Abstract
The synthesized biochars derived from sawdust (SD) SD ozone (SDO) biochar, purified SD (PSD) biochar, and sonicated SD (SSD) biochar, which was employed in the confiscation of methylene blue (MB) dye ion, were characterized employing “Brunauer–Emmett–Teller (BET), scanning electron microscope (SEM), Fourier Transform Infrared (FTIR), and Thermal gravimetrical analysis (TGA).” The impact of various factors, such as pH, biochar dosage, and initial concentration, on MB dye sequestration, was tested in this study. It was found that the biosorption of MB dye to the various biochars was dependent on the solution pH, with optimum confiscation of MB observed at pH 12 for all biochars. Pseudo-second-order (PSO), Freundlich (FRH)- (SDO and SSD biochars), and Langmuir (LNR)- (PSD biochar) models were used to best describe the biosorption process of MB dye to various biochars. Based on the LNR model fitting to the experimental data, the optimum sorption capacities obtained using SDO, SSD, and PSD biochars were 200, 526, and 769 mg/g, respectively. Electrostatic interaction and hydrogen bonding played an important role in the interaction mechanism between the various biochars and MB dye. Hence, these studied SDO, PSD, and SSD biochars prepared from cheap, easily accessible, biodegradable, and non-hazardous agro-waste materials can be effectively used for the removal, treatment, and management of MB dye as well as other industrial effluents before their disposal into the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental effluences ensuing as a result of the annihilation of the aquatic, terrestrial, and atmospheric ecosystems by the fast and aggressive increase in industrial activities and the global population are critical to the existence of man and other living organisms as well as the sustenance of the ecosystem [1,2,3,4,5,6,7,8,9,10]. According to several research reports, these environmental effluences have extremely affected the quality of water due to the untreated wastes being disposed of, released, or discharged into the aquatic environments, mostly by industries via the production processes of textiles, paper, leather, petroleum, and paint, as well as metal plating, mining, and other industrial production processes [11, 12], consequently, contributing immensely to climate change and global warming [13,14,15], health complications [16,17,18], food shortage (agricultural shortfall) [19,20,21], etc.
The majority of these industries that use dyes (cosmetics, textiles, paper, leather, and paint industries) have a high infecting trail, and it is projected that up to 2.00 × 105 tons of dyes are disposed of, released, or discharged into the aquatic environments owing to the ineffective wastewater management and treatment processes [22,23,24,25,26,27]. Amongst these toxic and aromatic effluents being disposed of, released, or discharged into the aquatic environments without preceding proper treatment, the cosmetics, textiles, paper, leather, and paint industries are major users of synthetic and toxic dyes, basically aimed at the coloring of their products [28,29,30].
The global dye market is currently valued at over 32 billion US dollars (USD) and is expected to reach 42 billion USD in 2021 [31]. Synthetic dyes are produced in excess of 7.00 × 105 tons per year, with approximately 15.00% of these synthetic dyes being released into water bodies (aquatic environments) [32]. These dyes are carcinogenic, toxic, and xenobiotic and they are divided into solvent, reactive, basic, vat, and direct dyes based on their chromophore structure. These synthetic dyes are exclusively decomposable in 47.00% of cases [28, 33]. Hence, dye has been considered one of the foremost sources of severe environmental pollution owing to the fast and aggressive industrial advancement and extensive utilization of its products [34,35,36].
Industrial effluents, instigated by the production processes of the cosmetics, textiles, paper, leather, and paint industries, are known to have a significant quantity of toxic aromatic dyes, mostly the thiazine cationic dyes such as methylene blue (MB) [37,38,39,40]. Notwithstanding the valuable uses of MB (C16H18ClN3S) in various scientific domains, the dye has detrimental effects on humans and other living organisms as well as the environment owing to its water solubility tendency [41, 42]. It is toxic, a mutagenic reagent, and is supposed to be a cancer-instigating dye [24]. The colored effluents disposed of, released, or discharged during the production processes of the cosmetics, textiles, paper, leather, and paint industries into the ecosystem are one of the predominant causes of the ensuing environmental pollution as well as awkwardness in the water bodies (aquatic environment) in particular [24, 29, 43, 44]. The MB is a characteristic industrial effluent, and it is one of the prevalent commercial thiazine and azo dyes [38, 39, 43, 45].
Also, MB dye is conceivably detrimental to human health and contributes to instigating chronic toxicity, mostly to the central nervous system [24]. The effluents and wastes generated by the cosmetics, textiles, paper, leather, and paint industries are highly colored, contain salt concentrations in high quantities, and have high biological oxygen demand as well as chemical oxygen demand values. As a result, removing MB dye and other toxic industrial waste is critical for a better and healthier environment and well-being [24, 29, 46]. For the removal of MB dye and other industrial effluents (generated by the cosmetics, textiles, paper, leather, and paint industries), a variety of effluents and waste treatment and management technologies has been investigated, including adsorption, electrochemical, ion exchange, photocatalysis, oxidation–reduction, membrane filtration, catalytic, and biological/organic treatment and processes [47,48,49,50,51]. Amongst the aforementioned removal and management technologies, adsorption seems to be the most economical and efficient technique, owing to its inexpensiveness, easy operational principle, and high effluent uptake capacities for the removal of dyes and other industrial effluents from wastewater [24, 48, 52].
Some industrial processes usually utilize activated carbon (AC) as an adsorbing reagent (biosorbent) due to its high absorbency as well as its specific surface area. However, due to its high production cost for regeneration tenacities, AC acquired from the market is usually expensive. Hence, the concept of using nanomaterials (NMs) as adsorbing reagents (biosorbents) is now gaining traction [53]. Though some NMs, such as carbon nanotubes, nano zerovalent iron, and titania nanotubes, are believed to be toxic and ineffective at removing dyes [48]. Several efforts have been made to mitigate these limitations via the preparation of the NMs from cellulose-based, tannin-based, and lignin-based sources, all of which have been proven to have massive potential for the adsorption and treatment of dyes and other industrial effluents, and they are considered affordable, easily accessible, biodegradable, and non-hazardous materials for the amputation of effluents in general as well as cationic MB dye in particular, from wastewater [3, 24, 54, 55]. Reportedly, these cellulose-based, tannin-based, and lignin-based adsorbing reagents (biosorbents) are derived from the cells of plants, mostly from agro-waste materials such as waste from watermelon, sugarcane, orange, wheat straw, maize, jackfruit, rice, mango, cloves, palm fruits, coconut, wood, and other agroforestry ingredients [3, 24, 48].
