Abstract
The public health and environment are currently facing significant risks due to the discharge of industrial wastewater, which contains harmful heavy metals and other contaminants. Therefore, there is a pressing need for sustainable and innovative technologies to treat wastewater. The main objective of this research was to develop novel composites known as chitosan, Padina pavonica, Fe(III), and nano MgO incorporated onto pomegranate peel with the specific purpose of removing Cd (II) and Cu (II) ions from aqueous solutions. The characterization of these nanocomposites involved the utilization of several analytical methods, including Fourier-transform infrared spectroscopy, scanning electron microscopy, X-ray diffraction, thermal gravimetric analysis, and X-ray photoelectron spectroscopy. The efficiency of these nanocomposites was evaluated through batch mode experiments, investigating the impact of factors such as pH, initial concentration, contact time, and adsorbent dose on the adsorption of Cu(II) ions. The optimum conditions for the removal of ions were pH = 5 for Cu (II) and 6 for Cd (II), contact time: 120 min, adsorbent dosage: 0.2 g, initial metal ion concentration: 50 mg/L for each metal ion for the present study. The MgO@Pp demonstrated the highest removal efficiencies for Cu(II) and Cd(II) at 98.2% and 96.4%, respectively. In contrast, the CS@Fe-PA achieved removal efficiencies of 97.2% for Cu(II) and 89.2% for Cd(II). The modified MgO@Pp exhibited significantly higher total adsorption capacities for Cu(II) and Cd(II) at 333.3 and 200 mg/g, respectively, compared to CS@Fe-PA, which had capacities of 250 and 142 mg/g, respectively. The adsorption of Cd (II) and Cu (II) ions by MgO@Pp was found to be a spontaneous process. The R2 values obtained using the Freundlich and Redlich-Peterson models were the highest for the MgO@Pp composite, with values of 0.99, 0.988, 0.987, and 0.994, respectively, for Cu (II) and Cd (II). The pseudo-second-order equation was determined to be the best-fit kinetic model for this process. Reusability experiments confirmed that the adsorbents can be utilized for up to four regeneration cycles. Based on the findings of this study, MgO @ Pp is the most promising alternative and could be instrumental in developing strategies to address existing environmental pollution through adsorption.
Similar content being viewed by others
Introduction
The global water crisis is worsening, exacerbated by factors such as industrialization, climate change, and urbanization. These factors contribute to the production of hazardous materials and the increasing demand for water due to population growth and development1. Consequently, large amounts of wastewater are being released into the environment without proper reuse, leading to further contamination of our water resources2. One of the critical issues is the presence of heavy metal contaminants in wastewater, which poses a significant threat to human health. These pollutants can be found in industrial wastewater effluents and contaminated urban in the soil and can cause severe health issues when exposed to them3. In the long term, heavy metal toxicity can have a significant negative impact on both humans and the environment4.
To address this crisis, one promising strategy is the recycling of contaminated wastewater into clean water for agricultural and industrial purposes. Implementing this strategy can help reduce the misuse of natural water resources and prevent further environmental damage5. According to the World Health Organization (WHO), water scarcity is a looming global issue that could potentially affect up to 4 billion people by 20506. While heavy metals like cadmium are essential for life in small amounts, they can be poisonous if present in larger amounts in the environment. Humans are particularly susceptible to cadmium poisoning, and even small amounts of it can cause long-term health complications such as liver and lung damage, increased blood pressure, impaired renal function, birth defects, and cancer7. Cadmium is an especially hazardous heavy metal, and its essential limit for plants is between 5 and 30 µg per gram, while the usual content of cadmium in the soil is around one microgram per gram8. The human body can typically absorb only about 0.001 mg of cadmium per kilogram of body weight per day9. Furthermore, the accumulation of heavy metals in land-dwelling animals can diminish their competitiveness, viability, and population size, ultimately leading to ecological disturbances10. Copper is a heavy metal that is both essential and highly toxic in high doses. As a result, it is crucial to remove such metal ions from aquatic environments to protect biodiversity, ecosystems, and people11. A comprehensive strategy should be developed to reduce the risks of heavy metal contamination and ensure the safety and sustainability of our environment13. To achieve this, a variety of remediation methods have been developed. These include physical, chemical, and biological approaches. Physical remediation methods involve the use of granular activated carbon, membrane separation, and supercritical fluid extraction, while chemical remediation encompasses chemical precipitation13, Fenton oxidation14, electrocoagulation, and electrolysis15. These methods are effective in removing a broad spectrum of contaminants, but they are often costly, require a lot of energy and additional chemicals, and can even cause secondary pollution. Hence, it is imperative to devise effective and innovative technologies that can be combined with existing ones to efficiently and cost-effectively remove heavy metals from wastewater effluents16. In recent years, new nano adsorbents utilizing nanomaterials are being developed. Nanomaterials offer exceptional properties such as high specific surface area, minimal generation of flocculants, high adsorption capacity, rapid kinetics, and the ability to be regenerated and reused for multiple cycles17. Numerous studies have presented evidence supporting the practical application of nanotechnology in remediating metal pollution. This includes the use of molecularly imprinted polymers (MIP), nanocatalysts, bioactive nanoparticles, nanostructured catalytic membranes, nanosorbents, and carbon nanotubes for removing pollutants18. Chitosan is a natural biopolymer that is biocompatible, chemically stable, and biodegradable with nontoxic degradation products. It has shown great potential as an adsorbent for heavy metal ions and dyes in decontamination applications. The abundance of amino and hydroxyl groups in chitosan plays a crucial role in facilitating effective sorption processes19. Metal oxide nanoparticles, including magnesium oxide (MgO), have emerged as up-and-coming candidates for large-scale wastewater treatment in industrial settings. These nanoparticles possess advantageous characteristics such as cost-effectiveness, exceptional adsorption capacity, unique chemical, functional, and physical properties, small bandgap energy (2.4–2.8 eV), stable physicochemical properties, and strong photo corrosion stability in aqueous solution as well as stable recyclability potentials ease of separation, and improved stability. These properties enable them to remove heavy metals from wastewater efficiently20. The research on marine macro-algae exhibited the highest efficiency in removing heavy metal ions in comparison to other adsorbents studied21. The potential of pomegranate peel as an adsorbent for eliminating diverse pollutants from aqueous solutions has been extensively researched. Several studies have demonstrated the high adsorption capacity of pomegranate peel, which is primarily attributed to the presence of functional groups such as carboxyl, hydroxyl, and phenolic groups. These groups are capable of interacting with the adsorbate via electrostatic attraction, hydrogen bonding, and other mechanisms22,23.investigated the use of pomegranate peel as an adsorbent for the removal of lead and Cu2+ ions from aqueous solutions. The study found that pomegranate peel exhibited a high adsorption capacity for lead and copper ions, with a maximum removal efficiency of 90.2% and 80%, respectively. For example, Padina pavonica has shown a high uptake capacity of 2.74 mg/g for Cu2+ and 24.93 mg/g Pb(II) ions at pH 6.30, respectively24. The combination of chitosan and Padina pavonica as an adsorbent material has many advantages due to their low cost and eco-friendly nature, as well as their wide availability. Another research group17, found that the coating of chitosan on algae (BBC) enhanced the removal of Cr(VI) and Pb (II) from10 to 25.8% and 22.9 to 57.6%, respectively, compared to the unmodified algae. Innovatively combining synthetic nanoparticles, consisting of MgO with pomegranate peels and chitosan-coated Fe(III) Padina pavonica, holds great promise as an affordable and effective approach for eliminating heavy metals from wastewater. This could have a positive impact on safeguarding the environment and promoting human health. Prior research has established that effective loading of magnesium oxide onto pomegranate peels can increase the number of sorption sites and enhance the nanocomposite electrostatic attraction ability, anion exchange capacity, and precipitation. As a result, MgO- pomegranate peel composites have shown a high affinity for anionic contaminants in aqueous solutions, as confirmed by recent studies25. In this study, our objective was to create a multifunctional material by incorporating nanosized MgO into impregnated pomegranate peels and chitosan combined with Fe(III) Padina pavonica. This composite is aimed at serving as an effective adsorbent with high adsorption capacity and rapid metal ion removal in aqueous media. Additionally, the fabrication method we proposed is environmentally friendly, cost-effective, time-saving, and free from harmful chemicals. The nanosized MgO-impregnated pomegranate peels, chitosan, and Fe(III) Padina pavonica used in the composite are derived from natural sources, ensuring their safety for both humans and the environment. The synthesized adsorbent composites were thoroughly characterized using various techniques, including XRD, XPS, TEM, SEM/EDX, and FTIR. Suitable isotherm and kinetic models were employed to predict the adsorption process. The reusability and regenerative properties of the developed adsorbents were extensively investigated over multiple cycles.
Material and methods
Chemicals
Analytical grades of CuSO4.5H2O, CdSO4.H2O and MgCl2·6H2O were obtained from Merck, Egypt. Iron (III) chloride hexahydrate was purchased from Sigma-Aldrich Co., Ltd. Chitosan (80–100 mesh) 90% acetylation degree, glutaraldehyde solution and sodium hydroxide (NaOH) were ordered from Pioneers for Chemical Co., Ltd., Egypt.
Synthesis
Synthesis of MgO nanoparticles by plant extract
The synthesis of MgO nanoparticles involves a few steps that must be followed in order to produce the nanoparticles. The first step is to prepare a feedstock consisting of dried pomegranate peels at 100 °C, which should be ground in a ball mill and followed by sieving to achieve particle sizes of 100 mm. The second step involves the preparation of Mulberry leaf extract. The Mulberry leaves were washed thoroughly using distilled water to eliminate dust and dirt. The plant collection and use were in accordance with all the relevant guidelines. The mulberry leaves and pomegranate peel used in this study were purchased from the supermarket. Eleven grams of leaves were boiled in 120 mL of deionized water at 100 °C for 5 min. After that, the solution was allowed to cool, filtered, and stored at a temperature of 4 °C. In the third step, the Padina pavonica underwent activation by being treated with a solution of pure CaCl2 and H2O in a ratio of 2:4 (v/v) for 24 h. Subsequently, the Padina pavonica pulp was once again crushed and then subjected to lyophilization for 24 h to ensure complete drying of the Padina pavonica26. In the fourth step, mix 50 L of a 1 M solution of MgCl2·6H2O with 20 mL of Mulberry leaf extract. Slowly add 1 M NaOH drop by drop while continuously stirring the mixture using a magnetic stirrer for 2 h. Additionally, add 4 g of pomegranate peels to the flask. The mixture must then be stirred for 6 h at a temperature of 80 °C. After this, the solid–liquid dispersion must be centrifuged at 4000 rpm for 14 min in order to remove any excess MgCl2 6H2O and residual organic molecules. It is necessary to rinse the residue with deionized water and subsequently dry it in an oven at a temperature of 70 °C for 2 h. Ultimately, to acquire the magnesium oxide nanoparticles (MgONPs), it is essential to subject them to a calcination process in a furnace, which involves heating at 500 °C for 3 h. This process results in the formation of the desired MgONPs. The MgONPs should then be sonicated at 40 °C for 1 h in an ultrasonic cleaner (Daihan-Scientific) in order to disperse the particles27.
Manufacture of CS@Fe-PA sample collection and bleaching treatment
Padina pavonica (L.) is an essential type of marine macroalgae found in the coastal zone of the Red Sea in Egypt. During the spring season, samples of this alga were collected from the coastal zone of Mousa coast, Ras Sedr government, Egypt. These samples had flattened fan-shaped thalli up to 15 cm in size and were typically found growing on submerged rocks up to 50 cm in depth. The first step in the preparation of Padina pavonica involved washing the samples under running tap water to remove any surface debris. The marine macro-algae were then air-dried for about 30 min and oven-dried at 50 °C. The Padina pavonica was activated through treatment with a solution comprising pure CaCl2 and H2O at a ratio of 2:4 (v/v) for 24 h. The dried biomass was then crushed with a blender and sieved with a 100 μm sieve before being stored in an airtight container at room temperature. The second step involved the deposition of Fe(III) particles onto the Padina pavonica (L.) surface using a modified method21. Eight grams of the prepared alga were submerged in a 250 mL solution of FeCl3·6H2O with a 0.1 mol/L concentration for 120 min, stirring continuously.
