Abstract
Recently, multi-drug resistant (MDR) bacteria are responsible for a large number of infectious diseases that can be life-threatening. Globally, new approaches are targeted to solve this essential issue. This study aims to discover novel antibiotic alternatives by using the whole components of the biofilm layer as a macromolecule to synthesize silver nanoparticles (AgNPs) as a promising agent against MDR. In particular, the biosynthesized biofilm-AgNPs were characterized using UV-Vis spectroscopy, electron microscopes, Energy Dispersive X-ray (EDX), zeta sizer and potential while their effect on bacterial strains and normal cell lines was identified. Accordingly, biofilm-AgNPs have a lavender-colored solution, spherical shape, with a size range of 20–60 nm. Notably, they have inhibitory effects when used on various bacterial strains with concentrations ranging between 12.5 and 25 µg/mL. In addition, they have an effective synergistic effect when combined with phage ZCSE9 to inhibit and kill Salmonella enterica with a concentration of 3.1 µg/mL. In conclusion, this work presents a novel biosynthesis preparation of AgNPs using biofilm for antibacterial purposes to reduce the possible toxicity by reducing the MICs using phage ZCSE9.
Similar content being viewed by others
Introduction
In 1928, the discovery of penicillin showed its capability to prevent the growth of Staphylococcus bacteria1, and it showed a distinct effect as an antibacterial agent on other Gram-positive bacteria2. Henceforth, using penicillin and its derivatives could limit the number of infections to be the golden era for reducing microbial infections3. A resistance had risen from either mutation, misusing antibiotics, or hosting foreign DNA from the environment4,5,6. Subsequently, various bacterial species acquired resistance to the most common antibiotic and turned them into superbugs7. Currently, MDR bacterial infections are difficult to be cured and might be life-threatening8. Therefore, further research is needed to find alternatives to limit MDR infections, such as using enzymes, immunostimulants, NPs, and organic acids9,10,11. As a result, scientists are struggling to formulate new antibiotics produced from natural products, synthetic products, or a combination of both of them12,13,14,15. Despite using phages and probiotics as novel approaches, which are alternatives to antibiotics, they might have undesirable results, such as recurrence and resistance16,17.
NPs have distinctive physical and chemical properties because of their high area-to-volume ratio and small size surfaces18,19. Accordingly, these properties enabled them to be beneficial in numerous applications in biotechnology, including environmental remediation, medical imaging, and drug delivery20,21. Practically, AgNPs are widely used and studied, despite their toxicity, due to their potent antibacterial22, antimicrobial23, and antifungal properties24,25. Furthermore, investigations on green synthesis have emerged as a new approach in nanoscience with significant implications in the biopharmaceutical and food industries26,27. One of the green synthesis techniques involves the implementation of microorganisms, such as fungi, algae, and bacteria, to reduce metal ions into NPs28,29,30,31. These techniques have several advantages, including using natural reducing agents and the potential for synthesizing NPs with unique properties that are difficult to achieve using traditional chemical methods32.
One of the highly effective alternative approaches is using phage to treat MDR bacterial infections33,34,35. Phages are viruses that target only a specific bacterial species in the environment and cause bacterial cell lysis36. Hence, they gained an interest in various medical applications due to their high specificity without affecting normal microbiota37,38,39. For instance, topical skin infections of Cutibacterium acnes40 and acute respiratory infections41 are treated using phages. Despite its high specificity, bacteria can resist it after a specific duration42,43. Furthermore, phage resistance results from some mutations in the bacterial genetic material44,45, which leads to the development of new virulence factors46,47. Correspondingly, the resistance increases while increasing the dosage of phage in the treatment period48,49. Some studies found that non-immunocompromised patients produce antibodies after the first month of treatment that reduce the effectiveness of the phage therapy50,51. Therefore, there is an urge to find a new approach to limit the development of such resistance.
Biofilms are complex bacterial communities ensconced within an extracellular matrix to protect them against host immune responses and environmental stressors52. Unexpectedly, biofilm layer synthesis is cost-effective, readily available, the synthesis process is eco-friendly, and does not use toxic chemicals, which makes them more advantageous over traditional chemical methods53. Biomolecules of biofilm of Pseudomonas aeruginosa (P. aeruginosa), such as proteins, lipids, and enzymes, are often utilized as reducing agents, independently, in the biosynthesis process of various NPs54,55. On the contrary, biofilm has been used in various beneficial manners for humanity, for instance, in breaking down toxic oils present in seas and oceans56, protecting plants’ roots from harmful bacteria57, and producing energy in bio-electrochemical systems while treating the used water58,59. Consequently, the unique combination and distinct properties of the ingredients present in the biofilm of Pseudomonas aeruginosa (P. aeruginosa) may offer benefits and advantages over the previously used biomolecules.
In this study, the biofilm layer of P. aeruginosa is used in the biosynthesis process of AgNPs. The outcomes of this study provide promising findings, suggesting that the ingredients present in the biofilm can effectively synthesize NPs in an eco-friendly and sustainable manner. Furthermore, the study findings may inspire further research to determine the potential of the biofilm of P. aeruginosa and its constituent molecules for the biosynthesis of NPs.
Material and methods
Materials
The materials used in this work are Tryptic Soybean Broth (TSB; MERCK, Germany), Tryptic Soy Agar (TSA; MERCK, Germany), silver nitrate (AgNO3; Techno Pharmachem, India) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Aldrich, US) , Dulbecco's Modified Eagle Medium (DMEM; Serana, Europe), Human Skin Fibroblast cell line (HSF), Human breast cancer cell line (MCF-7), Human liver cancer cell line (HepG2), Fetal Bovine Serum (FBS; Serana, Europe) and acidic isopropanol (Thermo fisher, US). In addition, Specific bacterial strains were used in this study to illustrate the effect of the biofilm-AgNPs. Salmonella enterica subsp. enterica serotype Typhi (S. enterica) NCTC 160 was grown at Xylose Lysine Deoxycholate agar (XLD; Oxoid, England), Escherichia coli (E. coli) ACTT 8739 was grown at Eosin Methylene blue (EMB; Oxoide, England), P. aeruginosa ACTT 27853 was grown at Cetrimide (MERCK, Germany), Staphylococcus aureus (S. aureus) ACTT 25923 was grown at Mannitol Salt Agar (MSA; Oxoide, England) and Bacillus cereus ACTT 14579 was grown at MYP agar (MERCK, Germany). In addition, phage ZCSE9 was used for S. enterica with an initial titer of 2 × 109 PFU/mL.
Methods
Preparation of biofilm-AgNPs
Preparation of the biofilm of P. aeruginosa
Briefly, the preparation of the biofilm of P. aeruginosa was executed as previously described60 with minor modifications. P. aeruginosa OL375153 was incubated in a 50 mL TSB flask at 37°C for 48 h. Then, after removing the planktonic cells, the biofilm layer was washed thrice with sterile distilled water to obtain a ring-shaped biofilm layer. The obtained biofilm was subsequently utilized for synthesizing AgNPs.
Biosynthesis of biofilm-AgNPs
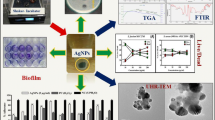
As for the AgNPs biosynthesis process, the biofilm layer of P. aeruginosa was used following the previously described procedures61 with minor modifications. Concisely, 50 mL of 1 mM AgNO3 was dissolved in sterilized deionized water and provided to the biofilm. After that, the solution was stirred continuously for approximately 4 h at room temperature. Finally, the solution was prepared for further characterization, as shown in Fig. 1.