Therefore, formulating innovative, easily accessible, and non-hazardous adsorbing reagents (biosorbents) with high-uptake proficiencies is a contemporary and continuous global issue for the adsorption and treatment of dyes and other industrial effluents from wastewater. Hence, this present study emphasizes and re-explores the prospects of using a new, affordable, easily accessible, biodegradable, and non-hazardous adsorbing reagent (biosorbents) prepared from sawdust (SD) agro-waste materials for the production of SD ozone (SDO) biochar, purified SD (PSD) biochar, and sonicated SD (SSD) biochar. The preparation, characterization, and morphological studies as well as the application of these produced SDO, PSD, and SSD biochars for the adsorption and treatment of MB dye from wastewater were considered. The batch adsorption studies for the adsorption and treatment of MB dye from industrial toxic effluents were characterized, and the assessment of the optimization of the various adsorption factors and parameters was systematically considered. Also, the calculation of the adsorption rate isotherms, thermodynamics, and kinetics was essentially highlighted.
2 Materials and methods
2.1 Materials and equipment
Wood sawdust was collected from a local wood market in Alexandria, Egypt, and was used as a raw material for the preparation of biochar. The standard stock solution was made from MB dye (Assay 99%) obtained from Sigma-Aldrich, USA. Pg instrument model T80 UV/visible high-performance double beam spectrophotometer matched with glass cells of 1 cm optical path, Shaker [A JS shaker (JSOS-500)], and pH meter JENCO (6173) was used in this study. The adsorption–desorption isotherm of PSD, SDO, and SSD biochars was carried out in an N2 atmosphere based on a thermodynamic model. The PSD, SDO, and SSD biochars’ surface area was calculated by N2 adsorption at 77 K with the aid of a surface area and pore analyzer (BELSORP – Mini II, BEL Japan, Inc.) [56, 57]. Surface area (SBET) (m2/g), monolayer volume (Vm) (cm3 (STP), average pore diameter (MPD) (nm), total pore volume (p0/p0) (cm3/g), and energy constant (C) values of PSD, SDO, and SSD biochars were calculated using the graph (Fig. 1). The mesoporous surface area (Smes), microporous surface area (Smi), mesoporous volume (Vmes), and microporous volume (Vmi) of PSD, SDO, and SSD biochars were evaluated using the Barrett–Joyner–Halenda (BJH) method. The BELSORP analysis program software was used to perform the calculations. The pore size dispersion was also determined from the desorption isotherm using the BJH technique [58]. With the help of a scanning electron microscope (SEM), the shape of the biochars surface was investigated (QUANTA 250). The functional groups on the surface of biochars’ were studied using Fourier transform infrared (FTIR) spectroscopy (VERTEX70) connected with ATR unit model V-100. IR-observable functional groups on the PSD, SDO, and SSD biochars’ surface were detected in the wavenumber range of 400–4000 cm−1. Thermal analyses were carried out using the SDT650-Simultaneous Thermal Analyzer device at a temperature range of 50–1000 °C and a ramped temperature of 5 °C/min.
2.2 Methods
2.2.1 SD Biochar preparation
The collected sawdust was thoroughly washed with tap water several times to remove dust and subsequently dried in an oven at 70 °C for 72 h. A total of 500 g of wood sawdust in 2000 ml solution of 80.00% H2SO4 was boiled in a refluxed system at 220 °C for 2 h, and then, the samples were filtered and washed with distilled water until the washing solution become neutral followed by washing with ethanol. The final product of biochar was dried in an oven at 120 °C, and its weight was then determined (220 g). The obtained biochar from this reaction was labelled (SD).
2.2.2 SSD biochar preparation
The prepared (SD) biochar was suspended in water and subsequently subjected to ultrasonic energy (Elma, TRANSSONIC NIC T890H/Germany, High Frequency peak 600 W, 35 kHz) for 60 min for physical activation to take place. The sonicated solution was filtered and washed with distilled water and dried at 120 °C. The obtained sonicated sawdust biochar from this treatment was labelled as SSD.
2.2.3 PSD biochar preparation
The SD biochar (30 g) was boiled in ethanol (200 ml) for 2 h and then collected and filtered. The solid biochar was dried at 120 °C for 24 h and labelled as PSD.
2.2.4 SDO biochar preparation
The prepared SD was subsequently subjected to ozone treatment in distilled water. For the treatment of SD with ozone, 50 g of prepared SD was ozonated in 200 ml distilled water for 2 h, then filtered, and washed with distilled water followed by ethanol. Finally, the ozonated SD biochar was dried at 120 °C for 48 h and labelled as SDO.
2.3 Adsorption measurement for MB dye
A stock solution of MB dye (1000 mg L−1) was prepared by dissolving 1.0 g of MB dye in 1000 ml of distilled water, and this solution was diluted to have the required concentration for the removal test and the standard curve. The batch adsorption experiments were employed to evaluate the adsorption capability, thermodynamic and kinetic parameters of SDO, SSD, and PSD which were prepared from SD. A series of Erlenmeyer flasks (300 ml) containing 100 ml of different concentrations of MB dye solution and different amounts of biochar was shaken at 200 rpm for a certain time. The sample pH was adjusted to the desired values with 0.1 M HCl or 0.1 M NaOH. About 0.5 ml of the solution in the Erlenmeyer flask was then separated from the adsorbent, and the concentration of MB dye was detected at different interval times and the equilibrium. The concentration of MB dye was determined by spectrophotometry at λmax 665 nm [38, 59].
Initially, the slope of the standard curve correlated between known MB dye concentrations and related absorbance has been used to calculate the unknown MB dye concentration (Ct) at time intervals from the absorbance value practically detected. Equation (1) shows the Ct calculation.
MB dye concentration at equilibrium Ce; at stable values of absorbance. Equation (2) shows the Ce calculation.
The adsorption capacities of MB dye at equilibrium (qe) were calculated from Eq. (3):
where qe is the amount of MB dye per unit of adsorbent at equilibrium (mg g−1); C0 and Ce are the initial and equilibrium concentrations of MB dye in the liquid phase (mg.L−1), respectively; V is the volume of the solution (L) and W is the mass of adsorbent (PSD, SDO, or SSD) in gram.
2.3.1 Effect of solution pH, initial MB dye concentration, adsorbent dosage, and contact time
The effect of pH was studied for SDO, SSD, and PSD using 100 ml of 100 ppm of initial MB dye concentration using solution pH (2–12). The pHPZC has been studied for the SDO, SSD, and PSD biochars following the literature-reported method [60].
The isotherm study for sawdust biochars was performed using various initial concentrations of MB dye solution (20, 40, 60, 80, 100, and 120 mg.L−1) using different doses of SDO and SSD biochars (05, 1.0, 2.0, 3.0, and 4.0 g.L−1) as well as the various concentrations of MB dye solution (100, 150, 200, 300, and 400 mg.L−1) using different doses of PSD biochar (0.5, 1.0, 1.5, 2.0, and 2.50 g.L−1). The samples were shaken at 200 rpm, and the MB dye concentration was analyzed at different time intervals at room temperature (25 ± 2 °C).
