Abstract
Silicon (Si) and/or proline (Pro) are natural supplements that are considered to induce plants' stress tolerance against various abiotic stresses. Sweet corn (Zea mays L. saccharata) production is severely afflicted by salinity stress. Therefore, two field tests were conducted to evaluate the potential effects of Si and/or Pro (6mM) used as seed soaking (SS) and/or foliar spray (FS) on Sweet corn plant growth and yield, physio-biochemical attributes, and antioxidant defense systems grown in a saline (EC = 7.14dS m−1) soil. The Si and/or Pro significantly increased growth and yield, photosynthetic pigments, free proline, total soluble sugars (TSS), K+/Na+ratios, relative water content (RWC), membrane stability index (MSI), α-Tocopherol (α-TOC), Ascorbate (AsA), glutathione (GSH), enzymatic antioxidants activities and other anatomical features as compared to controls. In contrast, electrolytes, such as SS and/or FS under salt stress compared to controls (SS and FS using tap water) were significantly decreased. The best results were obtained when SS was combined with FS via Si or Pro. These alterations are brought about by the exogenous application of Si and/or Pro rendering these elements potentially useful in aiding sweet corn plants to acclimate successfully to saline soil.
Similar content being viewed by others
Introduction
Salinity stress is a serious environmental problem that might affect agricultural output either directly or indirectly. Numerous plants cannot survive at low salinity because they are salt-sensitive. Low-quality irrigation water significantly increases the amount of dissolved salts in the soil, which reduces metabolism and shortens root shoots, inhibiting plant development and physiological processes1. Plant metabolism is constrained by salinity, which also lowers plants' capacity to profit from water. This affects plant growth and productivity.2. By generating more reactive oxygen species (ROS) such as O2⋅−, OH, and H2O2, which can alter plant metabolism due to osmotic stress and ion toxicity, salt also slows down plant development3,4,5,6. Reactive oxygen species are referred to be "by-products" of normal cellular metabolism when plants are subjected to salt stress and are required for the response of enzymatic inter- and intracellular coding7. Three effects of saline stress on plants include (1) a reduction in soil water content, which results in water shortages, (2) a specific adverse effect of Cl− and Na+ ions; and (3) a nutritional disproportion brought on by a reduction in the absorption of specific elements8. Ionic and osmotic stress caused by salt stress have a direct influence on plant physiology. It modifies the relationship between plants and water, which may lead to osmotic stress or drought physiology2,9. In the leaf apoplast, toxins and salt buildups encourage turgor loss and dehydration, which lead to tissue and cell death. The largest physiological impact of plant salt stress on photosynthesis is attributed to the reduction of chlorophyll pigment and stomata closure that lower CO2 pressure10,11 and inhibit rubisco12,13.
In terms of overall productivity and area planted, maize is a spectacular cereal crop that comes in second to wheat globally14. Zea mays L. saccharata, also known as sweet corn, is a hybridized type of corn that has been cultivated to contain more sugar. Around the world, sweet corn has a significant direct and indirect role in supplying humans with calories, protein, and several vitamins and minerals. Salinity has a detrimental effect on the production of maize, which is a susceptible crop to salt stress15. Therefore, in order to enhance plant growth and productivity, strategies for raising maize's tolerance to salinity must be developed16,17. The most effective method is to develop salt-tolerant maize hybrids, but doing so requires full knowledge of the mechanisms behind maize's reactions to salt stress. However, creating salt-tolerant hybrids requires a large investment in labor, time, and investment18. Consequently, certain methods, including the usage of osmoprotectant materials, can be helpful in this field19.
To boost stress tolerance, plant osmoprotectants can be added20. According to Yang et al.21 and Ihtisham et al.22, one of them, Proline (Pro), can be utilized to minimize the negative consequences of oxidative stress on plants. Plant growth is regulated by signaling molecules that start signaling cascades. Additionally adaptable is the amino acid23. Because they protect against acute ROS impacts24, plants that accumulate Pro are more resistant to the detrimental effects of stress25. It helps most plants because of its vital physics-mechanical functions. Increasing stress tolerance in plants helps to reduce the impacts of salt stress even while Si precipitation on cell walls stimulates the majority of physiological activities26,27. It lessens nutrient imbalances, lowers element toxicity, and increases photosynthetic activity28. To lessen ion toxicity, it also raises the K+/Na+ ratio and reduces Na+ uptake in plants under salt stress29. In order to sustain healthy development in the face of Na+ stress, Si may also affect Na+ transport and distribution in various plant tissues30.
In this work, exogenous silicon and/or proline were administered to maize plants under salt stress (EC = 7.14 dS m−1), and the effects on growth and yield, physio-biochemical components, and antioxidant defense mechanisms were examined. Increases in nutrient and osmoprotectant levels, as well as antioxidants' enzymatic and non-enzymatic activities that are important for reducing salt stress, are predicted to encourage plant yield and development, MSI, RWC, and leaf anatomical features when silicon and/or proline are exogenously applied, particularly seed soaking followed by a foliar spray.