Biofilm-AgNPs characterization
Various tests were used to identify the characteristics of the biosynthesized biofilm-AgNPs. Scanning Electron Microscope (SEM) figure was obtained as described before62 by using JEOL JSM-5300, SEM (Tokyo, Japan) instrument to identify the morphology. On the other hand, Transmission Electron Microscope (TEM) was used according to the previously described protocol63 by applying a single drop of the sample onto carbon copper grids in a JEOL 1230, 1230 JEOL (Tokyo, Japan) to determine the size and morphology. Moreover, EDX was used to detect the percentage of the elements in the sample while relying on JEOL JSM-5300 (Tokyo, Japan) at 20 kV for a 10 mm working distance64. The Zeta sizer and potential of the sample were measured using rapid, non-invasive dynamin light scattering (DSL) in (Zetasizer Nano ZS (Malvern, UK)) according to65 with several dilutions to identify their size. The UV–Vis spectrophotometer (Jenway 7200, Staffordshire, UK) was utilized with a wavelength range of 340–800 nm66. The sample was not sonicated and left to dry, then take the measurement. Distilled water was utilized as a blank. Moreover, the functional groups in the biofilm-AgNPs and biofilm layers were evaluated by Fourier Transform Infrared Spectroscopy (FTIR) analysis using the Agilent system Cary 360 FTIR model, which ranges from 4000 to 400 cm−1.
Turbidity assay
A turbidity assay was conducted to identify the potential impact of the biosynthesized biofilm-AgNPs on P. aeruginosa described before67 with minor modifications. Specifically, 100 μL of biofilm-AgNPs were incubated with TSB for one night at 37 °C to evaluate the viability of P. aeruginosa in the presence of the biosynthesized NPs. The results were analyzed to determine whether the synthesized biofilm-AgNPs have any antibacterial properties against P. aeruginosa.
Antimicrobial analyses
Disc and well diffusion assays
As for testing the antibacterial effect of the biosynthesized biofilm-AgNPs, disc and well diffusion tests were performed according to Iqbal et al. and Perveen et al. with minor modifications68,69. Initially, each bacterium was swabbed on TSA plate using a sterilized cotton swab. Then, each plate contained two sterilized filter-paper discs with a diameter of 6 mm and two induced wells with a diameter of 6 mm. After that, one disc was loaded with 10 µL, and one well was loaded with 20 µL of the 200 µg/mL of biofilm-AgNPs while the rest were loaded with double distilled water as a negative control. Moreover, the same volumes of 1 mM AgNO3 serve as positive controls. Finally, the prepared plates were dried and incubated overnight at 37 °C. The antimicrobial activity of biofilm-AgNPs was evaluated by measuring the inhibition zone around the discs or the wells as an indication of killing the bacteria.
MIC and MBC
Furthermore, antibacterial activity was determined using the microdilution method to undergo a minimum inhibitory concentration (MIC) test according to bakht Dalir et al.70 with some modifications. MIC was applied in a sterilized 96-well flat-bottom plate where the biofilm-AgNPs were diluted 1:1 v/v with the bacteria to start with 200 μg/mL of biofilm-AgNPs as a final concentration. Firstly, each bacterium was cultured at 37 °C in TSB to be used with initial titer as stated: S. enterica was 2.3 × 106 CFU/mL, E. coli was 1.8 × 106 CFU/mL, P. aeruginosa was 1.9 × 106 CFU/mL, S. aureus was 1.3 × 107 CFU/mL, and Bacillus cereus was 4 × 105 CFU/mL. Various controls were identified either by adding only bacteria or sterilized TSB. Secondly, the 96-well plate was incubated overnight at 37 °C. Then, the minimum bactericidal concentration (MBC) test is managed according to Loo et al.71 with modifications. First, the MBC test was applied by determining the clear or semi-clear wells in the previously prepared 96-well plate, and then 10 µL were taken from each of them to be cultivated on a TSA plate incubated at 37 °C overnight.
Time-Kill assay
In addition, the time-killing curve assay was measured regarding the methodology according to Abdelsattar et al.72 with modifications. The assay was performed by inoculating the bacteria in a 96-well plate with initial titer as stated: S. enterica was 2.3 × 106 CFU/mL, E. coli was 1.8 × 106 CFU/mL, P. aeruginosa was 1.9 × 106 CFU/mL, S. aureus was 1.3 × 107 CFU/mL, and Bacillus cereus was 4 × 105 CFU/mL. After that, biofilm-AgNPs were added with various concentrations of 100, 50, 25, 12.5, 6.3, 3.1 and 1.6 µg/mL. Then, their growth rate was identified at 37 °C through reading OD600 by a microplate reader (FLUOstar Omega, BMG LABTECH, Ortenberg, Germany). The readings and the extracted data were collected every 30 min throughout 11 h using the MARS Data Analysis Software package (version 3.42). The heatmap was created using Microsoft Excel Spreadsheet.
MTT assay
Finally, the MTT assay was applied on a 96-well plate with the bacteria incubated with biofilm-AgNPs overnight at 37 °C according to Makky et al.73 with modifications. First, each well was provided with 10 µL of MTT solution (5mg/mL) then the plate was incubated while shaking for 50 min at 37 °C. Second, 100 µL of acidic isopropanol 1.5% (v/v) was mixed into each well in the plate then it was incubated again while shaking for 50 min at 37 °C. Finally, the optical density of the bacteria was measured at OD570 by FLUOstar Omega Microplate Reader.
Cell membrane integrity
Cell membrane integrity test was applied to S. enterica in combination with ZCSE9 according to Townsend et al.74 with minor modifications. S. enterica was cultured in TSB at 37 °C for 10 min with ZCSE9 and biofilm-AgNPs with a concentration of 12.5 µg/mL. After that, 2.5% uranyl acetate stain was used to stain the whole sample, and then it was put on a carbon-coated Cu-grid until it dried. Finally, the sample was examined using TEM (1230 JEOL, Tokyo, Japan) at different magnification powers.
Cytotoxicity of biofilm-AgNPs
Cell viability for the HSF cell lines was identified by MTT assay as described previously according to Yan et al.75 with numerous modifications. First, HSF cells (passage 8) were seeded in sterilized 96 well plates with a density seeding of (8000 cells/well) in a complete DMEM medium with 10% FBS and 100 mg/mL of streptomycin for 24 h at 37 °C at a 5% CO2 incubator. After that, the serial concentrations of AgNPs were added, from the range 0.1 μg/mL to 16 μg/mL, negative control without treatment, and incubated for 24 h. Then, all the wells were provided with 100μL of (MTT reagent+DMEM medium), and cells were incubated for four hours at 37°C. Finally, the medium was discarded, and 100μL of DMSO was added and measured at OD570.
Analytical statistics
The data obtained from cytotoxicity was the means of two biological replications of three technical replications, and the standard deviation (SD) was determined as the error bar. Additionally, all microscopic, Zeta and EDX graphs were obtained three times at least with different fields to ensure the presented data is the average. Moreover, GraphPad Prism v5 software was used to produce the graphs and conduct all statistical analyses.