In the kinetics study, SDO and SSD biochars of various doses (0.5, 2.0, 3.0, and 4.0 g L−1) were added to 300-ml Erlenmeyer flasks containing 100 ml of initial MB dye solution (20, 40, 60, 80, 100, and 120 mg L−1), and PSD biochar of various doses (0.5, 1.0, 1.5, 2.0, and 2.5 g L−1) was added to 300-ml Erlenmeyer flasks containing 100 ml of initial MB dye solution (100, 150, 200, 300, and 400 mg L−1). The flasks were shaken at 200 rpm and samples were taken from the solution at different interval times for the analysis of the residual concentration of MB dye.
3 Results and discussion
3.1 Characterization of biochars
3.1.1 Estimation of biochar surface functional groups by FTIR
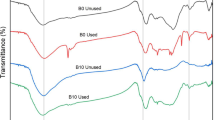
FTIR analysis was performed on SDO, SSD, and PSD biochars to identify the functional groups on their surfaces and to know the effect of modification on the disappearance or existence of a new functional group. Figure 1 shows the FTIR spectra of SDO, SSD, and PSD biochars. Bands at 3350 and 3215 cm−1 represent the O–H stretching vibration that existed in PSD, SDO, and SSD biochars, while the band at 2931.5 cm−1 represents –C–H stretch of the alkyl in PSD, SDO, and SSD biochars. The band from 1800–1450 cm−1 known as “overtones” as band 1695.2 cm−1 in PSD and SSD was dramatically reduced in SDO, whereas the band at 1592 cm−1 –C–C– stretch (in-ring) was unaffected by the different treatments applied to SD biochar [61]. Bands in biochars between 1470 and 900 cm−1 represent C-O functional groups and show no significant changes as a result of the different treatments applied to the SD biochar. The bands at 1027, 1029, and 1033 cm−1 in SDO, PSD, and SSD, respectively, are related to the C-O–H functional group [36, 37, 62].
Figure 2 shows the FTIR spectrum of PSD, SSD, and SDO biochars after they have been treated with MB dye for 3 h. It was discovered in all of the FTIR analyses of all tested biochars after they had been exposed to an MB dye removal process that there were bands at 1591, 1485, 1392, and 1340 cm−1, which correspond to the MB dye.
3.1.2 Biochars’ surfaces analysis
The influence of sonication and ammonia solution treatment on the surface properties of wood sawdust biochar was investigated by N2 adsorption–desorption. The Brunauer–Emmett–Teller (BET) and BJH methods were used to calculate the specific characteristic of biochar surfaces. Figure 3 shows that the BET-specific surface area of biochars decreased as SSD (4.0098 m2.g−1) > PSD (2.92 m2 g−1) > SDO (1.975 m2/g). It is noted that modifications affect the specific surface area and the effect of physical modification by sonication is more than chemical modification with ozone treatment. The mean pore diameter decreased with modifications in the order PSD (16.98 nm) > SSD (14.73 nm) > SDO (10.716 nm). The effect of ozone modification is greater than sonication on pore diameter decreasing due to the introduction of OH groups. Pore types are mesoporous. The total pore volume of PSD, SSD, and SDO biochars were 12.254 × 10−3, 14.77 × 10−3 and 5.291 × 10−3 cm3 g−1, respectively. Figure 3c shows the BJH data of PSD, SSD, and SDO biochars, and Table 1 includes their surface attributes.
3.1.3 Morphological properties of sawdust biochar surfaces
The surface morphology of sawdust raw material (RSD), PSD, SSD, and SDO biochars was investigated using SEM and reported in Fig. 4. As shown in Fig. 4a, b, the PSD and SSD biochars appear clean and free of any particulate impurities; besides, there is no damage in the pores of the SSD which was observed as a result of sonication. Also, a small increase in the pore volume and the number of pores in SSD biochar due to sonication treatment of PSD biochar, which explains the small increase in the surface area of SSD biochar. Figure 4c shows the SDO biochar that little pores existed which reflects the small surface area for SDO biochar. This proves our previous finding that the treatment of biochar with ozone in water led to a decrease in the surface area due to pore blocking [36, 37, 62]. It is obvious that new pores of different sizes are created in SSD biochar as a result of sonication treatment; thus, the surface areas of the sonicated biochar increased while treatment with ozone in water led to the blocking of the pore and decrease the surface area of the produced SDO biochar. The SEM image of sawdust raw material represented in Fig. 4d showed no pores.
3.1.4 Elemental analysis of sawdust biochars
Energy dispersive X-ray spectrometer (EDX) was used to analyze the chemical compositions of sawdust biochar. The chemical compositions of the samples were analyzed with a dispersive X-ray spectrometer (EDAX). The element percent of SD, SSD, and ASD biochars were examined and reported in Table 2, which shows the absence of nitrogen peak before the modification with the NH4OH reagent. The EDX analysis of ASD biochar proved the presence of about 7.63% nitrogen in sample weight.
3.1.5 Thermal characterization of biochars
Thermal gravimetrical analysis (TGA) or thermogravimetric analysis in general means the measurement of the weight loss during a user-defined temperature or heating process. Figure 5a and b show the decomposition of SD and SSD biochars, respectively, which takes place in three steps while the decomposition of the ASD biochar takes place in four steps (Fig. 5c). In SD biochar, the first step of decomposition occurs at temperatures ranging from 50 to 150 °C and involves the loss of surface-bound water and moisture present in the sample with an approximate weight loss of 6.76%. In the second step, temperatures ranging from 150 to 700 °C and an approximate weight loss of 56.30%, and in the third decomposition step, temperatures ranging from 700 to 1000 °C and an approximate weight loss of 22.55%. In SSD biochar, the first step of decomposition occurs at temperatures ranging from 50 to 180 °C, with an approximate weight loss of 12.21%; the second step, temperatures ranging from 180 to 675 °C and approximate weight loss of 32.31%; and in the third decomposition step, temperatures ranging from 675 to 1000 °C and approximate weight loss of 4.28%. While in ASD biochar, the first step of decomposition occurs at temperatures ranging from 50 to 180 °C and involves approximately weight loss of 13.21%; the second step, temperatures ranging from 180 to 250 °C and approximate weight loss of 3.99%; and in the third decomposition step, temperatures ranging from 250 to 700 °C and approximate weight loss of 30.08%; and in fourth step, temperatures ranging from 700 to 1000 °C and approximate weight loss of 2.96%.