After this, the Padina pavonica underwent a drying process in an oven set at 70 °C for 24 h. Subsequently, it was thoroughly rinsed multiple times with deionized water to eliminate any impurities before undergoing another round of oven-drying, rendering it ready for experimentation. Finally, a coating process was employed to synthesize a thin layer of chitosan on the surface of Padina pavonica. The process began by dissolving 3 grams of chitosan in 250 mL of 2.5% (v/v) acetic acid. Subsequently, 5 grams of the prepared Padina pavonica were introduced into the solution. The mixture was stirred continuously for 120 min. Next, 1.3% NaOH was added drop by drop to the homogeneous mixture until the pH level escalated to 9.5, which is the optimal condition for chitosan coating. The mixture was then allowed to sit at room temperature for 24 h, after which it was washed with DI water to eliminate any excess NaOH. The fourth step involved incorporating 200 mL of a 5% OHC(CH2)3CHO-solution and allowing the mixture to cure through cross-linking for 2.5 h. The C2H6O solution underwent a washing process, followed by filtration and subsequent drying in a desiccator to yield CS@Fe-PA beads28.
Preparation of Cu2+ and Cd2+ ions solutions
Initially, the appropriate quantities of CuSO4 5H2O and CdSO4 4H2O were dissolved in 1-liter volumetric flasks to prepare stock solutions with a concentration of 1000 mg/L for Cu2+ and Cd2+ ions.
Zero point of charge of nano composites
To ascertain the pHZPC values of MgO@Pp and CS@Fe-PA composites, a quantity of 0.2 grams of each sample was added to a 50 mL solution of 0.1 M NaCl, with an initial pH range spanning from 2 to 11. The mixture was vigorously shaken for 48 hours. Subsequently, the sample was separated from the solution, and the equilibrium pH values of the solution were measured. The discrepancy between the initial and equilibrium pH values was graphed against the initial pH of the solution. The point at which the graph intersected the x-axis was recognized as the pHZPC value, as described in the study by29.
Instrumentation
FTIR Spectrum (Model: Bruker-VERTEX 80 V) (400–4000 cm−1) was utilized to get FTIR spectra of adsorbents. The particle size of the nanoparticles was determined using a transmission electron microscope (model JEOL-JEM-2100 FS, Japan) operated at 200 kV. These adsorbents were imaged by a scanning electron microscope (SEM) (Model: Quanta 250) attached to an EDX unit (Energy Dispersive X-ray Analyses) with an accelerating voltage of 30K. XRD analysis was carried out using Bruker D8 with radiation Cu Kα (λ = 1.54 Å). The pH measurements were performed using a digital pH meter (Multi-9620IDS-pH-meter, WTW, Germany). Metal concentrations were determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) on the Perkin Elmer Sciex ELAN 9000. Thermo gravimetric and differential thermal analyses (TGA) were carried out using LABSYS evo TGA–DSC-analyzer units (Setaram- French). X-ray photoelectron spectroscopy (XPS) was performed on a K-ALPHA + XPS System (Thermo Fisher Scientific, U.SA.) with an X-ray Al- Kα 10 to 13,506 eV).
Adsorption studies
Several rounds of batch tests were carried out to evaluate the adsorption capacities of CS@Fe-PA and MgO-impregnated pomegranate peel composites for Cu2+ and Cd2+ ions. The experiments were carried out in 250 mL flasks, with each flask containing 100 mL of metal solution at varying concentrations (30–100 ppm) and adsorbent dosages (0.2–1.2 g). The mixtures were placed on a thermal shaker and agitated for 120 min at a speed of 210 rpm and a temperature of 25 °C. The initial concentration of Cu2+ and Cd2+ metals was optimized within the range of 30–100 ppm at a pH of 5, a temperature of 25 °C, and an adsorbent dosage of 0.2 g. Likewise, the impact of pH on the activity of the adsorbent was investigated by examining various pH levels (3.0, 5.0, and 6.0) while keeping the adsorptive concentration (50 ppm), contact time (120 min) and adsorbent dosage (0.2 g) constant. Additionally, the adsorbent dosage was investigated by conducting experiments at different dosage levels ranging from 0.2 to 1.2 g/L while maintaining a constant pH of 5 and adsorptive concentration of 50 ppm. Lastly, contact time optimization was explored by varying the contact time from 15 to 120 min while keeping the pH, adsorptive concentration, and adsorbent dosage constant at pH 5, 50 ppm, and 0. g, respectively. The removal efficiencies (RE%) of the adsorbents were calculated using the following equation:
Where A0 initial represents the initial concentration of Cu2+ and Cd2+ ions in the solution (mg/L) and Af final represents the residual concentration of Cu2+ and Cd2+ metal ions in the solution (mg/L).
The adsorption capacity (qe, mg/g) at equilibrium was determined using Eq. (2):
The concentrations of metal ions in the initial solution (Ci) and at equilibrium (Ce) are expressed in mg/L. The volume of the solution (V) is measured in liters, and the mass of the adsorbent (M) is measured in grams.
Adsorption kinetics
To investigate the kinetic adsorption process, experiments were performed on 50 ml flasks. A total of 0.2 g of CS@Fe-PA and MgO@Pp composites were added to a solution containing 50 ppm of Cu2+ and Cd2+. The flasks were placed in a shaker and agitated for various time intervals ranging from 15 min to 2 h. Following the agitation, the mixtures of Cu2+ and Cd2+with the sorbents were subjected to centrifugation at 4000 rpm for 10 min and subsequently filtered through a 0.45 μm filter. The concentration of total Cu2+ and Cd2+in the filtered solutions was then measured. The kinetics of Cu2+ and Cd2+ sorption onto the two sorbents were modeled using different approaches, including first-order30, pseudo- second order31, Intra-particle diffusion and Elovich16. In the equation, K1 represents the rate constant for pseudo-1st-order kinetics (min-1), while qe and qt denote the equilibrium and time-dependent amounts of adsorbed metal ions (mg/g), respectively. When the process deviates from linearity, it is referred to as the (PSO) model, which is characterized by the rate constant k2 (mg/g min)17.
Adsorption isotherms
The adsorption experiments were performed employing a batch system. In each experiment, 0.2 g of the respective adsorbents was mixed with Cu2+ and Cd2+solutions ranging from 30 to 100 mgL−1 in 200 mL flasks. The mixtures were agitated using a shaker for a predetermined equilibrium adsorption time of 2 h. After the agitation, the samples were subjected to centrifugation, filtration, and measurement of Cu2+ and Cd2+concentrations, following the same procedure described in the adsorption kinetics section. The obtained sorption data were analyzed using Langmuir, Freundlich32 and Dubinin-Radushkevich, Redlich–Peterson, and Temkin isotherm models and thermodynamic dynamics13.
Adsorbent reusability
The economic viability and reusability of CS@Fe-PA and MgO@Pp composites were assessed by regenerating the spent nanocomposite adsorbents and testing their effectiveness in practical applications. Typically, 1 g of CS@Fe-PA and MgO@Pp composites were subjected to shaking with 50 mg/L Cu2+ and Cd2+ solutions for 2 h using an orbital shaker. Once equilibrium was reached, the Cu2+ and Cd2+loaded CS@Fe-PA and MgO@Pp composites were dried and immersed in 50 mL of 0.1 M HCl, followed by shaking for 2 h at room temperature and drying. Following each treatment, the adsorbent material was separated from the Cu2+ and Cd2+solutions, rinsed with distilled water, and subsequently soaked in distilled water overnight. Following filtration, the solid residue underwent another cycle of agitation with a new 200 mL solution of Cu2+ and Cd2+ (50 mg/L). This procedure was repeated for up to 4 consecutive cycles, and the removal efficiency of Cu2+ and Cd2+was calculated after each cycle.
Results and discussion
Characterization analysis of nanocomposites
TEM and SEM analyses
A transmission electron microscope (TEM) was utilized to assess the size of the CS@Fe-PA and MgO@Pp composites. The synthesized CS@Fe-PA and MgO@Pp composites exhibited a maximum size of approximately 0.58 and 1.59 nm, respectively. TEM images clearly displayed the uniform distribution of MgO nanoparticles on the surface of the pomegranate peels. The graph from the TEM analysis indicated that the MgO nanoparticles were enveloped by a significant amount of pomegranate peels, resulting in a low percentage of Mg by weight in the MgO@Pp composite. Conversely, the TEM photograph revealed that the CS@Fe-PA particles predominantly consisted of aggregates of α-FeOOH (depicted as black areas) dispersed throughout the chitosan matrix, Fig. 1a,b. SEM and EDS analyses were conducted to compare the microstructure and surface morphology disparities between the CS@Fe-PA and MgO@Pp composites. The SEM images presented in Fig. 2a,c exhibited distinct morphological structures and varying particle sizes, confirming the successful cross-linking of chitosan with Fe(III) and the surface modification of algae with glutaraldehyde. The divergence in particle size and morphology resulted in variations in the physical and chemical properties of the nano-sorbents, which influenced. The physical and chemical characteristics of the nano-sorbents fluctuate due to variances in particle size and morphology, which have an impact on the sorption process and the availability of active sites for the adsorption of heavy metals. The SEM image shows a rough and porous surface with irregular shapes and particles of different sizes. The particles are densely packed, and there is no visible agglomeration, indicating a uniform distribution of MgO and Fe(III) in the composite. The surface characteristics and structure of the composite suggest that MgO has been well impregnated into the pomegranate peel, chitosan, and Padina pavonica have been well integrated into the Fe(III) particle matrix resulting in a highly porous and rough surface that can enhance the composite's adsorption capacity. The EDX spectra of CS@Fe-PA presented in Fig. 2b,c showed peaks corresponding to C, O, Cl, Mg, Al, Si, Ca, Fe, and S. These caused the loading of Fe (III) onto the surface of CS. C and O elements contribute 28.3% and 51.3%, respectively. This indicates a higher active carbon content in CS@Fe-PA, which can be attributed to the macro-algae cell wall, which possesses diverse functional groups such as phosphoryl, carboxyl, amino, hydroxyl, and sulfhydryl. This is further supported by the use of glutaraldehyde for cross-linking purposes. Meanwhile, the EDX analysis Fig. 2d revealed that the significant elements of the MgO@Pp composite were O, Mg, C, and Cl elements, contributing 59.6, 22.8, 6.9, and 9.12%, respectively. The more pronounced peaks for oxygen and carbon elements were attributed to the polyphenols, cellulose, and hemicellulose present in pomegranate peels. Additionally, the presence of Ca and Al elements originated from the pomegranate peel feedstock, as well as mulberry leaf extract. The presence of Mg confirmed the existence of MgO and indicated the successful incorporation of MgO into the pomegranate peel matrix. Moreover, the inclusion of base cations such as Ca and Mg could contribute to the adsorption of metal ions by the MgO@Pp composite, as suggested by previous studies32.