Results
Biofilm-AgNPs characterization
Color change, UV-Vis spectrum and FTIR of biofilm-AgNPs
The observable change of color of the prepared solution from a cloudy silver to a lavender was observed after four hours without heat, as shown in Fig. 2A. The alteration in color indicates the successfulness of reducing Ag+ to Ag0 in the AgNO3 solution and the success in the biofilm-AgNPs formation. Using the UV-Vis spectrophotometer indicates the possibility of the synthesized biofilm-AgNPs in the solution. The UV-Vis spectrum of the solution reveals a single peak of a maximum absorption rate at 364 nm, as shown in Fig. 2B. The FTIR was also conducted, and the results showed the same peaks for both the biofilm layer and biofilm-AgNPs. The resulting peaks at 1637.7 (for the biofilm layer) and 1637.51 (for biofilm-AgNPs) indicated the presence of the double bonds of C=O amides. However, the peaks at 3287.67 and 3283.81 indicated the presence of H–O bond in alcohols and phenolic compounds for both the biofilm layer and biofilm-AgNPs, respectively76,77.
TEM and SEM
TEM and SEM results are presented for the biosynthesized biofilm-AgNPs as shown in Fig. 3. TEM images reveal the formation of spherical AgNPs with a size range from 20 to 60 nm in length, as shown in Fig. 3A. In addition, they showed that biofilm-AgNPs are surrounded with the bacterial ghost in the biofilm as shown in Fig. 3B. SEM images indicate that biofilm-AgNPs are formed in the solution with the spherical shape around the bacterial ghost cells with damaged cells wall as shown in Fig. 3C and D. Moreover, these damages in the ghost bacterial cells and rupture are shown obviously in Fig. 3E where there is a disruption in the biofilm environment. Potentially, these results highlight their ability to penetrate the biofilm layer and their interaction with its components.
Illustrates TEM images of spherical AgNPs attached to ghost P. aeruginosa, where (A) shows the spherical structure of the formed NPs with a scale bar of 100 nm, and (B) shows its combination with the bacterial ghost with a scale bar of 1 µm. Moreover, SEM images of biofilm-AgNPs after their biosynthesis in combination with the bacterial ghosts in blue circles where (C), (D) with a scale bar of 500 nm, and (E) with a scale bar of 1 µm.
EDX and zeta sizer and zeta potential
The utilization of EDX enables many investigations into the metallic nature of the biosynthesized biofilm-AgNPs. The resulting EDX spectrum showcases a strong signal at 3 keV, thereby indicating the successful synthesis of AgNPs, as shown in Fig. 4A. Additionally, the EDX spectrum indicates the presence of Ag with percentage of 54.42%, carbon (C) with 17.88%, chlorine (Cl) with 17.68%, oxygen (O) with 9.11%, phosphorus (P) with 0.65% and aluminum (Al) with 0.27%. The analysis of surface charge exhibited that biofilm-AgNPs have a net negative charge. Moreover, the investigation of the biofilm-AgNPs on Zeta Potential shows a sharp peak at -19.1 mV, as shown in Fig. 4C. As the high negative or positive charge refers to the repulsion between the particles and the low aggregation level, it was suggested that the biofilm-AgNPs had a relatively low aggregation than other AgNPs preparations 78. In addition, the Zeta Sizer analysis of the biofilm-AgNPs indicates their uniformity of sizes within the range of 100 to 1000 nm, as shown in Fig. 4B. The size range shows two different peaks with two different ranges. The first peak is from 70 to 200 nm, and the most dominant size for this peak was 100 nm. Moreover, the second peak ranged from 200 to 700 nm, and the most dominant size in this peak was 400 nm for the prepared NPs.
Antimicrobial tests
Bacterial results
Disc and well diffusion tests
Disc and well diffusion tests were performed to detect the primary antibacterial activity of biosynthesized biofilm-AgNPs and Silver nitrate (positive control) on various bacterial stains, as shown in Figs. 5 and 6. The results represented in Table 1 illustrate the presence of an obvious antimicrobial activity for the biofilm-AgNPs with a concentration of 200 µg/mL. The highest effect of disc diffusion tests based on inhibition zones was 12.2 mm for S. enterica 10.7 mm for S. aureus, 10.4 mm for Bacillus cereus, 9.7 mm for P. aeruginosa and 8.9 mm for E. coli. The highest effect of well diffusion tests based on inhibition zones was 13.3 mm for S. aureus, 12.2 mm for Bacillus cereus, 11.8 mm for P. aeruginosa, 11.8 mm for S. enterica and 8.9 mm for E. coli. The inhibition zones were compared to the positive and negative controls.
MIC and MBC
Accordingly, MIC and MBC tests were applied to detect the effect of biofilm-AgNPs on these previously used bacterial strains. As shown in Table 1, the prepared biofilm-AgNPs have a clearance effect on the used bacteria with various concentrations. The lowest effect on MIC tests was 12.5 µg/mL for S. aureus, 25 µg/mL for Bacillus cereus and E. coli, and 100 µg/mL for P. aeruginosa. The lowest effect on MBC tests was 25 µg/mL for Bacillus cereus, 100 µg/mL for S. aureus and E. coli, in addition to 200 µg/mL for P. aeruginosa. The inhibitory and bactericidal effects were compared to several controls mentioned before.
Time-killing curve
Time-killing curve was conducted to detect a spectrum for bacterial growth under the effect of biofilm-AgNPs, and the variation in bacterial optical density (OD600), as shown in Fig. 7. These results were confirmed by the heat maps represented in Fig. 8, which shows the turbidity of the bacteria along 660 min continuously with golden yellow color for the less turbid wells and red color for the highly turbid wells. Several controls were applied, which are empty TSB wells shown as the blue line and bacterium without any treatment shown as the red line. At the concentration of 6.25 µg/mL, E. coli was inhibited for 420 min., and S. aureus was inhibited for 570 min. At the concentration of 12.5 µg/mL, Bacillus cereus was inhibited for 420 min., and E. coli and S. aureus were inhibited for 660 min. At the concentration of 25 µg/mL, Bacillus cereus was inhibited for 660 min. At the concentration of 50 µg/mL, P. aeruginosa was inhibited for 510 min. At the concentration of 100 µg/mL, P. aeruginosa was inhibited for 660 min.
Illustrates a heat map for the selected bacterial strains that indicates their growth under the effect of biofilm-AgNPs where the golden yellow color indicates low concentration while the red color indicates high concentration. (A) is Bacillus cereus, (B) is E. coli, (C) is P. aeruginosa, and (D) is S. aureus. The numbers 1, 2, 3, 4 & 5 represents the concentrations of biofilm-AgNPs 25, 12.5, 6.3, 3.1 and 1.6 µg/mL, respectively, and (B) is the bacteria without any treatment. The heatmap was created using Microsoft Excel Spreadsheet.
S. enterica with ZCSE9 results
MIC and MBC
MIC and MBC tests were applied to detect the effect of biofilm-AgNPs on S. enterica when it is combined with ZCSE9. As shown in Table 1, the prepared biofilm-AgNPs have a tangible effect on S. enterica. The lowest MIC effect was 3.1 µg/mL for S. enterica combined with ZCSE9, while it was 25 µg/mL for S. enterica alone. The lowest MBC effect was 3.1 µg/mL for S. enterica combined with ZCSE9, while it was 100 µg/mL for S. enterica alone.