3.2 Effect of pH on the adsorption of MB dye
The adsorption of MB dye to different biomaterials is extremely conditional on the pH of the solution and the biomaterial surface charge distribution. The electrostatic interactions of attractive or repulsive character between MB dye species and active sites on the surface of various biomaterials are affected by the solution pH. In a water-soluble solution, MB dye exists as undissociated molecules (MB0) and cationic species (MB1) (Fig. 6c). As observed in Fig. 6c, MB0 and MB1 (50% species each) coexist at pH = pKa = 3.8. While MB0 species (86%) and MB1 species dominate and are only present at pH 3 and pH > 6 [63]. The surface charge distribution (pHPZC) of SDO, SSD, and PSD biochars employed for the sequestration of MB dye ions is depicted in Fig. 6a. The determined pHpzc for SDO, SSD, and PSD showing the near association that exist between the surface groups of the biochars and their surface charge were 4.4, 4.9, and 4.4. Hence, the surface charge was positively or negatively charged when the solution pH was < or > the pHpzc. Figure 6b shows the impact of varying solution pH on MB dye confiscation and it was noticed that the % of MB dye ions sorbed to the SDO, SSD, and PSD biochars increased considerably as the solution pH was varied from pH 2 to 12, with optimum % removal of MB dye ions noticed at pH 12. The low % of MB dye confiscated to the various biochars at pH 2 was ascribed to the electrostatic repulsion between the negative surface charges and the cationic sorbate undissociated species (MB0), as surface sites on biochars are suggestive of having a considerably extra amount of negatively charged groups at low pH. Also, the increased competition between MB+ with the H+ and H3O+ ions for the biochar sorption sites leads to a reduced % of MB dye removed at acidic pH. The removal of MB dye to the various biochars at low pH values was due to interactions such as hydrogen bonding, π-π or π+-π interaction, and porous diffusion [64]. The improved % of MB dye removed to the various biochars was attributed to the electrostatic attraction between the dominant negatively surface charges (which increased with improving the solution pH) and the predominant MB dye species (MB1 dye). Improving the solution pH was reported to increase the amount of hydroxyl (-OH) and carboxylate anion (-COO-) groups on the biochar surface and hence improve the amount of negatively charged sites on the surface of the biochar [64]. A similar trend was also reported in the studies by Salazar-Rabago et al. [63], Phuong et al. [64], Al-Ghouti and Al-Absi [65], and Pandey et al. [60]. Electrostatic interaction and hydrogen bonding are suggested as the key biosorption mechanism of MB dye at acidic and basic pH [65].
3.3 Effect of biochar dosage on the biosorption of MB dye
An important factor that impacts the biosorption process, which assesses the biosorbent-sorbate system equilibrium, is the biosorbent dosage [54, 60]. The impact of biochar dosage on the sequestration of MB dye ions using SDO, SSD, and PSD biochars is reported in Fig. 7. The % of MB dye confiscated to SDO, SSD, and PSD biochars was observed to be directly comparative to the improved biochar dosage used and initial MB dye concentrations. These trends were ascribed to the surge in the accessible biosorption sites existing on the surface of each biochar. But elevated biochar dosage led to a saturation of the % of MB dye removal ascribed to particle aggregation which resulted in reduced biochar surface area available and improvement in the diffusion path length, thereby resulting in reduced removal. A comparable pattern was illustrated in the studies relating to MB dye removal to different biomaterials by [64, 66,67,68].
3.4 Impact of initial concentration on MB dye removal
The driving force offered by the dye concentration assists to stun the mass transfer resistance of molecules of dyes at the interfaces of a solid liquid [69, 70]. The impact of MB’s initial dye concentration was studied at various concentrations ranging from 20 to 120 mg/L using varying biosorbent dosages of 0.1–0.4 g/L at pH 12. It was observed in Fig. 8 that the % of MB dye ions confiscated onto the various biochars decreased with increasing MB dye concentrations (20–120 mg/L of SDO and SSD and 100–400 mg/L of PSD biochars) as contact time was improved from 0 to 180 min and 0 to 250 min. The optimum % of MB dye removed was obtained using MB dye concentrations of 20 and 100 mg/L. This decrease in the % of MB dye ions confiscated onto the biochars with increasing MB dye concentrations was attributed to the saturation of the accessible active sites on the surface of the biochars at elevated concentrations of MB dye [71].
3.5 Kinetic studies
The sorption kinetics is employed to study the change in sorption quantity over time that could offer a source for engineering process design and give an understanding of the mechanism of sorption [72]. To evaluate the kinetic data of MB dye adsorption by SDO, SSD, and PSD biochars under the experimental conditions examined, the pseudo-first-order (PFO), pseudo-second-order (PSO), film diffusion (FD), and intraparticle diffusion (ITD) models were employed. The linearized form of these models is given by Eqs. 4, 5, 6, 7.
qe, qt, t, K1, K2, F = qt/qe, kFD, Kdiff, and C indicate the equilibrium sorption capacity of the sorbent (mg g−1), and the sorption capacity (mg g−1) at time t, the contact time, the rate constants of the PFO and PSO models, fractional attainment of equilibrium, film diffusion rate coefficient (L/min), intraparticle diffusion rate constant (mg/gmin0.5), and the thickness of boundary layer [73,74,75].
The coefficient of regression (R2) closeness to unity (1) defines the extent the calculation model fits the data [76]. The linear plots of the PFO, PSO, FD, and INT models are given in Figs. 9, 10, and 11 and the determined parameters using these models are summarized in Tables 3, 4, 5, 6, 7, and 8. Comparing the R2 of the PFO and PSO models, it was found that the PSO model ideally demonstrates the sorption of MB to SDO, SSD, and PSD with R2 values which were > 0.9 (Tables 3, 5, and 7). The calculated qe (mg/g) values of SDO, SSD, and PSD biochars were very close to the obtained experimental values of qe (mg/g) using the PSO model, which validate the fitness of this model.
While Figs. 9, 10, and 11c and d show the linear plots of the FD and INT models. The determined parameters from these models are presented in Tables 4, 6, and 8. The plots of these models (INT and FD models) of the sorption process were not controlled by rate-determining steps and diffusion via liquid film surrounding the biochars, as their plots did not pass through the origin. The values of R2 of the FD and INT models were comparatively low and not above 0.9 for all values when compared to that of the PSO model [73, 77, 78]. Hence, the biosorption of MB dye to the various biochars was based on the assumption of a rate-limiting step of chemical sorption or chemisorption involving valency forces via sharing or electron exchange between the sorbate and the biosorbents [72, 79].
3.6 Isotherm studies
To evaluate the sorption mechanism, forecast the optimum sorption capacity of the biosorbents used, assess the relationship between the sorbate and the biosorbent, and improve the sorption system design, the sorption isotherm is employed [72]. In this study, linearized isotherm models were used to fit the sorption process, which includes Langmuir (LNR) and Freundlich (FRH) models. The linearized forms of these models are depicted in Eqs. 8–9. The linearized fitting of the curve of the confiscation MB dye to SDO, SSD, and PSD biochars is given in Fig. 12.