Fourier transforms infrared (FTIR) analysis
The FTIR spectrum of MgO@Pp composite exhibits several characteristic bands. Here's an interpretation of the prominent bands in the spectrum, 3697.3 cm−1, as shown in Fig. 3a: This broad and intense peak is attributed to the stretching vibration of O–H groups in the composite material, indicating the presence of hydroxyl groups. 3240.1 cm−1: This peak is also due to O–H stretching vibration but appears at a lower wavenumber than the previous peak33. It may arise from the presence of water molecules bound to the MgO surface or from aliphatic and phenolic hydroxyl groups in the pomegranate peels and extract plant 1445.1 cm−1: These peaks correspond to the bending vibrations of C–H bonds in the composite. They may arise from the methylene (–CH2–) groups in the pomegranate peels34.The deformation vibration of Mg–O–Mg, Si–O–Mg of Mg–O appeared at 1459 cm−1 and 410.1 cm−135, indicating that MgO was loaded on pomegranate peels successfully.1094.2 cm−1: This peak corresponds to the stretching vibration of Si–O bonds in the MgO particles, indicating the presence of the MgO component. 880.2 cm−1: This peak is attributed to the stretching vibration of C–O bonds in the composite, indicating the presence of carboxyl and ester groups in the pomegranate peels36. 690.6 cm−1, 642.9 cm−1, and 599.1 cm−1: These peaks correspond to the bending vibrations of C–H bonds in the aromatic ring of lignin or other aromatic compounds in the pomegranate peels. 444.1 cm−1 and 419.2 cm−1: These peaks correspond to the stretching vibration of Mg–O bonds in the MgO@Pp composite37. In contrast, the FTIR spectrum of CS@Fe-PA exhibits characteristic bands of the CS@Fe-PA shows distinctive bands that provide valuable information about its chemical composition and structure Fig. 3b. The bands observed at 3852.92, 3743.96, 3730.28, 3706.32, 3665.49, 3625.69, 3607.11, and 3384.11 cm−1 correspond to the stretching vibration of O–H and N–H bonds, indicating the presence of chitosan and water molecules in the composite structure. 2987.62 cm−1: This peak corresponds to the stretching vibration of C–H groups in the Padina pavonica component of the composite. 2901.28 cm−1: This peak corresponds to the stretching vibration of C–H groups in the chitosan component of the composite. 1637.02 cm−1: This peak corresponds to the amide I band in the chitosan component of the composite, indicating the presence of the C=O stretching vibration and N–H bending vibration38. 1551.95 cm-1: The observed peak corresponds to the amide II band in the chitosan component of the composite, suggesting the presence of N–H bending vibration and C–N stretching vibration39. 1408.31 cm−1: This peak corresponds to the stretching vibration of COO– groups in the chitosan component of the composite40. 1253.37 cm−1: This peak corresponds to the stretching vibration of C–O groups in the Padina pavonica component of the composite. 1150.04 cm−1, 1050.95 cm−1: These peaks correspond to the stretching vibration of Fe–OH bonds in the Fe(III) component of the composite. 1020.84 cm−1: This peak corresponds to the stretching vibration of C–O–C groups in the chitosan component of the composite.897.84 cm−1: This peak corresponds to the bending vibration of C–H groups in the Padina pavonica component of the composite. 801.48 cm−1, 701.48 cm−1: The observed peaks at 637.57 cm−1 in the FTIR spectrum correspond to the bending vibration of Fe–OH bonds present in the composite's Fe(III)-chitosan structure. This peak corresponds to the bending vibration of N–H groups in the chitosan component of the composite. 594.69 cm−1: This peak corresponds to the bending vibration of C–H groups in the biocarb component of the composite. 522.66–469.93 cm−1: The presence of the Fe–O–CHT group was validated by the characteristic peaks observed in the spectrum, specifically in the range of 522.66–469.93 cm−1. This provides evidence that the hydroxyl (OH) groups of chitosan actively participate in the complexation process with ferric ions, as indicated by18. This finding further supports the notion of a robust interaction between the loaded metal oxides and the amide groups within the chitosan matrix41.
Crystallographic characteristics
The X-ray diffraction (XRD) result obtained from CS@Fe-PA (shown in Fig. 4a) displays multiple diffraction peaks, which are located at 2θ values of 20.101° 24.068° 26.629° 29.508 (belong to chitosan),° 33.037° 35.550° 40.823° 49.343° 53.930° 62.293° 63.79 (belong to Fe(III) on chitosan). These peaks are associated with the crystal planes (110), (120), (130), (101), (200), (230), (240), and (640), respectively. These crystal planes are similar to those found in goethite minerals (ICDD PDF (No.69–221–1653). Based on this outcome, we can deduce that the primary form of Fe(III) found in the prepared beads is α-FeOOH42. The findings suggest that the NH2 and OH groups of CHT interact with Fe ions, supporting the favorable compatibility between the polymer matrix of CS@Fe-PA. Figure 4b shows the XRD pattern and the crystalline properties of the synthesized MgO@Pp composite. As the Fig 4b. demonstrates the emergence of five new diffraction peaks at 2θ approximately 37.677° 42.709° 62.042° 74.940° 76.289° 78.317 corresponds to the (111), (202),(222), (311), and (222) planes (JCPDS No. 96-101-1174), which reveals the successful decomposition of MgO crystallite onto pomegranate peels43,44. Diffraction peaks appeared at 2θ around 10.77° (1 1 0), 17.6° (0 0 2), 25.1° (1 2 0), 27.4° (1 0 3), 30.99° (200), and 35.9° (123) for pure pomegranate peels can be attributed to the reflection of carbon based on JCPDS file No. 96-101-1022, indicating the presence of amorphous carbon in pomegranate peels, as confirmed by18. However, in the MgO impregnated pomegranate peels composite, the intensity of diffraction peaks associated with amorphous carbon noticeably decreases due to the high loading of MgO onto the pomegranate peels. The broad peak observed in the spectrum can be ascribed to the existence of organic compounds derived from Pomegranate peels feedstock and Mulberries leaves extract, which play a role in coating and stabilizing the MgO within the Pomegranate peels matrix, as suggested by27. The peaks shown at angles 10.77 and 25.1 are related to carbon45.
Thermal gravimetric (TGA) analysis for characterization of nano composites
The thermograms of CS@Fe-PA and MgO@Pp composite adsorbents are represented in Fig. 5a,b. The thermal analysis of the CS@Fe-PA composite reveals three distinct stages of decomposition. The initial stage, occurring in the temperature range of 52-173 °C, exhibits a mass loss of 3.79%, corresponding to the release of hydrated water molecules. The second stage, with a weight loss of 27.83% within the temperature range of 186-350 °C, is attributed to the decomposition of the -NH2 and -CH2OH groups of chitosan41, the decline in wt% loss of CS@Fe-PA composite at temperatures 356–537 and 608–993 °C suggests significant thermal stability introduced in the CS@Fe-PA composite structure by chitosan46. In contrast, the thermal analysis curve of the MgO@Pp composite shows 40–227, 240–410, and 411–495 °C, with the percentage loss values corresponding to 6.6, 18.6, 8.376 and 3.68%, respectively. The first stage of thermal degradation primarily involves the desorption of water molecules, while the subsequent stages are associated with the partial decomposition of the MgO@Pp composite. The total weight loss percentage of this sorbent is measured at 28.88%, which is lower than that of the CS@Fe-PA composite (50.2%).
XPS analysis of nano composites
The surface chemical compositions of the CS@Fe-PA composite were assessed through XPS analysis, as depicted in Fig. 6a; the results indicated that the MgO@Pp composite consisted of C, N, O, Cu, and Cd elements, whereas the CS@Fe-PA composite contained C, N, O, Fe, and Si elements. The C1s spectrum of CS@Fe-PA Fig. 6b exhibited three peaks prior to adsorption. The first peak, at a binding energy of 284.77 eV, corresponded to C–C, C–H, and C–N bonds. The second peak, at 286.29 eV, was attributed to the C–O bond. The third peak, appearing at 287.36 eV, indicated the presence of C=O bonds in the amide group and C=N bonds 47. Upon comparing the C1s spectra after Cu2+ and Cd2+ adsorption, a decrease in the binding energy of the C–N, C–C, and C–H bonds (284.67 eV) and the binding energy of the C–O bond (286.26 eV), was observed, which may provide electronic energy for the Cu2+ and Cd2+ reduction43. Additionally, a slight increase in C=O and C=N in the bonds (287.86 eV) was noticed. The N1s spectrum (Fig. 6b–e) displayed two peaks at binding energies of 399.29 eV (N–H) and 401.58 eV (N–O)28. A noticeable change in chemical bonding was observed after the adsorption of Cu2+ and Cd2+ ions, evidenced by variations in the peak intensities of the N–H Cu, N–HCd bond and NH2 Cu2+, NH2 Cd2+ (398.36, 400.49 and 399.42 eV) and (400.13 and 399.08), respectively. The intensity of the N–O bond significantly increased, indicating chelation between the amino group and ferric ions due to the reduction in electron cloud density of N caused by electron shift in the outer layer, resulting in increased binding energy of N18,48,49. The high-resolution XPS spectra of Fe2p exhibited nine peaks at binding energies of 710.67, 725.26, 718.26, 728.72, 721.81, 732.86, 736.51, and 713.71 eV. The doublet at 710.67 and 725.26 eV corresponded to Fe(III) 2p3/2, while the peaks at 713.71 and 728.72 eV represented Fe(III) 2p1/250,51. The XPS analysis revealed the presence of a Fe(III) satellite peak at 718.26, 732.86, and 736.51 eV, confirming the presence of ferric ions near the surface of the CS@Fe-PA composite, as depicted in Fig. 6f48. The adsorption mechanism was elucidated by the shift in the position of the O1s peak before and after adsorption, as shown in Fig. 7a–c. Prior to adsorption, the O1s spectra of the CS@Fe-PA composite exhibited three peaks. The binding energy peaks at 532.59, 534.88, and 530.98 eV were assigned to C=O, C–O, and Fe–O, respectively. Following Cu2+ adsorption, the deconvoluted O1 s spectra of the CS@Fe-PA composite displayed two peaks decreased at 531.91 and 529.88 eV, corresponding to the groups C=O, Fe–OH and Fe–O, respectively. Following Cd2+ adsorption, the deconvoluted O1 s spectrum of the CS@Fe-PA composite also exhibited two peaks at 532.43 and 531.47 eV, attributed to the groups C=O and Fe–OH. Performing a split-peak fit to the Cu2p peak in the support material, seven refined peaks were observed at 932.76, 935, 940.72, 944.13, 952.37, 954.92, and 961.75 eV. The dominant peak at 932.59 eV and 954.92 corresponded to Cu2p and Cu2p1/2, respectively, indicating the presence of the divalent form Cu2p (OH), suggesting adsorption of Cu2+ on the surface through coordination bonds with the lone pair of electrons in the N atom of the moiety52. Regarding the support material, the Cd3d peak could be refined into two peaks at 405.41 eV and 412.14 eV. The peak at 405.9 eV indicated the electron binding energy of the divalent form Cd3/253, suggesting that Cd(II) formed coordination bonds with lone pairs of electrons in O or N in chitosan hydrogels. Cd2+ subsequently trapped these bonds. The new peak at 412.4 eV corresponded to the characteristic peak of metallic Cd3d3/2, indicating the formation of chelates between two or more active functional groups in Cd2+ and chitosan hydrogels54. The binding energy difference of approximately 7eV between the Cd3d and Cd3d(OH) peaks in the pure CS@Fe-PA composite further confirmed the +2 oxidation state of Cd25, as shown in Fig. 7d,e, and f. In the CS@Fe-PA composite, the Si2p spectra exhibited two SiOx signal peaks at 102.02 and 103.24 eV. However, after the adsorption of Cd2+ and Cu2+ ions, the interaction between Si and these metal ions caused a shift in the peak to higher binding energy, resulting in peaks located at 98.81, 102.02, 102.08, and 102.68 eV, as shown in Fig. 8a–c. The peak of S2p peak appeared after the adsorption of the Cd2+ ions36. This result showed that the composite films successfully adsorbed Cd2+ ion. The peak of S2p in Fig. 8d represents the two combination modes of Fe–S–O under 169.87 eV and 168.38 (–SO3–, –SO2–) functional groups55. This indicated that there was an ion exchange between the negative group (–SO3–) on the surface of the adsorbent and the Cd2+ ion. The XPS measurements of MgO@Pp before and after adsorbing Cu2+ and Cd2+. In the survey spectra of MgO@Pp shown in Fig. 9a,b, signals of Ca, Mg, and O elements were observed, and new signals of Cu2+ and Cd2+ elements appeared after adsorption, providing further evidence of effective Cu2+ and Cd2+ adsorption onto MgO@Pp. The high-resolution O1 s spectrum, depicted in Fig. 9c,d displayed two deconvoluted peaks at 531.48 eV and 532.52 eV. Which is assigned to O2– of MgO@Pp O or CuO and the absorbed water, respectively. After the adsorption of Cu2+, the two peaks shifted from 531.48 eV (O–H) and 532.51 eV (C–O–C) to 532.14 eV (O–H) and 531.5 eV (C–O–C), respectively56,57. The XPS results indicate that the surface of MgO@Pp presents massive hydroxyl groups, which are responsible for the Cu2+ and Cd2+ adsorption. Analyzing the C1s spectrum of MgO@Pp as shown in Fig. 9e,f, three fitting peaks were observed. The first peak at a binding energy of 284.75 eV was attributed to C–C, C–H, and C–N bonds. The second peak at 285.64 eV corresponded to the C=O bond in the amide group and the C=N bond47. The third peak at 289.30 eV indicated the M-CO3 bond58. Upon comparing the C1s spectra before and after adsorption, a slight increase in the binding energy of each chemical bond was observed, with values of 284.78 eV (C–N, C–C) and a slight decrease in C–H bond (285.4 eV for C–O). Notably, a significant increase in peak intensity was observed for the C=O/C=N bond, indicating the chelation between the amino group and the metal ion. Moreover, a considerable change in the intensity of the M-CO3 peak was observed after C1s adsorption, attributed to the adsorption interaction between the small particles of nanomaterials with a large specific surface area and active sites on the surface with metal ions. XPS spectrum of Cu2p, nine strong satellite peaks were observed at 934.82, 952.0, 940.37, 954.62, 962.76, 961.15, and 943.42 eV, indicating the presence of Cu2+ in the MgO@Pp composite59. The gap between Cu2p3/2 and Cu2p1/2 was approximately 29 eV, consistent with the CuO standard spectrum, as shown in Fig. 9g 60 The presence of Cu shakeup satellite peaks at 943.5 eV and 961.2 eV could be attributed to the Cu2O phase, which is in line with a similar finding reported by41. The XPS survey spectrum of Cd(II)- MgO@Pp in Fig10a,b shows peaks at ca. 531.34 eV for O1s and ca. 284.61 eV for C1s; two characteristic peaks of Cd(II) were observed in the Cd3d region at binding energies of 405.24 and 412.4 eV, corresponding to Cd3d5/2 and Cd3d3/2, respectively. The binding energy difference between the Cd3d5/2 and Cd3d3/2 peaks is approximately 7 eV for the MgO@Pp composite, further confirming the +2 oxidation state of Cd28. The C1s spectrum (Fig. 10b) of MgO@Pp exhibited three fitting peaks. The first component of binding energy at 284.75 eV was assigned to the C–C, C–H, and C–N bonds. The second component at 285.64 eV was attributed to the C–O bond. The last peak at 289.72 eV corresponded to the C=O bond. By comparing the spectra of C1s after adsorption, a slight decrease in the binding energy of each chemical bond, that is, 284.61 eV (C–N, C–C, and C–H), and 288.41 eV (O–C=O ) was observed. However, a considerable increase in the peak intensity was observed at the C=O bond because of the increase in C=O bonds, which indicated the chelation between the MgO@Pp and metal ion17.