Time-killing curve
A time-kill curve was conducted to detect the spectrum for S. enterica growth under the influence of biofilm-AgNPs alone and when combined with ZCSE9, the variation in bacterial optical density (OD600) as shown in Fig. 9. Several controls were applied which are empty TSB wells shown as the blue line and bacterium without any treatment shown as the red line. These results were confirmed by the heat maps represented in Fig. 10, which shows the turbidity of the bacteria for 11 h continuously, with a golden yellow color for the less turbid wells and a red color for the highly turbid wells. At the concentration of 12.5 µg/mL, S. enterica alone and in combination with ZCSE9 were inhibited for 660 min. At the concentration of 6.3 µg/mL, 11 h is needed to inhibit S. enterica in combination with ZCSE9 was inhibited for 660 min. and S. enterica only was inhibited for 480 min. At the concentration of 3.1 µg/mL, S. enterica, in combination with ZCSE9, was inhibited for 660 min, and S. enterica was inhibited for 240 min.
Illustrates heat maps for the selected bacterial strains that indicate their growth under the effect of biofilm-AgNPs, where the golden yellow color indicates low concentration, and the red color indicates high concentration. (A) is S. enterica with the NPs alone, while (B) is S. enterica with the NPs in combination with ZCSE9. The numbers 1, 2, 3, 4 and 5 represent the concentrations of biofilm-AgNPs 25, 12.5, 6.3, 3.1 and 1.6 µg/mL, respectively. (B) is the bacteria without any treatment, and (Φ) is ZCSE9. The heatmap was created using Microsoft Excel Spreadsheet.
Cell membrane integrity
The results of the integrity of bacterial cell membranes were illustrated using a TEM analysis device, as shown in Fig. 11. Incubating S. enterica with biofilm-AgNPs with a concentration of 0.5 MIC and ZCSE9 had various effects on the cell membrane integrity. Biofilm-AgNPs, in combination with ZCSE9, lead to disruption in the cell membrane, which leads to rupture in some parts, and the intracellular components come out of the cell, as shown in the blue circles and arrow in Fig. 11.
Illustrates that using biofilm-AgNPs with 0.5 × MIC concentration with ZCSE9 towards S. enterica leads to disruption in the cell membrane, and the intracellular components come out of the cell as shown in the blue circles and arrow where the scale bar of (A), (B), and (C) was at 200 nm while (D) was at 500 nm.
Cytotoxicity of biofilm-AgNPs
To assess the safety of biofilm-AgNPs, a cytotoxicity assay has been conducted on the HSF cell line with various concentrations. The results have shown in Fig. 12 that biofilm-AgNPs are not toxic and do not cause damage when exposed to the HSF cell line at concentrations 0.1 μg/mL to 8 μg/mL. When increasing the concentration to 16 μg/mL, it reduced the cell viability to 34.70%.
Discussion
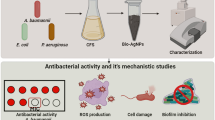
In 2022, the WHO reported that infection rates are continuously increasing. Statistically, more than 50% of bacterial infections around the world are due to MDR bacteria79. A long time ago, these MRD bacteria could transform, directly or indirectly, and cause a series of illnesses in humans and animals, such as S. aureus, E. coli, S. enteric, Bacillus cereus, and P. aeruginosa80,81,82,83. For instance, these reports demonstrated that more than 20% of clinically isolated E. coli are resistant to the first and second lines of treatments79,84. Therefore, tackling new approaches to treat or defeat MDRs is one of the universal goals85. In this study, biosynthesized NPs, biofilm-AgNPs, were prepared as a new approach to determine their antibacterial effect on the previously mentioned bacteria. Ag is the metallic ion used to synthesize the NPs due to its well-known antibacterial activity86,87. It is found that green synthesized and biosynthesized NPs have a higher antimicrobial effect than metallic ones88,89,90. In this study, the biofilm of P. aeruginosa is used in the biosynthesis process of AgNPs in a trial to determine its effect. In previous studies, some components of the biofilm layer were used as reducing agents in synthesizing NPs, such as extracellular polymer substances91, intracellular polymeric substances92 and various enzymes such as dehydrogenases93. Although these components were used independently, it is the first time to use all of the components altogether. It is hypothesized that using the whole biofilm layer might enhance the antimicrobial activity of the AgNPs.
The biosynthesized biofilm-AgNPs were prepared according to the steps illustrated in Fig. 1 and then characterized. There was a clear change in the color of the previously prepared solution from a cloudy silver color to a lavender color. This change occurred after four hours of continuous stirring for the solution without heat which is considered as a primary indicator for the synthesis of the NPs. According to previous studies, AgNPs can have various ranges of colors according to the natural product or substance used during its synthesis94,95. They can have brown, red, green or other rainbow colors96,97. The change in the color of the solution signifies the presence of a redox reaction where Ag+ is reduced to Ag0 to form NPs98,99. After the color change, UV-Vis spectroscopy analysis for the prepared solution identified a single peak of wavelength at 364 nm. Although previous studies had mentioned that the surface plasmon resonance (SPR) absorption of AgNPs ranges between 400 and 450 nm100,101,102, some studies documented that using a biological reducing agent will shift the peak to less wavelength range103,104. TEM and SEM results illustrated that the size of biofilm-AgNPs ranges between 20 and 60 nm with a spherical shape. These results are similar to previous studies where AgNPs have a size range of 20–90 nm with a spherical shape105,106,107. Here, it is suggested that the link between the biofilm components reduced the size of the formed NPs according to what was mentioned before108. As illustrated in Fig. 3, it is suggested that Ag+ could rupture the bacterial cells and penetrate the biofilm layer to use its components in the biosynthesis of the NPs. This might indicate its antibacterial effect, which is supported by previous studies that used bacteria in synthesizing NPs109,110. EDX results demonstrate the elemental analysis of the prepared sample. As presented in Fig. 4, about 54% of the sample contains Ag, while some elements represent the main components of natural products, such as carbon, with 17.88% and oxygen, with 9.11%. As detected in previous studies, these are the main components found in the biosynthesized NPs111,112. The results also show the presence of chlorine, which is represented as a source of contamination. The results of zeta illustrate the successful preparation and the possibility of being functional and stable. When compared to other studies, it is suggested that the more negative result might indicate more effect when applied on bacteria113,114,115. In addition, the zeta sizer illustrated two populations; it was suggested that the first population refers to the biofilm-AgNPs alone, and the second population refers to the biofilm-AgNPs bound to the P. aeruginosa. This finding is compatible with the results from TEM and SEM analysis (Fig. 3).