Ce (mg.L−1), Qm (mg.g−1), KL (L.mg−1), KF (mg.g−1) (L.mg−1)1/n, and 1/n (dimensionless; 0 < n < 10) represent the sorbate concentration in the solution at equilibrium, the determined saturated monolayer biosorption capacity of the sorbent under the given circumstances, and the LNR constant which relates to the affinity between the biosorbent and sorbate, FRH constant, and FRH intensity parameter, signifying the sorption driving force or surface heterogeneity magnitude [4, 60, 63].
Tables 3, 4, 5, 6, 7, and 8 depict the established parameters of isotherm models with their corresponding R2 relating to the most ideal model that explained the sorption of MB dye to SBO, SSB, and PSB models. It was noticed from the R2 values in Tables 9, 10, and 11 that the FRH model better described the sorption process of MB dye when compared to the LNR model using SBO (R2 = 0.957) and SSB (R2 = 0.986) biochars and the LNR model ideally describes the sorption process of MB dye with an R2 value of 0.999 employing PSB. The determined optimum sorption capacities from the LNR model were 200.0, 526.3, and 769.23 mg g−1. The values of n from the FRH model were greater than 1, suggesting that SDO and SSB biochars were suitable for MB dye confiscation from water-soluble solutions. High KF values using these biochars also showed that the various biochars could effortlessly sorb MB dye. The sorption capacities of the SDO, SSB, and PDB biochars were comparatively high when associated with other biosorbents employed for the confiscation of MB dye (Table 12). The sorption of MB dye to various biochars demonstrates monolayer sorption with uniform energy of a finite number of active sites and less sorbate transmigration in the plane surface using PSB biochar and multilayer layer sorption on a diverse surface with a diverse distribution of active sites and energies using SDO and SSB biochars. This was possibly due to their low cost, easy accessibility, greenest, and non-producing secondary contamination [76, 78, 80]. The adsorption mechanism of MB dye using the SDO, SSB, and PDB biochars may be understand via electrostatic attraction between cationic MB dye molecules and negatively charged biochar surface, a hydrogen bonding attraction force between MB dye molecules and biochar surface, and/or surface to pore diffusion of dye molecules [81].
4 Conclusion
The possibility of exploring and using new, cheap, easily accessible, biodegradable, and non-hazardous biosorbents prepared from SD agro-waste materials for the production/synthesis of SDO, PSD, and SSD biochars in the treating and removing of MB dye was considered and emphasized. The characterization of the synthesized/produced SDO, PSD, and SSD biochars was carried out using BET, SEM, FTIR, and TP. The biosorption of the MB dye using SDO, PSD, and SSD biochars was observed to be dependent on the solution pH, with optimum removal of MB observed at pH 12 for all (SDO, PSD, and SSD) biochars. Also, the biosorption process of MB to the SDO, PSD, and SSD biochars was ideally defined using the PSO, FRH-SDO, and SSD biochars and LNR-PSD biochar models. Based on the LNR model fitting to the experimental data, the optimum sorption capacities obtained using SDO, SSD, and SSD biochars were 200, 526, and 769 mg/g respectively. It was also observed that the electrostatic interaction and hydrogen bond played a critical role in the interaction mechanism between the various (SDO, PSD, and SSD) biochars and MB. In conclusion, this study has again shown that adsorbing reagents (biosorbents) prepared from cheap, easily accessible, biodegradable, and non-hazardous agro-waste materials such as SDO, PSD, and SSD biochars can effectively be useful for the removal of dyes such as MB and other industrial toxic wastes and effluents before their disposal into the environment. Therefore, it is suggested that further research on the use of cheap, easily accessible, biodegradable, and non-hazardous agro-waste materials for the control and mitigation of environmental effluences vis-à-vis industrial toxic wastes and effluent management, and research on the toxicity (toxicity assays) of synthesized biochars using at least cell lines or animal models should be given considerable attention.
a Determined pHPZC of SDO, PSD, and SSD biochars b impact of pH on MB sorption to biochars and c the speciation and molecular structure diagram (insert) of MB was adapted from [63]
Data availability
Data sharing does not apply to this article.
References
El Nemr A (2010) Impact, monitoring and management of environmental pollution in Pollution Science, Technology and Abatement Series. Nova Science Publishers, Inc. Hauppauge New York, USA, pp. 638
El Nemr A (2011) Environmental pollution and its relation to climate change in Pollution Science, Technology and Abatement Series. Nova Science Publishers, Inc. Hauppauge New York, USA, pp. 694
El-Nemr MA, Aigbe UO, Hassaan MA, Ukhurebor KE, Ragab S, Onyancha RB, Osibote OA, El Nemr A (2022) The use of biochar-NH2 produced from watermelon peels as a natural adsorbent for the removal of Cu(II) ion from water. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02327-1
Ukhurebor KE, Aigbe UO, Onyancha RB, Nwankwo W, Osibote OA, Paumo HK, Ama OM, Adetunji CO, Siloko IU (2021) Effect of hexavalent chromium on the environment and removal techniques: a review. J Environ Manag 280:111809
El Nemr A, Hassaan MA, Madkour FF, Idris AM, Said TO, Sahlabji T, Alghamdi MM, El-Zahhar AA (2021) Advanced oxidation of AY-11 dye effluent: detoxification and degradation mechanism. Toxin Rev 40(4):1472–1480
El Nemr A, Moneer AA, Khaled A, El Sikaily A (2012) Contamination and risk assessment of organochlorines in surface sediments of Egyptian Mediterranean coast. Egypt J Aquat Res 38:7–21
Salem DMS, Khaled A, El Nemr A (2013) Assessment of pesticides and polychlorinated biphenyls (PCBs) in sediments of the Egyptian Mediterranean Coast. Egypt J Aquat Res 39:141–152. https://doi.org/10.1016/j.ejar.2013.11.001