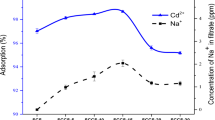
Effects of initial pH on Cu2+ and Cd2+ adsorption
The pH of the solution plays a crucial role in the removal of metal ions from water, as it impacts the surface charge of the adsorbent and consequently alters the adsorption capacity. In this study, the impact of pH on the adsorption efficiency of CS@Fe-PA and MgO@Pp composites for Cu2+ and Cd2+ ions was evaluated by preparing a solution containing Cu2+ and Cd2+ ions (50 mg/L) and adding 200 mg of nano-adsorbents, while adjusting the solution's pH within the range of 4–9. The removal percentages of metal ions were plotted against pH in Fig. 11a. The displayed result shows the maximum uptake for Cu2+ (98.3%) and (96.3%) at pH 5, and for Cd2+ (97.1%) and (89.2%) at pH6 for CS@Fe-PA and MgO impregnated pomegranate peels composites, respectively, were obtained. The pH of the zero point of charge (pHzpc) was found to be 7.5 and 7.1 for CS@Fe-PA and MgO@Pp composites, respectively. The surfaces of CS@Fe-PA and MgO@Pp composite sorbents are negatively charged when pH > pHzpc, which favors metal ions adsorption. The adsorption mechanisms observed on the adsorbents were primarily attributed to the ionic interactions between metal ions and the functional groups present in the nanocomposites61. At low acidic pH values, the adsorption efficiency was relatively poor due to the protonation of specific functional groups on the nanocomposite surfaces, leading to electrostatic repulsion between the protonated groups and the metal ions, thereby reducing metal ion adsorption on the CS@Fe-PA and MgO@Pp composites. A lower pH leads to an abundance of hydronium ions (H3O+) in the solution that causes competition between hydronium ions and Cu2+or Cd2+ ions (copper and cadmium ions are present as Cu2+, Cu(OH)+, Cd2+ and Cd(OH)+ at lower pH) for adsorption onto nanoparticles thereby lowering the overall adsorption efficiency of these metal ions at lower pH8.
On the contrary, the highest level of adsorption was observed at pH 6. This can be attributed to the deprotonation of functional groups, such as carboxylic acid, hydroxyls, and phenol, on the nanoparticle surface. This deprotonation results in the creation of harmful sites on the nanoparticles, leading to an electrostatic attraction between these negatively charged adsorption sites and the positively charged Cu2+ and Cd2+ ions. Consequently, this electrostatic interaction enhances the adsorption of these ions62. Similar findings have been reported in other studies examining the adsorption of Cu2+ onto various adsorbents. For instance, the synthetic Saccharomyces sp on the surface of CS/magnetic nanoparticles had an adsorption efficiency of 95.1% at pH 4.5563. In comparison, chitosan-modified magnetic kelp Padina pavonica had an efficiency of 90.4% at pH521. Zeolite-13X-immobilized Chlorella-Alginate showed maximum adsorption of Cu2+ (65.6%) at pH5, and magnetically activated carbon nanocomposite (MAC) had an efficiency of 90.2% for Cd2+ at pH664,65. However, compared to these studies, CS@Fe-PA and MgO-impregnated pomegranate peels performed better in the treatment of Cu2+ and Cd2+ in solution. It can be assumed that it was the presence of different functional groups on the surface of activated carbon that led to the increased binding of more ions, as suggested by49. Therefore, CS@Fe-PA and MgO impregnated pomegranate peels have a good potential to be utilized as effective adsorbents for the treatment of heavy metals.
Effects of adsorbent dosages on Cu2+ and Cd2+ adsorption
The adsorption of Cu2+ and Cd2+ ions from the aqueous solution is dependent on the adsorbent dosage. This study used a range of doses, from 0.2 to 1.2 g/L, to evaluate this impact. The dosage of the adsorbent determines the availability of potential active adsorption sites and, subsequently, impacts the uptake of metal ions. The adsorbent dosage was investigated to evaluate the adsorption capacity of CS@Fe-PA and MgO@Pp composites for the removal of Cu2+ and Cd2+ from aqueous solutions. The adsorption efficiency of Cu2+ and Cd2+ ions by the adsorbents at various dosages is presented in Fig. 11b. The adsorption capacities of both CS@Fe-PA and MgO@Pp composites showed a slight increase with rising dosage, as presented in Fig. 11b. At dosages of 0.2 g/L, the CS@Fe-PA composite exhibited removal efficiencies of 73.4% and 58.4% for Cu2+ and Cd2+, respectively. At 1.2 g/L, the efficiencies increased to 96.06% and 89.6%. The efficiency of MgO@Pp showed an increase in Cu2+ from 77.6% to 98.8% as the dosages increased from 0.2 to 1.2 g/L. In contrast, the removal efficiency for Cd2+ ions reached 69.4% at a dosage of 0.2 g/L and increased to 97.07% at a dosage of 1.2 g/L. The incorporation of iron into the adsorbent brings about changes in physicochemical interactions, including aggregation, ion exchange, redox reactions, and hydroxylation, resulting in the continuous precipitation of copper and Cd hydroxides43. The inclusion of Fe (III) in the composite improved the chitosan-supported padina povince required dosage per the quantity of Cu2+ and Cd2+ adsorptive. This improvement can be attributed to the creation of negatively charged surface complexes at pH five and the influence of Fe(III) speciation, which promotes the rate of Cd2+ adsorption from the test solution66. Furthermore, an increase in Cu2+ uptake was observed due to the augmentation of active pore spaces on the adsorbent surfaces. Figure 11b illustrates a sharp increase in the Cu2+ ion removal trend for both the MgO@Pp and CS@Fe-PA composites within the dosage range of 0.2–1.2 g/L. By comparing the adsorption percentages of the two composites, it is evident that the MgO@Pp composite exhibited higher adsorption rates even at lower quantities of the composite. The enhanced adsorption of the MgO-impregnated pomegranate peel composite can be attributed to its greater surface area and crystallite size when compared to the CS@Fe-PA composite. According to studies conducted by67, increasing the adsorbent dose provides more additional internal pore space, facilitating the adsorption of metals and resulting in the effective removal of Cu2+, Cd2+ and lead. Similarly7, reported an increase in the Cd2+ removal rate from 81.6 to 99.8% when the sorbent dose of chitosan impregnated with nanoparticles of zero-valent iron was increased from 0.2 to 0.7 g/L65. Found that the maximum removal efficiency uptake of Cd2+ ions by MAC was 65% at an adsorbent dosage of 0.2 g/L52. Reported that at a concentration of 0.5 g/L and a pH of 5, the removal efficiency rate of Cu2+ by Fe2O3 in microalgae was 78.88%51. Reported that at a concentration of 0.5 g/L and a pH of 5, the removal efficiency rate of Cu2+ by Fe2O3 in microalgae.
Influence of contact time on nano composites
In this study, the contact time between the adsorbent and adsorbate was optimized to determine the efficiency of CS@Fe-PA and MgO@Pp composite adsorbents in removing Cu2+ and Cd2+ ions from aqueous solutions. The contact time was varied within the range of 15 min to 120 minutes, and the results are presented in Fig. 11c. Initially, the adsorption rate increased rapidly within the first 15 minutes (shorter time), resulting in removal efficiencies of 78.04, 55.4, 66.8, and 50.3% for Cu2+ and Cd2+ in the MgO@Pp and CS@Fe-PA composites, respectively. Subsequently, the adsorption rate slowed down until equilibrium was achieved within 120 minutes (a longer time), leading to removal efficiencies of 98.2, 97.5, 96.7, and 90.09% for Cu2+ and Cd2+ in the MgO@Pp and CS@Fe-PA composites, respectively. After reaching equilibrium, the sorption system remained relatively constant with increasing contact time, indicating the saturation of biosorption. Therefore, a contact time of 120 min was deemed optimal for subsequent experiments. It was also observed that the adsorption changes in the initial stage were slightly higher in the MgO@Pp composite compared to the CS@Fe-PA composite (Cu2+ and Cd2+ per 15 minutes of contact time). This can be attributed to the larger surface area of the adsorbent and the availability of numerous vacant active adsorption sites for biosorption. The slower sorption rates observed in the later stages can be attributed to the rest of the sites being difficult to occupy due to repulsive forces between the solute molecules on the solid and bulk phases68. Comparable investigations have reported similar findings: a 68.6% adsorption of Cd2+ onto graphene oxide/iron(III) oxide nanocomposites within 20 min61, as well as a 57% adsorption of Cu2+ onto (FeNPs@HC) in the first 30 min27. Furthermore, Cu2+ exhibited faster adsorption and higher percentage removal compared to Cd2+. This difference may be attributed to variations in hydrolysis constants, ionic radius69 electrode potential, and solubility of these ions70. It is probable that Cu2+ ions diffuse more rapidly through the pores of MgO@Pp and are more readily adsorbed compared to Cd2+ ions, leading to the quicker and higher removal of Cu2+ compared to Cd2+ ions in this study. Similar findings have been reported in previous literature61 concerning Cd2+ adsorption on chitosan-graphene oxide-iron(III) oxide hydroxide, where a maximum adsorption efficiency of 68.7% for Cd2+ removal was achieved within 20 min.