Biofilm-AgNPs were tested on Gram-positive bacteria, such as Bacillus cereus and S. aureus, and Gram-negative bacteria, such as E. coli, S. enterica, and P. aeruginosa. Previous studies demonstrate that AgNPs have a strong antibacterial effect on Gram-positive and Gram-negative bacteria116,117,118. The disc and well diffusion test results showed that the inhibitory zones vary on Gram-positive and Gram-negative bacteria when biofilm-AgNPs were used with a concentration of 200 µg/mL. Afterward, MIC results highlighted that biofilm-AgNPs have the lowest inhibitory effect of 12.5 µg/mL for S. aureus, 25 µg/mL E. coli, Bacillus cereus and S. enterica and 100 µg/mL for P. aeruginosa. Besides, they have the lowest bactericidal effect of 25 µg/mL for Bacillus cereus, 100 µg/mL for E. coli, S. aureus and S. enterica, 200 µg/mL for P. aeruginosa. Yet, when biofilm-AgNPs were combined with ZCSE9, which is specific for S. enterica119, its inhibitory and bactericidal effects were enhanced to have a higher effect in lower concentration, which is 3.1 µg/mL. These results were confirmed using a killing curve, which showed that when using biofilm-AgNPs with its MIC, it would inhibit the growth of the bacteria for up to 11 h. This long period of time might indicate the long-lasting effect of the biofilm-AgNPs. This might enable them to be used as a detergent or a disinfectant for surfaces in hospitals and other places. Studies show that the effectiveness of the daily used detergents ranges from a few minutes to a few hours120,121,122. When biofilm-AgNPs are used with a concentration less than the bacterial MIC, 12.5 µg/mL for Bacillus cereus, E. coli, and S. enterica, 6.3 µg/mL for S. aureus and 50 µg/mL for P. aeruginosa, the inhibitory effect will last for less time but still effective at inhibiting their growth. These results were confirmed with the MTT assay, as shown in Fig. 13, in which the MTT assay was conducted to confirm the antibacterial effect of biofilm-AgNPs. The obvious effect of biofilm-AgNPs is conducted from the ability of AgNPs to penetrate the bacterial cell membrane and destroy it123,124. Its accessibility to the membrane indicates that the effect on Gram-negative bacteria is more than the effect on Gram-positive ones125,126. In this study, it is suggested that AgNPs affect various bacterial strains by producing ions that interact with cellular organelles, causing their damage127,128,129. Several studies explained the mechanism based on breaking down the cell membrane, entering the cell through porin proteins, disrupting mitochondrial function, generating Reactive Oxygen Species (ROS), breaking apart ribosomes, denaturing proteins, stopping ATP production and causing damage to DNA14,130,131,132,133. Here, we suggest that one of the mechanisms of biofilm-AgNPs could be a result of attaching NPs to the cell membrane and then interrupting bacterial selective permeability, causing the release of cellular contents, as in Fig. 11.
Shows the colorimetric change after using the MTT assay lanes containing (A) S. aureus, (B) E. coli, (C) S. Typhimurium, (D) S. enterica, (E) S. enterica+ZCSE9, (F) Bacillus cereus, and (G) P. aeruginosa. In addition, the rows from 1 to 8 contain biofilm-AgNPs with concentrations from (100, 50, 25, 12.5, 6.25, 3.1, 1.6 and 0.8 µg/mL), respectively.
The results indicated that whenever biofilm-AgNPs are used with low concentrations, 8 µg/mL, on a normal HSF cell line, they do not reduce the viability of the cells, as shown Fig. 12. These results are compatible with the results of adding biofilm-AgNPs with low concentration, 10 µg/mL, on cancer cell lines, MCF-7 and HepG2 cell lines, they do not reduce the viability of both cell lines as shown in Fig. 14. It was illustrated in Fig. 14 that using biofilm-AgNPs with higher concentrations reduces the viability of the cells to about 20–30%. Their effect on cancer cell lines, as shown in Fig. 14, are similar to other biosynthesized AgNPs, such as biosynthesized AgNPs using Bacillus sp, which induced the apoptosis on MCF-7 by 61% at 50 μg/mL134. Another study illustrated the cytotoxicity effect of the dragon fruit peel aqueous extract fabricated AgNPs on HEPG2 cell lines with (IC50) 38 μg/mL135.
These previously mentioned results could indicate a variety of things. The results represented the effective synergistic effect of using biofilm-AgNPs in combination with ZCSE9 to kill S. enterica with a concentration of 3.1 µg/mL. The concentration of 3.1 µg/mL, as demonstrated previously in Fig. 12, can be effective while not reducing the viability of the normal mammalian cells. Moreover, using biofilm-AgNPs with a concentration of 6.25 µg/mL to inhibit E. coli, and S. aureus. In this study, it is suggested that biofilm-AgNPs can be used in treating S. enterica, E. coli, and S. aureus infections caused by contaminated food, water, and hands136,137,138. This study demonstrates that it is unlikely to treat P. aeruginosa and Bacillus cereus infections because they require higher concentrations of biofilm-AgNPs to be inhibited, which reduces the viability of normal human cells and cancerous cell lines, as shown in Fig. 14. Nevertheless, biofilm-AgNPs show promising results in treating several bacterial infections rather than using antibiotics or phages alone. In addition, they have high effectiveness and antibacterial function with low concentration, which can be used as a disinfectant agent or topical treatment. They cannot be used to treat cancerous cells because they need higher concentrations, which will be toxic to normal cells, as IC50 values were 11.3 and 18.7 μg/mL against MCF7 and HEPG2 cell lines, respectively. In the end, it is suggested that further investigations are needed to identify the effect of combining biofilm-AgNPs with phages specific to other bacteria.
Conclusion
In this study, and for the first time, the biofilm was utilized to generate biosynthesis AgNPs and acts as a capping and stabilizing agent for the formation process. UV-vis spectroscopy, FT-IR, SEM-EDX and TEM were conducted as characterization analyses to confirm the formation of spherical biofilm-AgNPs with size range < 100 nm and bind to bacterial ghosts. Elemental analysis of synthesized NPs by EDX illustrates that the percentage of silver > 54% with the presence of organic elements. biofilm-AgNPs can be considered as one of the novel approaches as an alternative to antibiotics and other treatments to reduce MDR bacterial infections, especially those that are initiated from surfaces of hospitals. In addition, using it with a low concentration, 8 µg/mL, could not reduce the viability of mammalian cells. Furthermore, it gives more promising, synergistic results when combined with ZCSE9, where bacteria are inhibited and killed before developing resistance to the phage. Eventually, this work opens the door for using the phages with metallic nanoparticles as a combination with synergistic activity.
Data availability
Data will be made available on request.
References
Nicolaou, K. C. & Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 71, 153–184 (2018).
Gaynes, R. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg. Infect. Dis. 23, 849 (2017).
Chokshi, A., Sifri, Z., Cennimo, D. & Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 11, 36 (2019).
Schulz, F. et al. Towards a balanced view of the bacterial tree of life. Microbiome 5, 1–6 (2017).
MacGowan, A. & Macnaughton, E. Antibiotic resistance. Medicine 45, 622–628 (2017).
Aslam, B. et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 11, 1645 (2018).
Larsson, D. G. J. & Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257–269 (2022).
MacLean, R. C. & San Millan, A. The evolution of antibiotic resistance. Science 365, 1082–1083 (2019).
Mehdi, Y. et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 4, 170–178 (2018).
Van, T. T. H., Yidana, Z., Smooker, P. M. & Coloe, P. J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 20, 170–177 (2020).
Dawood, M. A. O., Koshio, S. & Esteban, M. Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Revi. Aquac. 10, 950–974 (2018).
Öztürk, D. & Mıhçıokur, H. Removal of lansoprazole one of the most prescribed drugs in Turkey from an aqueous solution by innovative magnetic nanomaterial Tween 85® PEI@ Fe3O4. J. Water Process Eng. 52, 103527 (2023).
Said, A., Abu-Elghait, M., Atta, H. M. & Salem, S. S. Antibacterial activity of green synthesized silver nanoparticles using Lawsonia inermis against common pathogens from urinary tract infection. Appl. Biochem. Biotechnol. https://doi.org/10.1007/s12010-023-04482-1 (2023).