Salem DMSA, El Sikaily A, El Nemr A (2014) Organocholorines and their risk in marine shellfish collected from the Mediterranean coast. Egypt Egypt J Aquat Res 40:93–101. https://doi.org/10.1016/j.ejar.2014.03.004
Salem DMSA, Morsy FA-EM, El Nemr A, El-Sikaily A, Khaled A (2014) The monitoring and risk assessment of aliphatic and aromatic hydrocarbons (PAHs) in sediments of the Red Sea. Egypt Egypt J Aquat Res 40:333–348. https://doi.org/10.1016/j.ejar.2014.11.003
Salem DMSA, Khaled A, El Nemr A, El-Sikaily A (2014) Comprehensive risk assessment of heavy metals in surface sediments along the Egyptian Red Sea coast. Egypt J Aquat Res 40:349–362. https://doi.org/10.1016/j.ejar.2014.11.004
Ragab S, El Sikaily A, El Nemr A (2016) Concentrations and sources of pesticides and PCBs in surficial sediments of the Red Sea Coast. Egypt Egypt J Aquat Res 42:365–374
El Nemr A, Khaled A, Moneer AA, El Sikaily A (2012) Risk probability due to heavy metals in bivalve from Egyptian Mediterranean coast. Egypt J Aquat Res 38:67–75. https://doi.org/10.1016/j.ejar.2012.11.001
Nwankwo W, Olayinka SA, Ukhurebor KE (2020) Green computing policies and regulations: a necessity? Inter J Scient Tech Res 9:4378–4383
Nwankwo W, Ukhurebor KE (2019) An x-ray of connectivity between climate change and particulate pollutions. J Adv Res Dyn Control Syst 11:3002–3011
Ukhurebor KE, Singh KRB, Nayak V, Eghonghon G UK (2021) Influence of SARS-CoV-2 pandemic: a review from the climate change perspective. Environ Sci: Process Impacts 23:1060–1078
Nwankwo W, Ukhurebor KE (2021) Nanoinformatics: opportunities and challenges in the development and delivery of healthcare products in developing countries. IOP Conf Ser: Earth Environ Sci 655:012018
Nwankwo W, Olayinka AS, Ukhurebor KE (2020) Nanoinformatics: why design of projects on nanomedicine development and clinical applications may fail? Proceedings of the 2020 International Conference in Mathematics, Computer Engineering and Computer Science (ICMCECS), Lagos, Nigeria, IEEE Xplore 1–7. https://doi.org/10.1109/ICMCECS47690.2020.246992
Kerry RG, Ukhurebor KE, Kumari S, Maurya GK, Patra S, Panigrahi B, Majhi S, Rout JR, Rodriguez-Torres MDP, Das G, Shin H-S, Patra JK (2021) A comprehensive review on the applications of nano-biosensor based approaches for non-communicable and communicable disease detection. Biomater Sci 9:3576–3602
Ukhurebor KE, Aidonojie PA (2021) The influence of climate change on food innovation technology: review on topical developments and legal framework. Agric Food Secur 10:1–14
Nwankwo W, Ukhurebor KE (2021) Big data analytics: a single window IoT-enabled climate variability system for all-year-round vegetable cultivation. IOP Conf Ser: Earth Environ Sci 655:012030
Siloko IU, Ukhurebor KE, Siloko EA, Enoyoze E, Bobadoye AO, Ishiekwene CC, Nwankwo W (2021) Effects of some meteorological variables on cassava production in Edo State, South-South, Nigeria via density estimation. Scient Afric 13:e00852
El Nemr A (2012) Textiles: types, uses and production methods in Materials Science and Technologies Series. Nova Science Publishers, Inc. Hauppauge New York. Hard cover, pp. 621. https://novapublishers.com/shop/textiles-types-uses-and-production-methods/
El Nemr A (2012) Non-Conventional textile waste water treatment in Pollution Science, Technology and Abatement Series. Nova Science Publishers, Inc. Hauppauge New York. pp. 267. https://www.amazon.com/Non-Conventional-Treatment-Pollution-Technology-Abatement/dp/1621000796
Kara HT, Anshebo ST, Sabir FK, Workineh GA (2021) Removal of methylene blue dye from wastewater using periodiated modified nanocellulose. Inter J Chem Eng 9965452:1–16
Hassaan MA, El Nemr A, Madkour FF (2017) Testing the advanced oxidation processes on the degradation of direct blue 86 dye in wastewater. Egypt J Aquat Res 43:11–19. https://doi.org/10.1016/j.ejar.2016.09.006
Hassaan MA, El Nemr A (2017) Madkour FF (2017) Advanced Oxidation Processes of Mordant Violet 40 Dye in Freshwater and Seawater. Egypt J Aquat Res 43:1–9. https://doi.org/10.1016/j.ejar.2016.09.004
Eldeeb TM, El Nemr A, Khedr MH, El-Dek SI (2021) Novel bio-nanocomposite for efficient copper removal. Egypt J Aquat Res 47:261–267. https://doi.org/10.1016/j.ejar.2021.07.002
Sudarni DHA, Aigbe UO, Ukhurebor KE, Onyancha RB, Kusuma HS, Darmokoesoemo H, Osibote OA, Balogun VA, Widyaningrum BA (2021) Malachite green removal by activated potassium hydroxide clove leaves agro-waste biosorbent: characterization, kinetics, isotherms and thermodynamics studies. Adsorpt Sci Tech 1145312:1–15
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS (2021) Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J Mater Res Tech 14:2751–2774
Ltaief S, Jabli M, Saber Ben Abdessalem SB (2021) Immobilization of copper oxide nanoparticles onto chitosan biopolymer: application to the oxidative degradation of Naphthol blue black. Carbohyd Polym 261:117908
Aigbe UO, Ukhurebor KE, Onyancha RB, Okundaye B, Pal K, Osibote OA, Esiekpe EL, Kusuma HS, Darmokoesoemo H (2022) A facile review on the sorption of heavy metals and dyes using bionanocomposites. Adsorpt Sci Technol 8030175:1–36
Eldeeb TM, Aigbe UO, Ukhurebor KE, Onyancha RB, El-Nemr MA, Hassaan MA, Osibote OA, Ragab S, Okundaye B, Balogun VA, El Nemr A (2022) Biosorption of acid brown 14 dye to mandarin-biochar-CO-TETA derived from mandarin peels. Biomass Convers Biorefin 2022:1–21
Hassaan MA, El Nemr A (2020) Pesticides pollution: classifications, human health impact, extraction and treatment techniques. Egypt J Aquat Res 46:207–220. https://doi.org/10.1016/j.ejar.2020.08.007
Anwer H, Mahmood A, Lee J, Kim K, Park J, Yip A (2019) Photocatalysts for degradation of dyes in industrial effluents: opportunities and challenges. Nano Res 12:955–972
Ali I, Peng C, Ye T, Naz I (2018) Sorption of cationic malachite green dye on phytogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid. RSC Adv 8:8878–8897
El-Nemr MA, Abdelmonem NM, Ismail IMA, Ragab S, El Nemr A (2020) The efficient removal of the hazardous azo dye acid orange 7 from water using modified biochar from Pea peels. Desal Water Treat 203:327–355