Influence of initial concentration of Cu2+ and Cd2+ ions
The initial concentration of metal ions has a significant impact on the effectiveness of metal uptake. In this study, initial concentrations ranging from 30 to 100 mg/L were chosen to compare the removal of Cu2+ and Cd2+. The impact of the initial metal ion concentration on the adsorption activity of the MgO-impregnated pomegranate peels and CS@Fe-PA composites is depicted in Fig. 11d. As the concentration of metal ions increased, the removal efficiency of Cu2+ and Cd2+ by the adsorbents decreased. The CS@Fe-PA composite showed less sensitivity to increasing initial adsorbate concentrations compared to the MgO@Pp composite. The removal efficiency of Cu2+ and Cd2+ by the MgO@Pp composite decreased from 98.02 to 81.2% and 96.4 to 68.2%, respectively, when the initial concentrations were increased from 30 to 100 ppm. On the other hand, the CS@Fe-PA composite achieved maximum removal efficiencies of 97.2 to 73.4.9% for Cu2+ and 89.7 to 32.08 % for Cd2+ when the concentration increased from 30 to 100 ppm. At lower concentrations, specific sites on the adsorbents are utilized for metal adsorption. However, at higher metal concentrations, these sites may become saturated, and the exchange sites can be occupied. This phenomenon has been observed in previous studies71. The adsorbent's capacity to hold adsorbate ions remains unchanged at a fixed adsorbent quantity, but with increasing adsorbate concentration, the number of adsorbate ions to be accommodated increases. Consequently, adsorption becomes more rapid at higher initial adsorbate concentrations72. The findings are congruent with previously published studies of Cd2+ adsorption73 confirmed that the titanium-modified ultrasonic Padina pavonica removed 59% of cadmium from the aqueous solution. In comparison to the research of74, it was reported that the maximum removal efficiencies of Cd2+ ions by the corn cobs supporting nano-zero valent iron were 89.0% and 60.1%, respectively, ranging from 25 to 200 mg/L, while the maximum removal efficiencies of Cu2+ ions by the macro-algae adsorbent with (CS) and ferric oxide were 48–98%, ranging from 50 to 250 mg/L21,48. Found that the maximum removal efficiency of Cu2+ ions by the ZnO modified Padina pavonica was 61.02%. These findings are consistent with previous research on Cd2+ adsorption73. Demonstrated that titanium-modified ultrasonic Padina pavonica removed 59% of cadmium from aqueous solutions. Similarly74, reported maximum removal efficiencies of Cd2+ ions ranging from 89.0% to 60.1% using corn cobs supported by nano-zero valent iron at concentrations ranging from 27 to 200 mg/L. For Cu2+ ions21, achieved removal efficiencies of 48% to 98% using a macro-algae adsorbent with CS/Fe2O3 at concentrations ranging from 50 to 250 mg/L. Additionally75, reported a maximum removal efficiency of 61.02% for Cu2+ ions using ZnO-modified Padina pavonica.
Kinetic study
In order to study the adsorption kinetics mechanism of Cu2+ and Cd2+ions onto over-surface CS@Fe-PA and MgO@Pp composites. Four kinetic models were utilized to evaluate the adsorption kinetics, including the pseudo-first-order kinetic model (Eq. 3)41, pseudo-second-order kinetic model (PSO) Eq. (4) 12, Elovich model Eq. (5), and intraparticle diffusion Eq. (6)76. These models were used to assess the suitability of each model based on the goodness of fit of the straight line (R2) and the agreement between experimental and predicted values of qe. The Weber-Morris Eq. (6) is used to test whether the metal ion species can penetrate the interior sites of the adsorbent particles. The formula for the equation is as follows: Kp is the intraparticle diffusion rate constant, 't' denotes the agitation time in minutes, 'α' denotes the initial adsorption rate (mg/g min), and 'β' represents the desorption constant grams per milligram during any given experiment. The parameter C (mg/g) represents the influence of the boundary layer effect, while Ki (mg g−1 min−1/2) signifies the rate constant within the intra-particle diffusion model, as described by77. The results obtained from fitting data using the pseudo-1st-order, PSO, and Elovich models for CS@Fe-PA and MgO@Pp composites are presented in Table 1. For the pseudo-1st-order model using Eq. (3), values of K1 (0.032), qe (41.3), and R2 (0.892) were obtained for Cu2+, while K1 (0.025), qe (26.5), and R2 (0.874) were obtained for Cd2+ in the CS@Fe-PA composite. In contrast, for the MgO@Pp composite, K1 (0.018), qe (48.1), and R2 (0.926) were obtained for Cu2+, and K1 (0.054), qe (26.2), and R2 (0.844) were obtained for Cd2+, as depicted in Fig. S1a–d(supplementary materials). These values were calculated by analyzing the linear form of the pseudo-1st order kinetic model using Eq. (4). The PSO kinetic is based on mass transfer dynamics. Based on the nonlinear form of the pseudo-2nd-order kinetic model using Eq. (4) and the data presented in Table 1, Cu2+ and Cd2+ ions in CS@Fe-PA exhibited K2 values of 0.0032 and 0.022, qe values of 45.4 and 47.6 mg/g, and R2 values of 0.989, and 0.978, respectively. In comparison, Cu2+ and Cd2+ ions in MgO@Pp demonstrated K2 values of 0.0038 and 0.014 and qe values of 47.6 and 50 mg/g. The observed R2 values for Cu2+ and Cd2+ were 0.99, 0.990, 0.98, and 0.97, respectively, for CS@Fe-PA and MgO@Pp composites as depicted in Fig. S2a–d (supplementary materials).
According to the k2 values in Table 1, it was deduced that MgO@Pp adsorbed Cu2+ and Cd2+ faster than CS@Fe-PA. However, in terms of adsorption capacity, MgO@Pp exhibited higher values (qe = 47.6 and 50 mg/g, respectively), whereas the corresponding values for CS@Fe-PA were 45.5 and 47.6 mg/g, respectively. It was found that the highest initial adsorption rate (h) was achieved when utilizing MgO@Pp, as indicated in Table 1. These values indicate that the adsorption of metal ions in CS@Fe-PA and MgO@Pp composites followed the pseudo-2nd order kinetic model. Considering these findings, it can be deduced that the optimal percentage of MgO in the composite with pomegranate peels contributes to enhanced pollutant adsorption. Therefore, the MgO@Pp composite proves to be a superior material for the adsorption of metal ions.
Elovich model
The Elovich equation is a highly regarded model for analyzing activated chemisorptions. It is defined by equation (5). By plotting qe against ln(t), graphs can be generated to calculate α, β, and the correlation coefficient (R2) using Table 1. According to78, the chemical reaction of Cu2+ and Cd2+ with the adsorbent surface is the rate-controlling step of the Elovich model, and the removal process involves multilayer adsorption. The results reveal that the initial Cu2+ and Cd2+ adsorption rates onto CS@Fe-PA and MgO@Pp were studied. The Elovich values (α) of Cu2+ and Cd2+ were determined to be 0.69, 1.7, 0.48, and 0.44 mg min-1, respectively, for MgO@Pp and CS@Fe-PA composites. The values of Cu2+ and Cd2+ of β, which denotes the number of adsorption sites, were 3.6, 3.8, 3.4, and 3.5 for MgO@Pp and CS@Fe-PA, respectively. The adsorption of Cu2+ and Cd2+ onto MgO@Pp and CS@Fe-PA follows the Elovich equation because of the high R2 values. Fig. S3a–d (Supplementary Materials) and Table 1 indicate that the Elovich equation can describe the adsorption of Cu2+ and Cd2+ on MgO@Pp and CS@Fe-PA. These findings suggest the heterogeneity of adsorbent surfaces and support the prediction of a chemisorption process as indicated by the PSO model79.
Intra-particle diffusion model
The transfer of solutes in the solid-liquid adsorption process is commonly analyzed using an intra-particle diffusion model, as depicted in equation (6). By plotting qt against t1/2, the slope and intercept of the linear graph can be used to determine the Ki and C values from equation (6), as shown in Table 1. The Ri value is divided into four zones: weakly initial adsorption (zone 1) for 1 > Ri > 0.9, intermediately initial adsorption (zone 2) for 0.9 > Ri > 0.5, strongly initial adsorption (zone 3) for 0.5 > Ri > 0.1, and approaching completely initial adsorption (zone 4) for Ri < 0.1. Table 1 presents the collected and estimated intraparticle diffusion constants for the adsorption of Cu2+ and Cd2+ onto MgO@Pp and CS@Fe-PA composites. The first step exhibited excellent linearization (R2 = 0.99, 0.99, 0.97 and 0.98) with the first intraparticle rate constant (Kid1) of 0.297, 0.237, 0.374 and 0.05 mg/ (g/min^0.5), respectively, for indicating a rapid process. This initial step could be attributed to film diffusion, where Cu2+ and Cd2+ ions molecules diffuse from the solution onto the external surfaces of the MgO@Pp and CS@Fe-PA composites, possibly forming a hydrodynamic boundary layer. In contrast, the subsequent steps demonstrated a decline in the second intraparticle rate constant (Kid2) of 0.126, 0.106, 0.08 and 0.026 mg/(g/min^0.5) and the third intraparticle rate constant (Kid3) of 0.041, 0.033, 0.005 and 0.03 mg/(g/min^0.5), along with lower R2 values (R2 = 0.66,0.75,0.67 and 0.68) from the Webber–Morris equation. The initial sharper part corresponds to the fast diffusion of Cu2+ and Cd2+ ions from the solution bulk to the external surface of CS@Fe-PA and MgO@Pp composites, or the boundary layer. The second stage of the adsorption process encompasses film diffusion and intra-particle diffusion mechanisms, where solutes gradually migrate from the boundary layer to the surface of the adsorbent. Subsequently, they attach themselves to the active sites within the pores. The last linear segments represent the equilibrium period, where the intraparticle diffusion rates of cations (kint, 3) significantly decrease, and equilibrium is gradually attained80. The recorded C values for the adsorption of Cu2+ and Cd2+ ions on CS@Fe-PA were 32.3, 36.8, and 41.7 mg/g, and 46.4, 47.02, and 48.08 mg/g, respectively. Similarly, MgO@Pp exhibited values of 46.4, 47.02, and 48.08 mg/g for Cu2+ ions and 39.6, 41.6, and 44.6 mg/g for Cd2+ ions. These values provide insights into the thickness of the boundary layer. It is noted that the influence of the boundary layer becomes more significant as the intercept increases81. Both Ki and C values of the MgO@Pp composite were the highest, indicating that the diffusion rate in this composite was faster, and surface sorption contributed more than in the case of CS@Fe-PA18.The observed phenomenon can be attributed to the relatively smaller pore size of the MgO-impregnated pomegranate peels composite, allowing for easier penetration of the metal ion molecules. Moreover, the initial adsorption of metal ions over MgO@Pp and CS@Fe-PA composite adsorbents belonged to zone 3 with 0.5 > Ri > 0. 1, initial solid adsorption, and zone 4, Ri < 0.1, complete initial adsorption. These classifications were determined based on the calculated Ri values82.