Elakraa, A. A., Salem, S. S., El-Sayyad, G. S. & Attia, M. S. Cefotaxime incorporated bimetallic silver-selenium nanoparticles: Promising antimicrobial synergism, antibiofilm activity, and bacterial membrane leakage reaction mechanism. RSC Adv. 12, 26603–26619 (2022).
Al-Rajhi, A. M. H., Salem, S. S., Alharbi, A. A. & Abdelghany, T. M. Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab. J. Chem. 15, 103927 (2022).
Talebi Bezmin Abadi, A., Rizvanov, A. A., Haertlé, T. & Blatt, N. L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience 9, 778–788 (2019).
Grujović, M. et al. Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. Food Saf. 21, 1537–1567 (2022).
León-Buitimea, A., Garza-Cárdenas, C. R., Garza-Cervantes, J. A., Lerma-Escalera, J. A. & Morones-Ramírez, J. R. The demand for new antibiotics: Antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front. Microbiol. 11, 1669 (2020).
Yaqoob, A. A., Umar, K. & Ibrahim, M. N. M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 10, 1369–1378 (2020).
Han, X., Xu, K., Taratula, O. & Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 11, 799–819 (2019).
Tharani, S., Bharathi, D. & Ranjithkumar, R. Extracellular green synthesis of chitosan-silver nanoparticles using Lactobacillus reuteri for antibacterial applications. Biocatal. Agric. Biotechnol. 30, 101838 (2020).
Veisi, H., Azizi, S. & Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 170, 1536–1543 (2018).
Kalwar, K. & Shan, D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism–a mini review. Micro Nano Lett. 13, 277–280 (2018).
Zhou, L. et al. Antifungal activity of silver nanoparticles synthesized by iturin against Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 105, 3759–3770 (2021).
Tortella, G. R. et al. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 390, 121974 (2020).
Rafique, M., Sadaf, I., Rafique, M. S. & Tahir, M. B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells, Nanomed., Biotechnol. 45, 1272–1291 (2017).
Wei, S. et al. A novel green synthesis of silver nanoparticles by the residues of Chinese herbal medicine and their biological activities. RSC Adv. 11, 1411–1419 (2021).
El Shafey, A. M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 9, 304–339 (2020).
Salem, S. S., Ali, O. M., Reyad, A. M., Abd-Elsalam, K. A. & Hashem, A. H. Pseudomonas indica-mediated silver nanoparticles: Antifungal and antioxidant biogenic tool for suppressing mucormycosis fungi. JoF 8, 126 (2022).
Abdelsattar, A. S., Dawoud, A. & Helal, M. A. Interaction of nanoparticles with biological macromolecules: A review of molecular docking studies. Nanotoxicology 15, 66–95 (2021).
Salem, S. S. Baker’s yeast-mediated silver nanoparticles: Characterisation and antimicrobial biogenic tool for suppressing pathogenic microbes. BioNanoScience 12, 1220–1229 (2022).
Salem, S. S. & Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 199, 344–370 (2021).
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: From biological mechanisms to future directions. Cell 186, 17–31 (2023).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285 (2020).
Dan, J. M. et al. Development of host immune response to bacteriophage in a lung transplant recipient on adjunctive phage therapy for a multidrug-resistant pneumonia. J. Infect. Dis. 227, 311–316 (2023).
Suh, G. A. et al. Considerations for the use of phage therapy in clinical practice. Antimicrob. Agents Chemother. 66, e02071-e2121 (2022).
Melo, L. D. R., Oliveira, H., Pires, D. P., Dabrowska, K. & Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 46, 78–99 (2020).
Hatfull, G. F., Dedrick, R. M. & Schooley, R. T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 73, 197–211 (2022).
Uyttebroek, S. et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(21)00612-5 (2022).
Rimon, A. et al. Topical phage therapy in a mouse model of Cutibacterium acnes-induced acne-like lesions. Nat. Commun. 14, 1005 (2023).
Roach, D. R. et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38–47 (2017).
Nilsson, A. S. Pharmacological limitations of phage therapy. Upsala J. Med. Sci. 124, 218–227 (2019).
Berryhill, B. A., Huseby, D. L., McCall, I. C., Hughes, D. & Levin, B. R. Evaluating the potential efficacy and limitations of a phage for joint antibiotic and phage therapy of Staphylococcus aureus infections. Proc. Natl. Acad. Sci. 118, e2008007118 (2021).
Lebeaux, D. et al. A case of phage therapy against pandrug-resistant Achromobacter xylosoxidans in a 12-year-old lung-transplanted cystic fibrosis patient. Viruses 13, 60 (2021).
Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 10, 351 (2018).
Gaborieau, B. & Debarbieux, L. The role of the animal host in the management of bacteriophage resistance during phage therapy. Curr. Opin. Virol. 58, 101290 (2023).
Hantke, K. Compilation of Escherichia coli K-12 outer membrane phage receptors–their function and some historical remarks. FEMS Microbiol. Lett. 367, fnaa013 (2020).
Chang, R. Y. K. et al. The effects of different doses of inhaled bacteriophage therapy for Pseudomonas aeruginosa pulmonary infections in mice. Clin. Microbiol. Infect. 28, 983–989 (2022).
Molleston, J. M. & Holtz, L. R. Fighting the wrong enemy: Antibacteriophage immunity in phage therapy. J. Infect. Dis. 227, 309–310 (2023).
Dedrick, R. M. et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat. Med. 27, 1357–1361 (2021).
Dedrick, R. M. et al. Phage therapy of mycobacterium infections: compassionate use of phages in 20 patients with drug-resistant mycobacterial disease. Clinic. Infect. Dis. 76, 103–112 (2023).
Moser, C. et al. Immune responses to Pseudomonas aeruginosa biofilm infections. Front. Immunol. 12, 625597 (2021).
Skariyachan, S., Sridhar, V. S., Packirisamy, S., Kumargowda, S. T. & Challapilli, S. B. Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol. 63, 413–432 (2018).
Riquelme, S. A. & Prince, A. Airway immunometabolites fuel Pseudomonas aeruginosa infection. Respir. Res. 21, 1–10 (2020).
Brindhadevi, K. et al. Biofilm and quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Biochem. 96, 49–57 (2020).
Muhammad, M. H. et al. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 11, 928 (2020).
Hashem, A., Tabassum, B. & Abd Allah, E. F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 26, 1291–1297 (2019).
Ren, L., McCuskey, S. R., Moreland, A., Bazan, G. C. & Nguyen, T.-Q. Tuning Geobacter sulfurreducens biofilm with conjugated polyelectrolyte for increased performance in bioelectrochemical system. Biosens. Bioelectron. 144, 111630 (2019).
Bajracharya, S. et al. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 98, 153–170 (2016).
Pavissich, J. P., Li, M. & Nerenberg, R. Spatial distribution of mechanical properties in Pseudomonas aeruginosa biofilms, and their potential impacts on biofilm deformation. Biotechnol. Bioeng. 118, 1545–1556 (2021).
Tailor, G., Yadav, B. L., Chaudhary, J., Joshi, M. & Suvalka, C. Green synthesis of silver nanoparticles using Ocimum canum and their anti-bacterial activity. Biochem. Biophys. Rep. 24, 100848 (2020).