El-Nemr MA, Abdelmonem NM, Ismail IMA, Ragab S, El Nemr A (2020) Removal of acid yellow 11 dye using novel modified biochar derived from Watermelon Peels. Desal Water Treat 203:403–431
El Nemr A, Shoaib AG, El Sikaily A, Mohamed AEDA, Hassan AF (2021) Evaluation of cationic methylene blue dye removal by high surface area mesoporous activated carbon derived from Ulva Lactuca. Environ Process 8(1):311–332
Kuang Y, Zhang X, Zhou S (2020) Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 12(2):587
Hasanzadeh M, Jorfi S, Ahmadi M, Jaafarzadeh N (2020) Hybrid sono-photocatalytic degradation of acid brown 14 using persulphate and ZnO nanoparticles: Feasibility and kinetic study. Inter J Environ Analyt Chem 1–14. https://doi.org/10.1080/03067319.2020.1790543
Soury R, Jabli M, Latif S, Alenezi KM, El Oudi M, Abdulaziz F, Teka S, El Moll H, Haque A (2022) Synthesis and characterization of a new meso-tetrakis (2,4,6-trimethylphenyl) porphyrinto) zinc(II) supported sodium alginate gel beads for improved adsorption of methylene blue dye. Int J Biol Macromol 202:161–176
Ammar C, El-Ghoul JM (2021) Characterization and valuable use of Calotropis gigantea seedpods as a biosorbent of methylene blue. Int J Phytorem 23:1085–1094
Ba OS, Ka H, Shoe T, Ya H, Yo N (2020) Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Scient Rep 7824:1–10
Ibrahima E, Moustafab H, El-molla SA, Halim SD, Ibrahim SS (2021) Integrated experimental and theoretical insights for malachite green dye adsorption from wastewater using low-cost adsorbent. Water Sci Technol 84(12):3833–3858. https://doi.org/10.2166/wst.2021.489
Pajaie SH, Archin S, Asadpour G (2018) Optimization of process parameters by response surface methodology for methylene blue removal using cellulose dusts. Civil Eng J 4:620
Alswat AA, Ahmad MB, Saleh TA (2016) Zeolite modified with copper oxide and iron oxide for lead and arsenic adsorption from aqueous solutions. J Water Supply: Res Tech-Aqua 65:465–479
Lin Q, Wang K, Gao M, Bai Y, Chen L, Ma H (2017) Effectively removal of cationic and anionic dyes by pH-sensitive amphoteric adsorbent derived from agricultural waste-wheat straw. J Taiwan Inst Chem Eng 76:65–72
Kara HT, Anshebo ST, Sabir FK (2021) Preparation and characterization of functionalized cellulose nanomaterials (CNMs) for Pb (II) ions removal from wastewater. J Chem 5514853:1–18
Ama O, Wilson A, Ray S (2018) Photoelectrochemical degradation of methylene blue dye under visible light irradiation using EG/Ag-ZrO2 nanocomposite electrodes. Inter J Electrochem Sci 14:9982–10001
Putro JN, Santoso SP, Soetaredjo FE, Ismadji S, Ju Y-H (2019) Nanocrystalline cellulose from waste paper: adsorbent for azo dyes removal. Environ Nanotech Monit Manag 12:100260
Tang Y, Lin T, Jiang C, Zhao Y, Ai S (2021) Renewable adsorbents from carboxylate-modified agro-forestry residues for efficient removal of methylene blue dye. J Phys Chem Solids 149:109811
Titchou FE, Akbour RA, Assabbane A, Hamdani M (2020) Removal of cationic dye from aqueous solution using Moroccan pozzolana as adsorbent: isotherms, kinetic studies, and application on real textile wastewater treatment. Groundw Sustain Dev 11:100405
Al-Ghamdi YO, Jabli M, Soury R, Ali Khan S (2021) Synthesis of copper oxide nanoparticles using Pergularia tomentosa leaves and decolorization studies. Int J Phytorem 24:118–130
Aigbe UO, Osibote OA (2021) Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: a review. Environ Nanotech Monitor Managem 100578. https://doi.org/10.1016/j.enmm.2021.100578
Duan C, Ma T, Wang J, Zhou Y (2020) Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. J Water Process Eng 37:101339
Gregg SJ, Sing KSW (1982) Adsorption Surface Area and Porosity, 2nd edn. Academic Press INC., London
Rouquerol F, Rouquerol J, Sing KSW (1999) Adsorption by powders and porous solids. Academic Press INC., London
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Amer Chem Soc 73:373–380
El Nemr A, El-Sikaily A, Khaled A (2010) Modeling of adsorption isotherms of methylene blue onto rice husk activated carbon. Egypt J Aquat Res 36:403–425
El Nemr A, Aboughaly RM, El Sikaily A, Masoud MS, Ramadan MS, Ragab S (2022) Microporous activated carbons with a high surface area of type I adsorption isotherm derived from sugarcane bagasse impregnated with zinc chloride. Carbon Lett 32:229–249. https://doi.org/10.1007/s42823-021-00270-1
Salem AMS, Harraz FA, El-Sheikh SM, Hafez HS, Ibrahima IA, Mottaleb MSAA (2015) Enhanced electrical and luminescent performance of porous silicon/MEH-PPV, nanohybrid synthesized by anodization and repeated spin coating. RSC Adv 5:99892–99898. https://doi.org/10.1039/C5RA18407J
El-Nemr MA, Ismail IMA, Abdelmonem NM, El Nemr A, Ragab S (2021) Amination of biochar derived from watermelon peel by triethylenetetramine and ammonium hydroxide for toxic chromium removal enhancement. Chinese J Chem Eng 36:199–222. S1004–9541(20)30464-X
Salazar-Rabago JJ, Leyva-Ramos R, Rivera-Utrilla J, Ocampo-Perez R, Cerino-Cordova FJ (2017) Biosorption mechanism of methylene blue from aqueous solution onto white pine (Pinus durangensis) sawdust: effect of operating conditions. Sustain Environ Res 27:32–40
Phuong DTM, Loc N, Miyanishi T (2019) Efficiency of dye adsorption by biochars produced from residues of two rice varieties, Japanese Koshihikari and Vietnamese IR50404. Desalin Water Treat 165:333–351
Al-Ghouti MA, Al-Absi RS (2020) Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Scient Rep 10:1–18
Pandey D, Daverey A, Dutta K, Yata VK, Arunachalam K (2021) Valorization of waste pine needle biomass into biosorbents for the removal of methylene blue dye from water: kinetics, equilibrium and thermodynamics study. Environ Technol Innovat 25:102200. https://doi.org/10.1016/j.eti.2021.102200
Mathew M, Desmond RD, Caxton M (2016) Removal of methylene blue from aqueous solutions using biochar prepared from Eichhorrnia crassipes (Water Hyacinth)-molasses composite: kinetic and equilibrium studies. Afric J Pure Appl Chem 10:63–72
