–127 (2009).
Shoaib, A. G. M., El-Sikaily, A., El Nemr, A., Mohamed, A. E. & Hassan, A. A. Testing the carbonization condition for high surface area preparation of activated carbon followed Type IV from green alga Ulva lactuca. Biomass Convers. Biorefinery 12, 3303–3318. https://doi.org/10.1007/s13399-020-00823-w (2022).
Shoaib, A. G. M., El-Sikaily, A., El Nemr, A., Mohamed, A.E.-D.A. & Hassan, A. A. Preparation and characterization of highly surface area activated carbons followed Type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Convers. Biorefinery. 12, 2253–2265. https://doi.org/10.1007/s13399-020-00760-8 (2022).
Nazir, M. A. et al. Synthesis of porous secondary metal-doped MOFs for removal of Rhodamine B from water: Role of secondary metal on efficiency and kinetics. Surfaces Interfaces 25, 101261 (2021).
Abdelwahab, O., El Sikaily, A., Khaled, A. & El Nemr, A. Mass-transfer processes of Chromium (VI) adsorption onto Guava seeds. Chem. Ecol. 23(1), 73–85 (2007).
El-Nemr, M. A., Ismail, I. M. A., Abdelmonem, N. M., Ragab, S. & El Nemr, A. Ozone and ammonium hydroxide modification of biochar prepared from Pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ. Processes 7(2020), 973–1007. https://doi.org/10.1007/s40710-020-00455-2 (2020).
El Nemr, A. et al. Microporous nano activated carbon type I derived from orange peel and its application for Cr(VI) removal from aquatic environment. Biomass Conver. Bioref. 12, 5125–5143. https://doi.org/10.1007/s13399-020-00995-5 (2022).
Yılmaz, M. et al. The use of mandarin-biochar-O3-TETA (MBT) produced from mandarin peels as a natural adsorbent for the removal of acid red 35 (AR35) dye from water. Environ. Processes 9, 44. https://doi.org/10.1007/s40710-022-00592-w (2022).
El-Nemr, M. A., Ismail, I. M. A., Abdelmonem, N. M., El Nemr, A. & Ragab, S. Amination of biochar surface from watermelon peel for toxic chromium removal enhancement. Chin. J. Chem. Eng. 36, 199–222 (2021).
El Nemr, A., El-Sikaily, A. & Khaled, A. Modeling of adsorption isotherms of Methylene Blue onto rice husk activated carbon. Egypt. J. Aquatic Res. 36(3), 403–425 (2010).
El Eleryan, A. et al. Feasible and eco-friendly removal of hexavalent chromium toxicant from aqueous solutions using chemically modified sugarcane bagasse cellulose. Toxin Rev. 40(4), 835–846. https://doi.org/10.1080/15569543.2020.1790606 (2021).
El Nemr, A., Shoaib, A. G. M., El Sikaily, A., Mohamed, A.E.-D.A. & Hassan, A. F. Evaluation of cationic Methylene blue dye removal by high surface area mesoporous nano activated carbon derived from Ulva lactuca. Environ. Process. 8(1), 311–332. https://doi.org/10.1007/s40710-020-00487-8 (2021).
Sahlabji, T. et al. High surface area microporous activated carbon from Pisum sativum peels for hexavalent chromium removal from aquatic environment. Toxin Rev. 41(2), 639–649. https://doi.org/10.1080/15569543.2021.1908361 (2022).
Nazir, M. A. et al. Enhanced adsorption removal of methyl orange from water by porous bimetallic Ni/Co MOF composite: A systematic study of adsorption kinetics. Int. J. Environ. Anal. Chem. 103, 1–16 (2021).
El Nemr, A., Helmy, E. T., Arafa, E., Eldafrawy, S. & Mousa, M. Photocatalytic and biological activities of undoped and doped TiO2 prepared by Green method for water treatment. J. Environ. Chem. Eng. 7(5), 103385 (2019).
Hosain, A. N. A., El Nemr, A., El Sikaily, A., Mahmoud, M. E. & Amira, M. F. Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb(II), Cu(II) and Cd(II) removal. J. Environ. Chem. Eng. 8(5), 104316. https://doi.org/10.1016/j.jece.2020.104316 (2020).
El Nemr, A. et al. Utilization of green alga Ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of Direct Red 23 dye from aquatic environment. Carbon Lett. 32, 153–168. https://doi.org/10.1007/s42823-021-00262-1 (2022).
Shoaib, A. G. M., El Nemr, A., El Sikaily, A., Masoud, M. S. & Ramadan, M. S. Amidoxime modification of poly acrylonitrile/Pterocladia capillacea derived activated carbon composite for adsorption of toxic chromium from aquatic environment. Carbon Lett. 32, 513–535. https://doi.org/10.1007/s42823-021-00281-y (2022).
El-Nemr, M. A. et al. Microporous activated carbon prepared from Pisum sativum pods using different activation methods for adsorptive removal of Acid Orange 7 dye from water. Molecules 27, 4840. https://doi.org/10.3390/molecules27154840 (2022).
El-Nemr, M. A. et al. Adsorption of Cr6+ ion using activated Pisum sativum peels decorated with triethylenetetramine. Environ. Sci. Pollut. Res. 29, 91036–91060. https://doi.org/10.1007/s11356-022-21957-6 (2022).