Materials and methods
Experimental site and plant material
Two field tests were conducted in Yibin University's greenhouse over the course of two successive growing seasons (2021 and 2022). Before each agricultural season, soil samples from the research site were collected, as shown in Table 1, and evaluated in accordance with Black et al.31 and Jackson32. Dahnke and Whitney33 classified the soil's EC values as mildly saline. 0.1 percent HgCl2 and deionized water were used to surface sterilize the grains.
According to the best time for maize growth in the area, sweet corn (Zea mays L. saccharata) seed was sown on May 1 in both seasons. The seeds were cleansed with deionized water, rinsed with filtered water, and then sterilized for two minutes in 10 ml l−1 NaClO. They were then left to dry at room temperature (21 °C). Air-dried seeds were grown in plots 3.0 m long and 3.50 m wide, generating an area of 10.5 m2 in hills with rows 60 cm apart, after soaking in tap water (TW) and/or silicon or proline. The hills are 15–20 cm apart. The cultivation of maize in the area followed the recommended standard agronomic practices, such as drip irrigation and pest and disease control. Before sowing, 33 kg P unit of Ca (H2PO4)2 (15.5% P2O5) and 95 kg K unit of K2SO4 (48% K2O) were added to each ha. Additionally, five split applications of 300 kg N unit ((NH4)2SO4; 21% N) per ha were made seven days following seeding.
Application or silicon and/or proline
Silicon was used at a concentration of 6 mM Si (in Stable Water-Soluble Silicic Acid Potassium Salt, Potassium Silicate powder (K2SiO3) at pH 11.3; Code 1312-76-1 at Henan Daken Chemical Co., LTD, China) and proline was used at a concentration of 6 mM Pro (Pure L-Proline Powder Compliant with US Pharmacopoeia (USP) Quality Standard, NuSci Brand). Three times, at 25, 40, and 55 days following planting to run-off, silicon or proline was applied as a foliar spray (FS), with the addition of a few drops of Tween-20 as a surfactant to ensure effective and complete spray solution penetration.
Maize Morphological Traits and yield component
After 65 days, the plants were harvested. To measure plant height (cm) and other physiological traits, ten maize plants from each treatment were removed. Samples were gathered during harvest from ten randomly selected plants to determine the number of grains per row, the number of rows per ear, the grain yield (kg/ha), the bio yield (kg/ha), and the 1000-grain weight.
Determination of chlorophyll content, PSII quantum yield, and CO2 fixation rate
Avron34 eliminated all of the carotenoids and chlorophyll from the fresh leaf using only pure acetone. The net photosynthetic rate (Pn), rate of transpiration (Tr), and stomatal conductance (gs) of leaves were measured using a portable photosynthesis system (LF6400XTR, LI-COR, USA). Between nine in the morning and eleven in the morning, the tests were given.
Determinations of RWC, MSI, EL, MDA, Leaf soluble sugars, and proline
Barrs and Weatherley35 described the relative water content (RWC), whereas Premchandra et al.36 created the membrane stability index (MSI). Sullivan and Ross published a technique for calculating the total ions in leafy tissue in 1979. Electrical conductivities (EC1, EC2, and EC3) of a 20-leaf tissue disc solution were determined 3 times: before heating, during a 30 min. heating period at 45–55 °C, and after ten min. of boiling at 100 °C. EL was calculated using the following well-known formula:
Malondialdehyde (MDA; mol/g of leaf FW) concentration was evaluated to evaluate lipid peroxidation. Utilizing the same H2O2 extracts, MDA was assessed. The MDA concentration was calculated using a coefficient of molar extinction of 0.155 10−3 M−1 cm−137.
Irigoyen et al.38 procedure was used to extract and assess the total soluble sugar content. A dried leaf sample (0.2 g) was homogenized in 5 ml of 96% (v/v) ethanol before being rinsed in 5 ml of 70% (v/v) ethanol. Before measurement, centrifuging the extract at 3500g for 10 min. was necessary, and then the supernatant was put to store at 4 °C. Anthrone reagent, which is created by mixing 150 mg of anthrone with 100 ml of 72% (v/v) sulphuric acid, was added to 0.1 ml of the ethanolic extract in order to determine the amount of soluble sugar contained. After that, the mixture was cooked for 10 min in a bath of boiling water. After cooling, absorbances at 625 nm were measured using a Bauschand Lomb-2000 Spectronic Spectrophotometer (ThermoSpectronic, Mercers Row, Cambridge, UK).
The proline amounts in 0.5g dehydrated leaf specimen were determined using fast colorimetry and the Bates et al.39 technique. Following the extraction in 10 ml of 3% (v/v) sulphosalicylic acid, a 10-min. centrifugation at 10,000g was carried out. After the reaction was halted with a cold bath, the extraction was done with 5 ml of toluene and 15 s of vortex mixing. The toluene and aqueous phases were separated in the shadows for 20 min. at room temperature. The top toluene phase was carefully gathered and then the absorption at 520 nm was measured.