Singh R, Singh TS, Odiyo JO, Smith JA, Edokpayi JN (2020) Evaluation of methylene blue sorption onto low-cost biosorbents: equilibrium, kinetics, and thermodynamics. J Chem 8318049. https://doi.org/10.1155/2020/8318049
Amin MT, Alazba AA, Shafiq M (2017) Effective adsorption of methylene blue dye using activated carbon developed from the rosemary plant: isotherms and kinetic studies. Desal Water Treat 74:336–345
Aigbe UO, Ukhurebor KE, Onyancha RB, Ama OM, Okundaye B, Esiekpe EL, Osibote OA, Kusuma HS, Osifo PO (2021) Utility of bionanocomposites for wastewater treatment. Bionanomaterials for Environmental and Agricultural Applications, by Singh, Ravindra Pratap; Singh, Kshitij RB. Bristol, UK: IOP Publishing pp. 8-1-8-25. https://doi.org/10.1088/978-0-7503-3863-9ch8
Khaleghi H, Esmaeili H, Jaafarzadeh N, Ramavandi B (2022) Date seed activated carbon decorated with CaO and Fe3O4 nanoparticles as a reusable sorbent for removal of formaldehyde. Korean J Chem Eng 39:146–160
Liu L, Fan S, Li Y (2018) Removal behavior of methylene blue from aqueous solution by tea waste: kinetics, isotherms and mechanism. Inter J Environ Res Public Health 15:1321
Salam MA, El-Shishtawy RM, Obaid AY (2014) Synthesis of magnetic multi-walled carbon nanotubes/magnetite/chitin magnetic nanocomposite for the removal of Rose Bengal from real and model solution. J Indust Eng Chem 20:3559–3567
Guarin JR, Moreno-Pirajan JC, Giraldo L (2018) Kinetic study of the bioadsorption of methylene blue on the surface of the biomass obtained from the algae D. antarctica. J Chem. pp. 12. https://doi.org/10.1155/2018/2124845
Chakraborty TK, Islam MS, Zaman S, Kabir AHME, Ghosh GC (2020) Jute (Corchorus olitorius) stick charcoal as a low-cost adsorbent for the removal of methylene blue dye from aqueous solution. SN Appl Sci 2:1–10
Zein R, Hevira L, Fauzia S, Ighalo JO (2022) The improvement of indigo carmine dye adsorption by Terminalia catappa shell modified with broiler egg white. Biomass Conversd Bioref1-18. https://doi.org/10.1007/s13399-021-02290-3
Tarawou T, Wankasi D, Jnr MH (2012) Equilibrium sorption studies of basic blue-9 dye from aqueous medium using activated carbon produced from water hyacinth (Eichornia Crassipes). J Nepal Chem Soc 29:67–74
Kavci E, Erkmen J, Bingöl MS (2021) Removal of methylene blue dye from aqueous solution using citric acid modified apricot stone. Chem Eng Communicat 1-16. https://doi.org/10.1080/00986445.2021.2009812
Sun L, Wan S, Luo W (2013) Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: characterization, equilibrium, and kinetic studies. Bioresour Technol 140:406–413
Yuan N, Cai H, Liu T, Huang Q, Zhang X (2019) Adsorptive removal of methylene blue from aqueous solution using coal fly ash-derived mesoporous silica material. Adsorpt Sci Technol 37:333–348
Oraon A, Prajapati AK, Ram M, Saxena VK, Dutta S, Gupta AK (2022) Synthesis, characterization, and application of microporous biochar prepared from Pterospermum acerifolium plant fruit shell waste for methylene blue dye adsorption: the role of surface modification by SDS surfactant. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02320-8
El-Sikaily A, Khaled A, El Nemr A, Abdelwahab O (2006) Removal of methylene blue from aqueous solution by marine green alga Ulva lactuca. Chem Ecol 22:149–157
Mbarki F, Kesraoui A, Seffen M, Ayrault P (2018) Kinetic, thermodynamic, and adsorption behavior of cationic and anionic dyes onto corn stigmata: nonlinear and stochastic analyses. Water Air Soil Poll 229:1–17
Amrhar O, Nassali H, Elyoubi MS (2015) Modeling of adsorption isotherms of methylene blue onto natural illitic clay: nonlinear regression analysis. Moroccan J Chem 3:582–593
Li D, Yan J, Liu Z (2016) Adsorption kinetic studies for removal of methylene blue using activated carbon prepared from sugar beet pulp. Int J Environ Sci Tech 13:1815–1822
Yilmaz P, Gunduz D, Ozbek B (2021) Utilization of low-cost bio-waste adsorbent for methylene blue dye removal from aqueous solutions and optimization of process variables by response surface methodology approach. Desal Water Treat 224:367–388
Nworie FS, Nwabue FI, Oti W, Mbam E, Nwali BU (2019) Removal of methylene blue from aqueous solution using activated rice husk biochar: adsorption isotherms, kinetics and error analysis. J Chilean Chem Soc 64:4365–4376
Üner O, Geçgel Ü, Bayrak Y (2016) Adsorption of methylene blue by an efficient activated carbon prepared from Citrullus lanatus rind: kinetic, isotherm, thermodynamic, and mechanism analysis. Water Air Soil Poll 227:1–15
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135:264–273
Boumediene M, Benaïssa H, George B, Molina S, Merlin A (2018) Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study. J Mater Environ Sci 9:1700–1711
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was partially funded by the Science and Technology Development Fund (STDF) of Egypt (Projects Nos. CB-4874 and CB-22816).
Author information
Authors and Affiliations
Contributions
Mr. T. M. Eldeeb, Eng. M. A. El-Nemr conducted the experimental part, calculation, and theoretical models. M. A. Hassaan conducted the calculation and characterization part, Dr. S. Ragab read the manuscript and supervised the experimental work. Dr. U. O. Aigbe, Dr. K. E. Ukhurebor, Dr. R. B. Onyancha, and Dr. O. A. Osibote wrote the original manuscript. Prof. A. El Nemr supervised the experimental work, completed the manuscript, and provided financial support for the experiment work.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The adsorption of MB dye by SDO, PSD, and SSD is best modelled by LNR and PSO.
• SDO, PSD, and SSD are effective and cheap biosorbents for the confiscation of MB dye.
• The impact on the sorption efficiency of SDO, PSD, and SSD is influenced by the pH.
• The Qm for MB dye onto SDO, SSD and PSD were 200, 526 and 769 mg/g, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldeeb, T.M., Aigbe, U.O., Ukhurebor, K.E. et al. Adsorption of methylene blue (MB) dye on ozone, purified and sonicated sawdust biochars. Biomass Conv. Bioref. 14, 9361–9383 (2024). https://doi.org/10.1007/s13399-022-03015-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03015-w