Mohamed, M. E., Ezzat, A. & Abdel-Gaber, A. M. Fabrication of eco-friendly graphene-based superhydrophobic coating on steel substrate and its corrosion resistance, chemical and mechanical stability. Sci. Rep. 12, 10530 (2022).
AlSalhi, M. S. et al. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 11, 4439 (2016).
Mahmoud, M. E., Hassan, S. S. M., Kamel, A. H. & Elserw, M. I. A. Fast microwave-assisted sorption of heavy metals on the surface of nanosilica-functionalized-glycine and reduced glutathione. Bioresour. Technol. 264, 228–237 (2018).
Kulikouskaya, V. et al. Chitosan-capped silver nanoparticles: A comprehensive study of polymer molecular weight effect on the reaction kinetic, physicochemical properties, and synergetic antibacterial potential. SPE Polym. 3, 77–90 (2022).
Aygün, A., Özdemir, S., Gülcan, M., Cellat, K. & Şen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 178, 112970 (2020).
Surmeneva, M. A. et al. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf. B: Biointerfaces 156, 104–113 (2017).
Perveen, R. et al. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J. Mater. Res. Technol. 9, 7817–7827 (2020).
Iqbal, H. et al. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly (vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 144, 921–931 (2020).
Bakht Dalir, S. J., Djahaniani, H., Nabati, F. & Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 6, e03624 (2020).
Loo, Y. Y. et al. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 9, 1555 (2018).
Abdelsattar, A. S., Yakoup, A. Y., Safwat, A. & El-Shibiny, A. The synergistic effect of using bacteriophages and chitosan nanoparticles against pathogenic bacteria as a novel therapeutic approach. Int. J. Biol. Macromol. 228, 374–384 (2023).
Makky, S. et al. Characterization of the biosynthesized Syzygium aromaticum-mediated silver nanoparticles and its antibacterial and antibiofilm activity in combination with bacteriophage. Results Chem. 5, 100686 (2023).
Townsend, E. M. et al. Isolation and characterization of Klebsiella phages for phage therapy. Ther., Appl., Res. 2, 26–42 (2021).
Yan, T. et al. Exosomal miR-10b-5p mediates cell communication of gastric cancer cells and fibroblasts and facilitates cell proliferation. J. Cancer 12, 2140 (2021).
Hong, T., Yin, J.-Y., Nie, S.-P. & Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem.: X 12, 100168 (2021).
Gong, C.-P., Li, S.-C. & Wang, R.-Y. Development of biosynthesized silver nanoparticles based formulation for treating wounds during nursing care in hospitals. J. Photochem. Photobiol. B: Biol. 183, 137–141 (2018).
Kamel, A. G., Abdelsattar, A. S., Badr, T. & El-Shibiny, A. The antibacterial and cytotoxicity effect of silver ferrite nanocomposite biosynthesized using Allium sativum plant extract. Mater. Lett. 348, 134677 (2023).
WHO. Report signals increasing resistance to antibiotics in bacterial infections in humans and need for better data. https://www.who.int/news/item/09-12-2022-report-signals-increasing-resistance-to-antibiotics-in-bacterial-infections-in-humans-and-need-for-better-data (2022).
Antibiotic resistance threats in the United States, 2019. https://doi.org/10.15620/CDC:82532(2019).
Stocker, M. D. et al. Persistent patterns of E. coli concentrations in two irrigation ponds from 3 years of monitoring. Water, Air, Soil Pollut. 232, 1–15 (2021).
Wendel, A. F. et al. Genomic-based transmission analysis of carbapenem-resistant Pseudomonas aeruginosa at a tertiary care centre in Cologne (Germany) from 2015 to 2020. JAC-Antimicrob. Resist. 4, dlac057 (2022).
Hemeg, H. A. Determination of phylogenetic relationships among methicillin-resistant Staphylococcus aureus recovered from infected humans and companion animals. Saudi J. Biol. Sci. 28, 2098–2101 (2021).
Mukubwa, G. K. et al. Resistance profiles of urinary Escherichia coli and Klebsiella pneumoniae isolates to antibiotics commonly prescribed for treatment of urinary tract infections at Monkole Hospital Center, Kinshasa, Democratic Republic of the Congo. Afr. J. Clinic. Exp. Microbiol. 24, 51–60 (2023).
Schrader, S. M., Vaubourgeix, J. & Nathan, C. Biology of antimicrobial resistance and approaches to combat it. Scie. Transl. Med. 12, 6eaaz6992 (2020).
Das, C. G. A. et al. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatal. Agric. Biotechnol. 27, 101593 (2020).
Keshari, A. K., Srivastava, R., Singh, P., Yadav, V. B. & Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 11, 37–44 (2020).
Garibo, D. et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 10, 1–11 (2020).
Shinde, B. H., Inamdar, S. N., Nalawade, S. A. & Chaudhari, S. B. A systematic review on antifungal and insecticidal applications of biosynthesized metal nanoparticles. Mater. Today: Proc. 73, 412–417 (2023).
Govindarajan, M. et al. Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: A potent eco-friendly tool against malaria and arbovirus vectors. J. Photochem. Photobiol. B: Biol. 161, 482–489 (2016).
Feng, L.-J. et al. Role of extracellular polymeric substances in the acute inhibition of activated sludge by polystyrene nanoparticles. Environ. Pollut. 238, 859–865 (2018).
Huangfu, X. et al. A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates. Chemosphere 219, 766–783 (2019).
de Gonzalo, G. & Paul, C. E. Recent trends in synthetic enzymatic cascades promoted by alcohol dehydrogenases. Curr. Opin. Green Sustain. Chem. 32, 100548 (2021).
Nguyen, T. T. T. et al. Passion fruit peel pectin/chitosan based antibacterial films incorporated with biosynthesized silver nanoparticles for wound healing application. Alex. Eng. J. 69, 419–430 (2023).
Riazunnisa, K. Antimicrobial activity of Biosynthesized silver nanoparticles of Bauhinia racemosa leaf extracts. Res. J. Pharm. Technol. 16, 745–749 (2023).
Fatima, R., Priya, M., Indurthi, L., Radhakrishnan, V. & Sudhakaran, R. Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 138, 103780 (2020).
Yousaf, H., Mehmood, A., Ahmad, K. S. & Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng.: C 112, 110901 (2020).
Santhoshkumar, J., Rajeshkumar, S. & Kumar, S. V. Phyto-assisted synthesis, characterization and applications of gold nanoparticles–A review. Biochem. Biophys. Rep. 11, 46–57 (2017).
Menon, S., Rajeshkumar, S. & Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour.-Effic. Technol. 3, 516–527 (2017).
Che Sulaiman, I. S. et al. A review on colorimetric methods for determination of organophosphate pesticides using gold and silver nanoparticles. Microchimica Acta 187, 1–22 (2020).
Jaffri, S. B. & Ahmad, K. S. Biomimetic detoxifier Prunus cerasifera Ehrh. silver nanoparticles: Innate green bullets for morbific pathogens and persistent pollutants. Environ. Sci. Pollut. Res. 27, 9669–9685 (2020).
Luna-Sánchez, J. L. et al. Green synthesis of silver nanoparticles using Jalapeño Chili extract and thermal lens study of acrylic resin nanocomposites. Thermochim. Acta 678, 178314 (2019).
Hamedi, S., Ghaseminezhad, M., Shokrollahzadeh, S. & Shojaosadati, S. A. Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif. Cells, Nanomed. Biotechnol. 45, 1588–1596 (2017).