Adsorption isotherms
The Langmuir and Freundlich model can be expressed in its linear form, along with the separation factor RL (Eqs. 7 and 9). The composite's adsorption capacity is denoted as qe, expressing the quantity of metal in milligrams per gram of dry adsorbent. Meanwhile, Ce, measured in milligrams per litre, represents the concentration of metal ions following the treatment. Qmax, expressed in milligrams per gram, corresponds to the Langmuir constant. KL, measured in liter per milligram, is the constant associated with the free energy or net enthalpy of adsorption. KF represents the Freundlich constant, which is measured in milligrams per gram and is related to the adsorption capacity. The Freundlich exponent, denoted as n and dimensionless, serves as an empirical parameter indicating the intensity of adsorption. For favorable adsorption, the value of n should be within the range of 1 < n < 10. Co represents the initial amount of adsorbate, measured in milligrams per liter. The RL parameter provides a more dependable indication of adsorption and can take on four potential values. By computing the slope and intercept from the Langmuir plot of Ce versus Ce/qe in Fig. S4a–d (supplementary materials), the Langmuir constants Qo and b were determined. The Langmuir models disclosed that the maximum adsorption capacities (Qm) of the MgO@Pp composite for Cu2+ and Cd2+ were 333.3 and 200 mg/g, respectively. In contrast, the Qm values for the CS@Fe-PA composite was 250 and 142.8 mg/g for Cu2+ and Cd2+, respectively. The Qm of the MgO@Pp composite to Cu2+ and Cd2+ was greater than that of the CS@Fe-PA composite. The Langmuir R2 values were very high, being almost 0.971 and 0.944 for Cu2+ and Cd2+ by the MgO@Pp as compared to the CS@Fe-PA composites (0.95 for Cu2+ and 0.92 for Cd2+). The RL values were calculated for the nanoparticles studied using Eq. (8), which takes into account b (L mg−1) and Ci (mg L−1). For the Cu2+ and Cd2+ ions in the present system, the RL values ranged from 0.1 to 0.09, and 0.4 to 0.3, respectively, for the MgO@Pp and CS@Fe-PA composites, indicating a favourable adsorption process Table 2. The linear form of the Freundlich adsorption model is represented by Eq. (9). The determination of the Freundlich constants Kf and n involved analyzing the slope and intercept of the plot depicting ln qe versus ln C e, as presented in Fig. S5(supplementary materials). The adsorption intensity (n) values were observed to be greater than 1, confirming the favorable adsorption of the two metal ions investigated using CS@Fe-PA and MgO@Pp composites, as demonstrated in Table 2. Furthermore, the values of Freundlich constants n for Cu2+ (1.6) and Cd2+ 2.4) for MgO@Pp, as well as constants n for Cu2+ (1.4) and Cd2+ (1.2), are greater than 1. The determination coefficient values (R2) obtained from the Freundlich model for Cu2+ and Cd2+ using the MgO@Pp composite were found to be 0.993 and 0.988, respectively. The determination coefficient values (R2) obtained from the Freundlich model for Cu2+ and Cd2+ using the CS@Fe-PA composite was found to be 0.97 and 0.95, respectively. These values were higher compared to the Langmuir model, indicating a better fit of the data to the Freundlich model and confirming the occurrence of multilayer adsorption. From the analysis shown in Fig. S5a–d(supplementary materials), it can be observed that the Freundlich model demonstrated a stronger correlation with the experimental data for both the MgO@Pp and CS@Fe-PA composites in comparison to the Langmuir model. The composites of MgO@Pp and CS@Fe-PA displayed a higher adsorption capacity compared to numerous other absorbents that have been documented in the literature. Temkin isotherm model is mathematically represented by Eq. (10)83. The Temkin plot Fig. S6a–d (supplementary materials) and Table 2 provided estimated values for Cu2+ and Cd2+ of the MgO@Pp composite, with AT = 2.3 L/g, B = 33.18 J/mol, and AT = 2.1 L/g, B = 36.06 J/mol, respectively. For the CS@Fe-PA composite, the estimated values for Cu2+ were AT = 2.06 L/g, B = 37.02 J/mol, while those for Cd2+ were AT = 2.02 L/g, B = 37.86 J/mol. These values suggest a physical adsorption process, as the Temkin isotherm model with a high correlation coefficient of 0.986 and 0.983 for Cu2+ and Cd2+ by MgO@Pp composite, respectively. The model was also applied to the CS@Fe-PA composite, resulting in correlation coefficients of 0.979 and 0.956 for Cu2+ and Cd2+, respectively. These results indicate that the Freundlich model is a better fit for the experimental data of Cu2+ and Cd2+ adsorption by CS@Fe-PA and MgO@Pp composites than the other two models.
Dubinin–Radushkevich isotherm
Equations (11, 12) present the non-linear version of the (D-Rh) isotherm84. The purpose of this approach was to differentiate between chemical and physical adsorptions of metal ions based on their mean free energy (E). According to Table 2, the sorption energy β for MgO@Pp was determined to be 0.003 (Cu2+) and 2.13 (Cd2+), while for CS@Fe-PA, β was reported to be 3.6 × 10–4 (Cu2+) and 6.3 × 10–4 (Cd2+). For the MgO@Pp composite, the E values for Cu2+ and Cd2+ were found to be 12.9 and 11.39, respectively, indicating that the sorption process follows an ion exchange mechanism. In contrast, for the CS@Fe-PA composite, the E values for Cu2+ and Cd2+ were 36.8 and 28.1, respectively. These positive E values suggest that the sorption process is chemisorption. Furthermore, it can be inferred that a higher solution temperature would promote the sorption process, as stated by85. The Redlich-Peterson model can be expressed by Eq. (13), as mentioned by45. Table 2 displays the heterogeneity (β) values for Cu2+ and Cd2+ ions, which fall within the range of 0.022 and 0.016 for MgO@Pp and 0.055 and 0.45 for CS@Fe-PA. Table 2 displays the αR (L/g) values for Cu2+ and Cd2+ ions, which fall within the range of 1.955 and 1.76 for MgO@Pp composite and 1.336 and 1.042 for CS@Fe-PA composite. The Redlich–Peterson isotherms showed the highest fitness to the adsorption data by generating the highest R2 values in the range of Cu2+ and Cd2+ ions 0.987–0.994 and 0.97–0.977, respectively, for MgO@Pp and CS@Fe-PA composites, as shown in Table 2.
Thermodynamic studies
Thermodynamic research is an essential tool for understanding the nature of adsorption processes. By analyzing the standard thermodynamic parameters, researchers can determine the nature of an adsorption process, distinguishing between physical and chemical adsorption. In recent studies, these parameters have been used to investigate the removal of Cu2+ and Cd2+ ions using chitosan @Fe PA and MgO@Pp composites. The assessment of thermodynamic characteristics such as changes in Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) associated with the adsorption process can be accomplished by employing both the rate Eq. (15) and the van't Hoff equation. The rate equation can be expressed as presented in previous works86,87, and T denotes the adsorption temperature in Kelvin (K). By plotting ΔG° against temperature, a linear correlation was observed. The slope and intercept of the plot enabled the calculation of ΔS° and ΔH° values. Additionally, plotting the natural logarithm of KL against 1/T yielded a straight line, with the slope and intercept corresponding to ΔH°/R and ΔS°/R, respectively. These values facilitated the computation of enthalpy and entropy changes. The equilibrium constant (K), absolute temperature (T), equilibrium concentration (Ce), and the amount of Cu2+ and Cd2+ adsorbed on the surface of the composites (qe) were also considered in the calculations, as per the equations presented in Eq. (16).The slopes and intercepts of the plot of ln Kc versus 1/T helped to determine the values of ΔH° and ΔS°, as shown in Fig. S6(supplementary materials). Further, ΔG° was calculated using the corresponding ΔH° and ΔS°. Table 3 provides the thermodynamic parameters for the adsorption of Cu2+ and Cd2+ on the CS@Fe-PA and MgO@Pp composites at different temperatures. The feasibility and spontaneity of the adsorption process are confirmed by the negative ΔG° values, which are further substantiated by the Langmuir separation factor RL and the Freundlich exponent n. The values of RL were between 0 and 1, and n was greater than 1. Raising the temperature caused a decline in the value of ΔG°, suggesting that an elevated temperature promotes the favorability of the adsorption process. The positive value of enthalpy confirms the endothermic nature of the interaction between the prepared MgO@Pp composite and the external boundary layer of Cu2+ and Cd2+. With an increase in temperature, the negative values of ΔG° became more negative, suggesting that the adsorption of Cu2+ and Cd2+on the MgO@Pp composite increased. On the other hand, positive values of ΔG° indicate that the adsorption of Cu2+ and Cd2+ on the CS@Fe-PA is a non-spontaneous process. For all the metals examined, the fact that the enthalpy value is negative indicates that the adsorption is an exothermic reaction. On the contrary, an increase in temperature results in increased mobility of the adsorbate molecules and a reduction in the solution's viscosity, making the subsequent stages of adsorption more favorable. The consistently observed negative enthalpy values confirm that the adsorption process for all the examined metals is indeed exothermic17.
Adsorption mechanism
The mechanism of CS@Fe-PA and MgO@Pp on heavy metals usually involves complexation, ion exchange, physical adsorption, precipitation, and the combined effects of these actions88. Adsorption mechanisms of Cd2+ and Cu2+ ions adsorption on the surface of CS@Fe-PA and MgO@Pp can be divided into two categories. The first category refers to physical adsorption (physisorption), in which a one-step reaction mechanism occurs through van der Waals force, which are interaction inversely proportional to the distance between atoms or molecules21. The ΔH values were < 40 kJmol1, proving that physical adsorption occurred during the adsorption of Cd2+ and Cu2+ ions onto CS@Fe-PA and MgO@Pp. The negative zeta potential of CS@Fe-PA and MgO@Pp in the pH range of 5–6 aids in the adsorption of positively charged heavy metal ions through electrostatic attraction. However, the effect of electrostatic attraction on the adsorption of Cd2+ and Cu2+ ions by CS@Fe-PA and MgO@Pp is relatively small21.The pseudo-second-order kinetics and Freundlich adsorption model for heterogeneous adsorbents exhibited excellent correlation with the Cd2+ and Cu2+ ions adsorption onto CS@Fe-PA and MgO@Pp, proving that the chemisorption process is mainly responsible for the adsorption of Cd2+ and Cu2+ ions onto CS@Fe-PA and MgO@Pp76. The ion-exchange process was attributed to the increase of equilibrium pH. In aqueous solution, the free Cd2+ and Cu2+ ions were hydrolyzed to produce H + (Eqs. (17) and (18)). During Cd2+ and Cu2+ ions adsorption process, more Mg2+ was released from the surface of MgO@Pp, and Cd2+ and Cu2+ ions were adsorbed on MgO@Pp through ion-exchange (Eqs. (19) and (20) (Fig. 12). Therefore, less H+ was produced from the hydrolysis. The considerable amount of MgO in the MgO@Pp consumed the existing H+ (Eq. (21). With the decreased H+ produce and the increased H + capture, the equilibrium pH was increased compared to the initial pH during Cd2+ and Cu2+ ions removal process.
The surface complexion depends on the electrostatic attraction between the surface functional groups on the CS@Fe-PA and MgO@Pp and the heavy metal ions in an aqueous solution. Positively or negatively charged groups on the surface, such as hydroxyl, carboxyl, ester, or amine groups, attract oppositely charged ions in the solution, forming the complexions through chemical bonding on the surface. When several different ions or molecules in the solution are adsorbed together on the surface, this process is called co-precipitation89. So, both surface complexion and co-precipitation are predominant in the case of CS@Fe-PA and MgO@Pp due to the addition of various functional groups on the surface of the CS@Fe-PA after the introduction of the chitosan layer. The role of surface complexation with these active groups in Cd2+ and Cu2+ ions sorption was evaluated by characterizing CS@Fe-PA and MgO@Pp with FTIR technique. XPS was also employed to understand the roles of functional groups in the Cd2+ and Cu2+ ions removal process. The binding energy and the changes of the surface chemical elements before and after adsorption are shown in Fig. 13a–c shows the XPS C1s spectra of CS@Fe-PA and MgO@Pp before and after Cd2+ and Cu2+ ions adsorption, where the spectra were deconvoluted into three peaks attributable to C–O, C=O, and O–C=O. It can be observed that C–O after adsorption changes significantly after CS@Fe-PA and MgO@Pp adsorbed Cd2+ and Cu2+ ions, and the binding energy of the corresponding characteristic peaks also shifts significantly. This may be related to the complexation of the Л-electron, which forms the Cd–Л bond between the Cd2+ and C=C double bond. It means that metal can form a σ bond with the s orbital of the C=C double bond, and the d orbital of metal can reversely contribute electrons to the Л orbital of the C=C double bond90. This result indicates that the C=C double bond may be vital to the adsorption of Cd2+. The C1s peak pattern after Cu2+ adsorption by CS@Fe-PA and MgO@Pp Fig. 13d–f differs from that before Cu2+ adsorption by CS@Fe-PA and MgO@Pp. The characteristic peaks corresponding to carbonyl, carboxyl, and hydroxyl groups change significantly, indicating that carbonyl, carboxyl, and hydroxyl are significant to Cu adsorption91.The XPS peaks of N1s elements in CS@Fe-PA and MgO@Pp after adsorption of Cd2+ and Cu2+ ions are in Fig. 13g–l. In addition, after CS@Fe-PA and MgO@Pp adsorbs Cd2+ and Cu2+, the characteristic peaks corresponding to these two N-containing functional groups also shift significantly. The obvious shift of these characteristic peaks may be due to complexes between N-containing functional groups and heavy metals. Studies have shown that when the covalent bond formed by N atoms and heavy metals obtains lone pair electrons from N atoms, the electron cloud density on N atoms decreases, thus changing the binding energy of the corresponding characteristic peaks92 and shifting them. Figure 13m–p show the XPS Cu2p and Cd3d spectra, respectively. The binding energies of Cu2p and Cd3d were observed after Cd2+ and Cu2+ ions adsorption, implying that CS@Fe-PA and MgO@Pp ions were successfully adsorbed on the CS@Fe-PA and MgO@Pp. The Cu2+/Cu–O were assigned to Cu2p, and Cd2+/Cd–O were assigned to Cd3d. At the same time, the Cu–O and Cd–O bonding contributed to the complexation with functional groups51, which was consistent with the results of the C1s spectra analysis. O1s spectra were deconvoluted into three peaks at 531.2, 532.1, and 533.3 eV are assigned to the lattice oxygen, bridging hydroxyl, terminal hydroxyl, and the surface adsorption of water, respectively attributable to Mg-O,-OH and C-O (Fig. S7) (supplementary materials). After the adsorption of CS@Fe-PA and MgO@Pp ions, the -OH and C-O groups decreased. This phenomenon is attributed to the ion- exchange between CS@Fe-PA and MgO@Pp and CS@Fe-PA and MgO@Pp ions93. To sum up, the mechanism for CS@Fe-PA and MgO@Pp ions adsorption by CS@Fe-PA and MgO@Pp, including precipitation, complexation, and ion-exchange, consequently the ion-exchange is the main mechanism during CS@Fe-PA and MgO@Pp ions adsorption process88.