Eleryan, A. et al. Mandarin Biochar-TETA (MBT) prepared from Citrus reticulata peels for adsorption of Acid Yellow 11 dye from water. Sci. Rep. 12, 17797 (2022).
Abdi, J. et al. Synthesis of magnetic metal-organic framework nanocomposite (ZIF-8@SiO2@MnFe2O4) as a novel adsorbent for selective dye removal from multicomponent systems. Microporous Mesoporous Mater. 273, 177–188 (2019).
Haque, E. et al. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J. Hazard. Mater. 181(1), 535–542 (2010).
Asouhidou, D. D. et al. Adsorption of reactive dyes from aqueous solutions by layered double hydroxides. J. Chem. Technol. Biotechnol. 87(4), 575–582 (2012).
Nazir, M. A. et al. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 190, 105564 (2020).
Darwish, A. A. A., Rashad, M. & Al-Aoh, H. A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigments 160, 563–571 (2019).
Khan, E. A., Shahjahan, & Khan, T. A. Adsorption of methyl red on activated carbon derived from custard apple (Annona squamosa) fruit shell: Equilibrium isotherm and kinetic studies. J. Mol. Liq. 249, 1195–1211 (2018).
Zaman, S. et al. Hen feather: a bio-waste material for adsorptive removal of methyl red dye from aqueous solutions. H2Open J. 4(1), 291–301 (2021).
Brahma, D. & Saikia, H. Surfactants assisted synthesis of CuAl-sodium dodecyl sulfate layered double hydroxide and its adsorptive removal of methyl red dye from aqueous solution. Inorgan. Nano-Metal Chem. 1–16 (2023).
Zaheer, Z., Al-Asfar, A. & Aazam, E. S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 283, 287–298 (2019).
El Nemr, A. Non-conventional textile waste water treatment. In Pollution Science, Technology and Abatement Series. Nova Science Publishers, Inc. Hauppauge New York. [Hard cover ISBN: 978–1–62100–079–2, e-book ISBN: 978–1–62100–228–4] 267 pages (2012). https://www.amazon.com/Non-Conventional-Treatment-Pollution-Technology-Abatement/dp/1621000796
El-Nemr, A. et al. Utilization of sugarcane bagasse/ZnCl2 for sustainable production of microporous nano activated carbons of type I for toxic Cr(VI) removal from aqueous environment. Biomass Convers. Biorefinery 13, 1581–1600. https://doi.org/10.1007/s13399-021-01445-6 (2023).
El Nemr, A. et al. Utilization of Citrus aurantium peels for sustainable production of high surface area type I microporous nano activated carbons. Biomass Convers. Biorefinery 13, 1613–1631. https://doi.org/10.1007/s13399-021-01457-2 (2023).
El Sikaily, A., El-Nemr, A., Khaled, A. & Abdelwahab, O. Removal of toxic Chromium from wastewater using green alga Ulva lactuca and its activated Carbon. J. Hazard. Mater. 148, 216–228 (2007).
El-Nemr, M. A., Abdelmonem, N. M., Ismail, I. M., Ragab, S. & El Nemr, A. Ozone and ammonium hydroxide modification of biochar prepared from Pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ. Process. 7, 973–1007 (2020).
El Nemr, A. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: kinetic and isotherm studies. J. Hazard. Mater. 161, 132–141 (2009).
Eleryan, A. et al. Mandarin Biochar-TETA (MBT) prepared from Citrus reticulata peels for adsorption of Acid Yellow 11 dye from water. Sci. Rep. 12, 17797. https://doi.org/10.1038/s41598-022-22359-x (2022).
El Nemr, A., Abdelwahab, O., Khaled, A. & El Sikaily, A. Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. J. Hazard. Mater. 161, 102–110 (2009).
Parshetti, G. K., Hoekman, S. K. & Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 135, 683–689 (2013).
Tsai, W. T. & Chen, H. R. Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manure-derived biochar. Int. J. Environ. Sci. Technol. 10, 1349–1356 (2013).
Xu, R. K., Xiao, S. C., Yuan, J. H. & Zhao, A. Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol. 102, 10293–10298. https://doi.org/10.1016/j.biortech.2011.08.089 (2011).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was partially funded by the Science and Technology Development Fund (STDF) of Egypt (Projects Nos. SCSE-31235).
Author information
Authors and Affiliations
Contributions
Dr A.E., and Dr. M.A.H. carried out the experiment, calculation and theoretical models. Dr. M.A.H. carried out the characterization, Dr S.R. read the manuscript and supervised the experimental work, Dr. M.A.N. and Dr. S.S.A.S. wrote the original manuscript. Prof. A.E.N. supervised the experimental work, completed the manuscript and provided financial support for the experiment work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eleryan, A., Hassaan, M., Nazir, M.A. et al. Isothermal and kinetic screening of methyl red and methyl orange dyes adsorption from water by Delonix regia biochar-sulfur oxide (DRB-SO). Sci Rep 14, 13585 (2024). https://doi.org/10.1038/s41598-024-63510-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63510-0
- Springer Nature Limited