Kanniah, P. et al. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver based chitosan nanocomposite. Int. J. Biol. Macromol. 189, 18–33 (2021).
Wu, M., Guo, H., Liu, L., Liu, Y. & Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. https://doi.org/10.2147/IJN.S201107 (2019).
Tang, S. & Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 7, 1701503 (2018).
Restrepo, C. V. & Villa, C. C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol., Monit. Manag. 15, 100428 (2021).
Qaeed, M. A. et al. The effect of different aqueous solutions ratios of Ocimum basilicum utilized in AgNPs synthesis on the inhibition of bacterial growth. Sci. Rep. 13, 5866 (2023).
Alavi, M. & Nokhodchi, A. Synthesis and modification of bio-derived antibacterial Ag and ZnO nanoparticles by plants, fungi, and bacteria. Drug Discov. Today 26, 1953–1962 (2021).
Afolayan, E. M., Afegbua, S. L. & Ado, S. A. Characterization and antibacterial activity of silver nanoparticles synthesized by soil-dwelling Bacillus thuringiensis against drug-resistant bacteria. Biologia 78, 2283–2292 (2023).
Gholamali, I., Asnaashariisfahani, M. & Alipour, E. Silver nanoparticles incorporated in pH-sensitive nanocomposite hydrogels based on carboxymethyl chitosan-poly (vinyl alcohol) for use in a drug delivery system. Regen. Eng. Transl. Med. 6, 138–153 (2020).
Hatipoğlu, A. Green synthesis of gold nanoparticles from Prunus cerasifera pissardii nigra leaf and their antimicrobial activities on some food pathogens. Prog. Nutr. 23, e2021241 (2021).
Gola, D. et al. Silver nanoparticles for enhanced dye degradation. Curr. Res. Green Sustain. Chem. 4, 100132 (2021).
Jain, A. et al. Multi dye degradation and antibacterial potential of Papaya leaf derived silver nanoparticles. Environ. Nanotechnol., Monit. Manag. 14, 100337 (2020).
Das, G., Patra, J. K. & Shin, H. S. Biosynthesis, and potential effect of fern mediated biocompatible silver nanoparticles by cytotoxicity, antidiabetic, antioxidant and antibacterial, studies. Mater. Sci. Eng.: C 114, 111011 (2020).
Domínguez, A. V., Algaba, R. A., Canturri, A. M., Villodres, Á. R. & Smani, Y. Antibacterial activity of colloidal silver against gram-negative and gram-positive bacteria. Antibiotics 9, 36 (2020).
Ji, H. et al. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng.: C 110, 110735 (2020).
Arifin, D. C. V., Saragih, D. I. & Santosa, S. J. Antibacterial activity of silver nanoparticles synthesized using tyrosine as capping and reducing agent. Int. J. https://doi.org/10.30534/ijeter/2020/34862020 (2020).
Abdelsattar, A. S. et al. The lytic activity of bacteriophage ZCSE9 against Salmonella enterica and its synergistic effects with kanamycin. Viruses 15, 912 (2023).
Bjørnestad, V. A. & Lund, R. Pathways of membrane solubilization: A structural study of model lipid vesicles exposed to classical detergents. Langmuir 39, 3914–3933 (2023).
Cremer, J. et al. Aging of industrial polypropylene surfaces in detergent solution and its consequences for biofilm formation. Polymers 15, 1247 (2023).
Liu, J., Zhang, J., Yi, W. & Tong, Y. Real-time sensing of disinfectant by a novel dielectric methodology. Sens. Actuators A: Phys. 357, 114350 (2023).
Mikhailova, E. O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 11, 84 (2020).
Skrzyniarz, K. et al. Mechanistic insight of lysozyme transport through the outer bacteria membrane with dendronized silver nanoparticles for peptidoglycan degradation. Int. J. Biol. Macromol. 237, 124239 (2023).
Phanjom, P. & Ahmed, G. Effect of different physicochemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae (MTCC No. 1846) and their antibacterial effect. Adv. Nat. Sci.: Nanosci. Nanotechnol. 8, 45016 (2017).
Thomas, T. & Thalla, A. K. Synthesis of silver nanoparticles using Myristica fragrans seed shell: Assessment of antibacterial, antioxidant properties and photocatalytic degradation of dyes. J. Environ. Chem. Eng. 11, 109585 (2023).
Lara, H. H., Garza-Treviño, E. N., Ixtepan-Turrent, L. & Singh, D. K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 9, 1–8 (2011).
Kim, S.-H., Lee, H.-S., Ryu, D.-S., Choi, S.-J. & Lee, D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Microbiol. Biotechnol. Lett. 39, 77–85 (2011).
Gevorgyan, S. et al. Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci. Rep. 12, 14077 (2022).
Abdelsattar, A. S., Kamel, A. G. & El-Shibiny, A. The green production of eco-friendly silver with cobalt ferrite nanocomposite using Citrus limon extract. Results Chem. 5, 100687 (2023).
Nasr, H. A., Nassar, O. M., El-Sayed, M. H. & Kobisi, A. A. Characterization and antimicrobial activity of lemon peel mediated green synthesis of silver nanoparticles. Int. J. Biol. Chem. 12(2), 56–63 (2019).
Aref, M. S. & Salem, S. S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 27, 101689 (2020).
De Lacerda Coriolano, D. et al. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 52, 267–278 (2021).
Almalki, M. A. & Khalifa, A. Y. Z. Silver nanoparticles synthesis from Bacillus sp KFU36 and its anticancer effect in breast cancer MCF-7 cells via induction of apoptotic mechanism. J. Photochem. Photobiol. B: Biol. 204, 111786 (2020).
Shyamalagowri, S. et al. In vitro anticancer activity of silver nanoparticles phyto-fabricated by Hylocereus undatus peel extracts on human liver carcinoma (HepG2) cell lines. Process Biochem. 116, 17–25 (2022).
Bolívar, A. et al. Cross contamination of Escherichia coli O157: H7 in fresh-cut leafy vegetables: Derivation of a food safety objective and other risk management metrics. Food Control 147, 109599 (2023).
Zhang, Y. et al. Combine thermal processing with polyvalent phage LPEK22 to prevent the Escherichia coli and Salmonella enterica contamination in Food. Food Res. Int. 165, 112454 (2023).
Guo, N., Bai, X., Shen, Y. & Zhang, T. Target-based screening for natural products against Staphylococcus aureus biofilms. Crit. Rev. Food Sci. Nutr. 63, 2216–2230 (2023).
Acknowledgements
The authors would like to thank Tarek Badr, Mariam Gerges, Afnan Hassan, Radwa Gamal and Ahmed Ossama.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was funded by Science and Technology Development Fund (STDF): grant #41909.
Author information
Authors and Affiliations
Contributions
A.Y.Y., A.S.A., A.G.K., Y.E. and A.E. contributed to the study design, data interpretation and the writing of the manuscript. All authors reviewed and approved the final version of the manuscript. All authors gave their consent for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yakoup, A.Y., Kamel, A.G., Elbermawy, Y. et al. Characterization, antibacterial, and cytotoxic activities of silver nanoparticles using the whole biofilm layer as a macromolecule in biosynthesis. Sci Rep 14, 364 (2024). https://doi.org/10.1038/s41598-023-50548-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50548-9
- Springer Nature Limited