Evaluating the adsorption capacity (qmax) of nano composites with diverse adsorbents
Table 4 presents a comprehensive comparison of the adsorption capabilities for Cu2+ and Cd2+ across various adsorbents documented in existing literature. The findings indicate that the utilization of CS@Fe-PA and MgO@Pp composites, as employed in this research, demonstrates significant potential as an efficient adsorbent for Cu2+ and Cd2+ ions. Notably, its performance surpasses that of several other adsorbents listed in the table above.
Reusability of the prepared adsorbent composites studies
Reusability is an indispensable indicator for evaluating the longevity of an adsorbent. In this study, a 0.2 mol/L HCl solution and deionized water were respectively used to regenerate CS@Fe-PA and MgO@Pp composites after adsorbing Cu2+ and Cd2+. The CS@Fe-PA and MgO@Pp composites were adsorbed, washed, and dried with deionized water multiple times to achieve the regeneration of CS@Fe-PA and MgO@Pp composites. Hydrochloric acid was chosen for eluting metal ions from adsorbents due to its widespread use in the industry, cost-effectiveness, and solubility of metal ions in it94,95. As depicted in Table 5, the removal efficiency dropped in subsequent cycles, reaching 97 to 92% for Cu2+ and 95.7% to 89.2% for Cd2+. In contrast, on CS@Fe-PA, the removal efficiency for Cu2+ and Cd2+ decreased from 90 to 81% and 87.2 to 68%, respectively, across the four regeneration cycles. Fig. 14a,b depicts that desorption efficiency decreased with each sorption-desorption cycle. The desorption efficiency of Cu2+ and Cd2+ was initially high for the first cycle, i.e., 94.6% and 90.9%, respectively. However, the performance of CS@Fe-PA declined, achieving desorption efficiencies of 87.3% for Cu2+ and 70.1% for Cd2+, which gradually decreased with subsequent cycles. The reduced efficiency of regenerated adsorbents may be attributed to the strong interaction between Cu2+ and Cd2+ and CS@Fe-PA and MgO@Pp composites. Water washing can only remove the adsorbates with low affinity to the surface of adsorbents, which are usually adsorbed by physical adsorption or weak chemical adsorption. Consequently, the number of active sites available on the surface of CS@Fe-PA and MgO@Pp composites decreased as the number of regenerations increased8.
Conclusions
The combination of different parent materials in the synthesis of composite adsorbents effectively merged their distinct properties, resulting in varying capabilities to remove Cu2+ and Cd2+ ions. XRD, SEM–EDX, and XPS characterization proved the formation of CS@Fe-PA and MgO@Pp composite materials. The effects of various experimental conditions (pH, adsorbent dosage, initial metal ion concentration and contact time) were examined. The SEM analysis confirmed the appropriateness of the material for the adsorption process, showcasing a rough surface and porous structure. The adsorption capacities of MgO@Pp showed much better sorption ability for Cu2+ and Cd2+ than the CS@Fe-PA. TGA demonstrated remarkable thermal stability of the synthesized MgO@Pp composite. The XPS analysis confirmed that the amino and hydroxyl groups chelated with metal ions. Thermodynamic parameters, including the change in free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°), were also evaluated. The findings indicated an endothermic removal of Cu2+ and Cd2+ ions by MgO@Pp, whereas CS@Fe-PA demonstrated an exothermic process. Desorption studies on metal-adsorbed MgO@Pp and CS@Fe-PA composites were done with 0.2 M HCl and distilled water. This study revealed that mineral acid HCl was sufficiently helpful for the regeneration of metal ions from adsorbents. These results demonstrate that low-cost CS@Fe-PA and MgO@Pp composite adsorbents can be fabricated in a facile and environmentally friendly way, which are considerable candidates for adsorption of Cu2+ and Cd2+ ions from aqueous solutions.
Data availability
All data generated or analyzed during this study are of our own work and it is our pleasure to be available on reasonable request. Connect with aurthor-mohamed_taha@nwrc.gov.eg.
References
Ayele, A., Haile, S., Alemu, D. & Kamaraj, M. Comparative utilization of dead and live fungal biomass for the removal of heavy metal: A concise review. 2021, (2021).
Elkhatib, E. A., Moharem, M. L., Saad, A. F. & Attia, F. A. Novel metal based nanocomposite for rapid and efficient removal of lead from contaminated wastewater sorption kinetics, thermodynamics and mechanisms. Sci. Rep. 12, 1–16 (2022).
Kalantari, K. et al. Rapid and high capacity adsorption of heavy metals by isotherms, and adsorption kinetics study. J. Taiwan Inst. Chem. Eng. 000, 1–7 (2014).
Alothman, Z. A. et al. Low cost biosorbents from fungi for heavy metals removal from wastewater. Sep. Sci. Technol. 0, 1–10 (2019).
Elgamal, A. M., El-ghany, N. A. A. & Saad, G. R. International Journal of Biological Macromolecules Synthesis and characterization of hydrogel-based magnetite nanocomposite adsorbents for the potential removal of Acid Orange 10 dye and Cr ( VI ) ions from aqueous solution. 227, 27–44 (2023).
Wang, Y. et al. Cadmium tolerance and accumulation hydroponic conditions †. RSC Adv. 8, 33383–33390 (2018).
Ahmadi, M., Foladivanda, M., Jafarzadeh, N., Ramavandi, B. & Kakavandi, B. Uncorrected Proof Uncorrected Proof. 1–15 (2017) doi:https://doi.org/10.2166/aqua.2017.027.
Jain, M. et al. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 20, 54–74 (2018).
Ba, B. et al. Insights into methyl orange adsorption behavior on a cadmium zeolitic-imidazolate framework Cd-ZIF-8: A joint experimental and theoretical study. Arab. J. Chem. 14, 102897 (2021).
Qu, J. et al. Iron/manganese binary metal oxide-biochar nano-composites with high adsorption capacities of Cd 2+: Preparation and adsorption mechanisms. J. Water Process Eng. 51, 103332 (2023).
Soleimani, M. & Siahpoosh, Z. H. Ghezeljeh nanoclay as a new natural adsorbent for the removal of chemical engineering thermodynamics. CJCHE https://doi.org/10.1016/j.cjche.2015.08.024 (2015).
Zeng, G. et al. Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis aeruginosa. (2022).
Zhang, Y. & Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 81, 1130–1136 (2020).
Quynh, H. G. et al. Rapid removal of methylene blue by a heterogeneous photo-Fenton process using economical and simple-synthesized magnetite–zeolite composite. Environ. Technol. Innov. 31, 103155 (2023).
El-enein, S. A. Adsorption of Selected Metals Ions in Solution Using Nano-Bentonite Particles : Isotherms and Kinetics. 463–477 (2020).
Shahnaz, T., Sharma, V., Subbiah, S. & Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 36, 101283 (2020).
Song, H., Liang, L. & Yang, K. Ac Ce p T. Chemical Engineering Research and Design (Institution of Chemical Engineers, 2014). doi:https://doi.org/10.1016/j.cherd.2014.04.027.
Xu, Y. et al. Adsorption of cadmium (II) in wastewater by magnesium oxide modified biochar. Arab. J. Chem. 15, 104059 (2022).
Hosain, A. N. A., El, A., El, A., Mahmoud, M. E. & Amira, M. F. Journal of Environmental Chemical Engineering Surface modi fi cations of nanochitosan coated magnetic nanoparticles and their applications in Pb ( II ), Cu ( II ) and Cd ( II ) removal. J. Environ. Chem. Eng. 8, 104316 (2020).
Abuhatab, S., El-qanni, A., Al-qalaq, H., Hmoudah, M. & Al-zerei, W. Effective adsorptive removal of Zn 2 þ, Cu 2 þ, and Cr 3 þ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manage. 268, 110713 (2020).
Son, E. et al. adsorbent with chitosan and ferric oxide for simultaneous efficient. Bioresour. Technol. https://doi.org/10.1016/j.biortech.2018.03.077 (2018).
Salmani, M. H. et al. Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J. Environ. Heal. Sci. Eng. 19, 603–612 (2021).
El-Ashtoukhy, E. S. Z., Amin, N. K. & Abdelwahab, O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 223, 162–173 (2008).
Ozudogru, Y. pavonica BIOSORPTION OF Cu ( II ) AND Pb ( II ) IONS BY USING MARINE BROWN ALGAE Padina pavonica. (2017).
Ahmed, M. F. et al. Hybrid beads of zero valent iron oxide nanoparticles and chitosan for removal of arsenic in contaminated water. 1–15 (2021).
Sassi, W. et al. Pomegranate peel-derived biochar as ecofriendly adsorbent of aniline-based dyes removal from wastewater. Clean Technol. Environ. Policy https://doi.org/10.1007/s10098-023-02522-2 (2023).
Khairy, G. M. et al. Green synthesis of a novel eco-friendly hydrochar from Pomegranate peels loaded with iron nanoparticles for the removal of copper ions and methylene blue from aqueous solutions. J. Mol. Liq. 368, 120722 (2022).
Liu, Y., Shan, H., Pang, Y., Zhan, H. & Zeng, C. International Journal of Biological Macromolecules Iron modified chitosan/coconut shell activated carbon composite beads for Cr (VI ) removal from aqueous solution. Int. J. Biol. Macromol. 224, 156–169 (2023).
Afshin, S., Rashtbari, Y., Vosough, M. & Dargahi, A. Application of box–behnken design for optimizing parameters of hexavalent chromium removal from aqueous solutions using Fe 3 O 4 loaded on activated carbon prepared from alga: Kinetics and equilibrium study. J. Water Process Eng. 42, 102113 (2021).
Ivanets, A. I., Srivastava, V., Kitikova, N. V., Shashkova, I. L. & Sillanpää, M. Kinetic and thermodynamic studies of the Co(II) and Ni(II) ions removal from aqueous solutions by Ca-Mg phosphates. Chemosphere 171, 348–354 (2017).
Pooladi, A. & Bazargan-lari, R. Simultaneous removal of copper and zinc ions by Chitosan/hydroxyapatite/nano-Magnetite composite. J. Mater. Res. Technol. 9, 14841–14852 (2020).
Abdallah, Y. et al. The Green Synthesis of MgO Nano-Flowers Using Rosmarinus officinalis L . ( Rosemary ) and the Antibacterial Activities against Xanthomonas oryzae pv . oryzae. 2019, (2019).
Rahimi, R., Bathaee, H. & Rabbani, M. Degradation of rhodamine B using Cr-doped TiO2under visible light irradiation. 1138 (2019) https://doi.org/10.3390/ecsoc-16-01138.
Gottipati, R. & Mishra, S. Process optimization of adsorption of Cr(VI) on activated carbons prepared from plant precursors by a two-level full factorial design. Chem. Eng. J. 160, 99–107 (2010).
Kim, N., Thi, N., Chau, T. & Hung, P. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Devices https://doi.org/10.1016/j.jsamd.2020.01.009 (2020).
Munir, R. et al. Biosynthesis of Leucaena Leucocephala leaf mediated ZnO, CuO, MnO2, and MgO based nano-adsorbents for reactive golden yellow-145 (RY-145) and direct red-31 (DR-31) dye removal from textile wastewater to reuse in agricultural purpose. Sep. Purif. Technol. 306, 122527 (2023).
Tarekegn, M. M. & Balakrishnan, M. zerovalent iron, nanoclay and iron impregnated. 30109–30131 (2021) doi:https://doi.org/10.1039/d1ra03918k.
Benamer-Oudih, S. et al. Sorption behavior of chitosan nanoparticles for single and binary removal of cationic and anionic dyes from aqueous solution. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-023-27907-0 (2023).
Subedi, N., Lähde, A., Abu-Danso, E., Iqbal, J. & Bhatnagar, A. A comparative study of magnetic chitosan (Chi@Fe3O4) and graphene oxide modified magnetic chitosan (Chi@Fe3O4GO) nanocomposites for efficient removal of Cr(VI) from water. Int. J. Biol. Macromol. 137, 948–959 (2019).
Liang, H. et al. Porous MgO-modified biochar adsorbents fabricated by the activation of Mg(NO3)2 for phosphate removal: Synergistic enhancement of porosity and active sites. Chemosphere 324, 138320 (2023).
Alhammadi, S. et al. Performance of graphene–CdS hybrid nanocomposite thin film for applications in Cu ( In , Ga ) Se 2 solar cell and H 2 production. (2020) doi:https://doi.org/10.3390/nano10020245.
Yang, J. et al. Synthesis of montmorillonite-supported nano-zero-valent iron via green tea extract: Enhanced transport and application for hexavalent chromium removal from water and soil. J. Hazard. Mater. 419, 126461 (2021).
Tarekegn, M. M., Balakrishnan, R. M., Hiruy, A. M., Dekebo, A. H. & Maanyam, H. S. Nano-clay and iron impregnated clay nanocomposite for Cu2+ and Pb2+ ions removal from aqueous solutions. Air, Soil Water Res. 15, (2022).
Terangpi, P. & Chakraborty, S. Adsorption kinetics and equilibrium studies for removal of acid azo dyes by aniline formaldehyde condensate. Appl. Water Sci. 7, 3661–3671 (2017).
Ahmad, M., Usman, A. R. A., Rafique, M. I. & Al-Wabel, M. I. Engineered biochar composites with zeolite, silica, and nano-zerovalent iron for the efficient scavenging of chlortetracycline from aqueous solutions. Environ. Sci. Pollut. Res. 26, 15136–15152 (2019).
Kong, D., He, L., Li, H. & Song, Z. Colloids and Surfaces A : Physicochemical and Engineering Aspects Preparation and characterization of graphene oxide/chitosan composite aerogel with high adsorption performance for Cr ( VI ) by a new crosslinking route. 625, (2021).
Gorzalski, A. S., Donley, C. & Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Memb. Sci. 522, 31–44 (2017).
Jiang, C., Zhang, T., Li, S. & Yang, Z. A comparative study on Fe(III)-chitosan and Fe(III)-chitosan-CTAB composites for As(V) removal from water: preparation, characterization and reaction mechanism. Environ. Sci. Pollut. Res. 29, 77851–77863 (2022).
Niu, Y., Ying, D., Li, K., Wang, Y. & Jia, J. Adsorption of heavy-metal ions from aqueous solution onto chitosan-modified polyethylene terephthalate (PET). Res. Chem. Intermed. 43, 4213–4225 (2017).
Caetano, A. A., Batista, M., Moratta, T. F. & Terra, J. C. S. Catalysis Science & Technology Nanostructured iron oxides stabilized by chitosan: Using copper to enhance degradation by a combined mechanism †. (2020) doi:https://doi.org/10.1039/d0cy00473a.
Shen, L. et al. A high-efficiency Fe2O3@Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 33, 101026 (2020).
Badruddoza, A. Z. M., Shawon, Z. B. Z., Tay, W. J. D., Hidajat, K. & Uddin, M. S. Fe 3O 4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 91, 322–332 (2013).
Zhang, F. et al. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manage. 153, 68–73 (2015).
Sun, H., Xia, N., Liu, Z., Kong, F. & Wang, S. Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere 236, 124370 (2019).
Wen, K., Li, Y., Zhang, S., Zhang, X. & Han, R. Adsorption of congo red from solution by iron doped PVA-chitosan composite film. Desalin. Water Treat. 187, 378–389 (2020).
Halim, A. et al. Environmental nanotechnology, monitoring & management removal of toxic metal ions from wastewater using ZnO @ Chitosan core- shell nanocomposite. Environ. Nanotechnol. Monit. Manag. 9, 67–75 (2018).
Cui, S. et al. Preparation of magnetic MnFe2O4-Cellulose aerogel composite and its kinetics and thermodynamics of Cu(II) adsorption. Cellulose 25, 735–751 (2018).
Dou, D. Jo ur o. (2021).
Shi, Q., Zhang, H., Shahab, A., Zeng, H. & Zeng, H. Ecotoxicology and Environmental Safety Efficient performance of magnesium oxide loaded biochar for the significant removal of Pb 2 + and Cd 2 + from aqueous solution. 221, (2021).
Kim, A., Muthuchamy, N., Yoon, C., Joo, S. H. & Park, K. H. MOF-Derived Cu @ Cu 2 O Nanocatalyst for Oxygen Reduction Reaction and Cycloaddition Reaction. Nanometerials 8, 1–13. https://doi.org/10.3390/nano8030138 (2018).
Parastar, M., Sheshmani, S. & Shokrollahzadeh, S. International Journal of Biological Macromolecules Cross-linked chitosan into graphene oxide-iron ( III ) oxide hydroxide as nano-biosorbent for Pd ( II ) and Cd ( II ) removal. Int. J. Biol. Macromol. 166, 229–237 (2021).
Baig, U., Kashif, M. & Gondal, M. A. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surfaces A 584, 124031 (2020).
Peng, Q. et al. Biosorption of copper ( II ) by immobilizing Saccharomyces cerevisiae on the surface of chitosan-coated magnetic nanoparticles from aqueous solution. J. Hazard. Mater. 177, 676–682 (2010).
Amirebrahim, S., Moghaddam, E., Harun, R. & Noriznan, M. Kinetic and equilibrium modeling for the biosorption of metal ion by Zeolite 13X-Algal-Alginate Beads (ZABs ). J. Water Process Eng. 33, 101057 (2020).
Kang, A. J., Baghdadi, M. & Pardakhti, A. Removal of cadmium and lead from aqueous solutions by magnetic acid-treated activated carbon nanocomposite. 3994, (2015).
Palansooriya, K. N. et al. Fe(III) loaded chitosan-biochar composite fibers for the removal of phosphate from water. J. Hazard. Mater. 415, 125464 (2021).
Sulaiman, S. et al. Adsorptive removal of copper (II) ions from aqueous solution using a magnetite nano-adsorbent from mill scale waste: Synthesis, characterization, adsorption and kinetic modelling studies. Nanoscale Res. Lett. 16, (2021).
Alnasrawi, F. A. & Mohammed, A. A. Enhancement of Cd2+ removal on CuMgAl-layered double hydroxide/montmorillonite nanocomposite: Kinetic, isotherm, and thermodynamic studies. Arab. J. Chem. 16, 104471 (2023).
Stietiya, M. H. & Wang, J. J. Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J. Environ. Qual. 43, 498–506 (2014).
Yavuz, Ö., Altunkaynak, Y. & Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 37, 948–952 (2003).
Sari, A. A., Fitrianingsih, F. & Nurdin, M. Immobilization of activated carbon on alginate beads as decolorization agent and its characterization for black Liquor wastewater. (2020).
El-Baz, A., Hendy, I., Dohdoh, A. & Srour, M. Adsorption technique for pollutants removal; current new trends and future challenges–a review. Egypt. J. Eng. Sci. Technol. 32, 1–24 (2020).
Luo, M. et al. Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour. Technol. 284, 333–339 (2019).
Younes, A. A., Abdulhady, Y. A. M., Shahat, N. S. & Farida, M. S. Removal of cadmium ions from wastewaters using corn cobs supporting nano-zero valent iron. Sep. Sci. Technol. 00, 1–13 (2019).
Kaur, M. & Kaur, H. Synergistic effect of biochar impregnated with ZnO nano-flowers for effective removal of organic pollutants from wastewater. Appl. Surf. Sci. Adv. 12, 100339 (2022).
Qin, J. et al. Journal of Environmental Chemical Engineering Highly efficient Cd 2+ and Cu 2+ removal by MgO-modified tobermorite in aqueous solutions. J. Environ. Chem. Eng. 11, 109534 (2023).
Sham, A. Y. W. & Notley, S. M. Adsorption of organic dyes from aqueous solutions using surfactant exfoliated graphene. J. Environ. Chem. Eng. 6, 495–504 (2018).
Tran, H. N., You, S. J., Hosseini-Bandegharaei, A. & Chao, H. P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 120, 88–116 (2017).
López-Luna, J. et al. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 1, (2019).
Hasani, N. et al. Theoretical, equilibrium, kinetics and thermodynamic investigations of methylene blue adsorption onto lignite coal. Molecules 27, (2022).
Sinkhonde, D., Rimbarngaye, A. & Kone, B. A multi-image analysis suitable to characterise structural parameters of mineral and chemical admixtures for sustainable construction. Clean. Eng. Technol. 11, 100566 (2022).
Sun, Y. X., Wu, Q. Y., Hu, H. Y. & Tian, J. Effects of operating conditions on THMs and HAAs formation during wastewater chlorination. J. Hazard. Mater. 168, 1290–1295 (2009).
Ma, Y. et al. Adsorption characteristics and mechanism for K 2 Ti 4 O 9 whiskers removal of Pb ( II ), Cd ( II ), and Cu ( II ) cations in wastewater. J. Environ. Chem. Eng. 9, 106236 (2021).
Uogintė, I., Lujanienė, G. & Mažeika, K. Study of Cu (II), Co (II), Ni (II) and Pb (II) removal from aqueous solutions using magnetic Prussian blue nano-sorbent. J. Hazard. Mater. 369, 226–235 (2019).
Azzam, A. M. Removal of Pb, Cd Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. https://doi.org/10.1016/j.jece.2016.03.048 (2016).
Banerjee, S. & Sharma, Y. C. Equilibrium and kinetic studies for removal of malachite green from aqueous solution by a low cost activated carbon. J. Ind. Eng. Chem. 19, 1099–1105 (2013).
Muinde, V. M., Onyari, J. M., Wamalwa, B., Wabomba, J. & Nthumbi, R. M. Adsorption of malachite green from aqueous solutions onto rice husks: Kinetic and equilibrium studies. J. Environ. Protect. 8, 215–230. https://doi.org/10.4236/jep.2017.83017 (2017).
Bian, P., Liu, Y., Zheng, X. & Shen, W. Removal and mechanism of cadmium, lead and copper in water by functional modification of silkworm excrement biochar. Polymers 14, 2889 (2022).
Dakroury, G. A., Abo-Zahra, S. F. & Hassan, H. S. Utilization of olive pomace in nano MgO modification for sorption of Ni(II) and Cu(II) metal ions from aqueous solutions. Arab. J. Chem. 13, 6510–6522 (2020).
Smolyakov, B. S., Sagidullin, A. K. & Chikunov, A. S. Removal of Cd(II), Zn(II), and Cu(II) from aqueous solutions using humic-modified moss (Polytrichum Comm.). J. Environ. Chem. Eng. 5, 1015–1020 (2017).
Feng, Z., Chen, N., Liu, T. & Feng, C. KHCO3 activated biochar supporting MgO for Pb(II) and Cd(II) adsorption from water: Experimental study and DFT calculation analysis. J. Hazard. Mater. 426, 128059 (2022).
Xu, X., Schierz, A., Xu, N. & Cao, X. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 463, 55–60 (2016).
Shi, Q. et al. Efficient performance of magnesium oxide loaded biochar for the significant removal of Pb2+ and Cd2+ from aqueous solution. Ecotoxicol. Environ. Saf. 221, 112426 (2021).
Le, G. H. et al. One-step synthesis of super-absorbent nanocomposite hydrogel based on bentonite. Mater. Res. Express 10, 15001 (2023).
Modwi, A. et al. Efficient removal of Cd ( II ) from aquatic media by heteronanostructure MgO @ TiO 2 @ g-C 3 N 4. 2022, (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohammed TahaMoustafa: significantly contributed to the inception and design of the research. Additionally, Sabah Ibrahim was involved in the procurement, analysis, and interpretation of the data. They took the initiative in drafting the manuscript and made substantial revisions to it.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamad, M.T.M.H., Ibrahim, S. Effective fabrication and characterization of eco-friendly nano particles composite for adsorption Cd (II) and Cu (II) ions from aqueous solutions using modelling studies. Sci Rep 14, 11767 (2024). https://doi.org/10.1038/s41598-024-61050-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61050-1
- Springer Nature Limited