Determinations of nutrient content
The following concentrations of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and sodium (Na) were estimated: digested 0.2 g of dried leaf with sulphuric acid in the presence of H2O240. Total Na, Ca, and K concentrations were measured directly using a Flame photometer41. Total N was determined using a microkjeldahl method, according to Chapman and Pratt42. Total P was determined calorimetrically using the ascorbic acid method43.
Determinations of antioxidant enzyme activities
The enzymes were extracted using the technique described by Vitoria et al.44. According to Chance and Maehly45, Fielding and Hall46, Sairam et al.47, and Rao et al.48, the enzymes ascorbate peroxidase (APX), peroxidase (POD), glutathione reductase (GR), catalase (CAT), and superoxide dismutase (SOD) were assessed.
Determination of non-enzymatic antioxidant compounds and oxidative stress (hydrogen peroxide; H2O2, and superoxide; O2 ⋅‒)
Kampfenkel and Van Montagu49 used a component of 30 mM potassium phosphate buffer pH 7.4 + 2.5% TCA + 8.4% H2PO4 + 0.8% bipyridyl + 0.3% FeCl3 to assess the concentration (mol g1 FW) of ascorbate asa. The reaction was run at 40 °C for 30 min while measuring the absorbance at 525 nm. Griffth50 reported that the amounts of total and reduced glutathione (GSH) (mol g1 FW) were measured. A reaction combination including leaf extract, 130 mM sodium phosphate buffer, pH 7.4 + 7 mM sodium phosphate buffer, pH 6.8 with 6 mM DTNB, and 5, 5-dithiobis-(2-nitrobenzoic acid) was used to measure the GSH concentration. Prior to measuring the absorbance at 412 nm, the reaction mixture was kept at 30 °C for 10 min.
The tocopherol content of each gram of leaf dry weight was calculated. The extraction solvent (n-hexane–ethyl acetate, n-hexane + 0.1 L of CH3–COO–CH2–CH3) included 0.9 L and 0.02 g of butylated hydroxytoluene (BHT). R-TOC was combined with 0.05 g of n-hexane in 0.1 L to create standard solutions (0.02–0.2 mg per mL). Then, an HPLC system with a phase ratio of 94 methanol /6 water, flow rate of 1.5 mL/min, and 292 smm UV detector were used to determine the α-TOC concentration51.
By homogenizing 0.25 g of garden-fresh leaf in 5 ml of 5% TCA, the H2O2 concentration (mol per g of leaf FW) was calculated. It took 15 min to complete a homogenate centrifugation (12,000g) at 4 °C. A reaction medium with 10 mM of buffer (potassium phosphate, pH 7.0) and 1 M of KI was added after the supernatant had been collected. According to Velikova et al.52, absorbance at 390 nm was measured using a spectrophotometer and an H2O2 standard.
In order to quantify O2⋅, 100 mg of a fresh, completely inflated maize leaf was divided into 1 mm-sized pieces. The pieces were then immersed for a further hour at room temperature in a solution of 10 mM K-phosphate buffer, pH 7.8, 0.05% NBT, and 10 mM NaN3. After 15 min of heating at 85 °C, a 2 ml immersed solution was instantly cooled. A580 g-1 FW was used as the O2⋅− concentration when the optical density was calorimetrically measured at nm53.
Anatomical studies
For comparative microscopy at 50 DAS, the main stem leaflet's median area from the 2021 (the second growing season) experiment was employed. Plants from the best (Put) treatment and the control, as well as three water levels, were used. The samples were fixed following the Paolillo and Zobel54 method, which calls for the use of the FAA solution (formalin, acetic acid, and alcohol). Using double-edged razor blades, the conserved leaflet and stem were cut into pieces (5 mm long), which were then cut into thin cross sections. After that, Johansen's pigments, fast green, and safranin were accustomed to coloring the cross sections55. The samples were cleaned with ethanol and xylene methyl salicylate (1:2, v/v) before being quickly examined under a microscope and prepared for comprehensive imaging. Using a Thermo Fisher Scientific EVOS FL Cell Imaging System, excellent images were captured.
Statistical analysis
The study's data were analyzed using SPSS software, version 19.0. The significant changes between the investigated treatments were displayed using the Least Significant Difference (LSD) at P 0.05.
Statement
We confirm that the plant material was collected by Yibin University guidelines and legislation. We follow the IUCN Policy Statement on Extinction-Risk Species Research and the Convention on International Trade in Endangered Species of Wild Fauna and Flora.
Results
Growth, yield, photosynthetic pigments, and photosynthetic efficiency
All Si and/or pro treatments performed better than the control (SS in FS + TW with TW). As an example, SS in FS + TW with Si, SS in FS + TW with Pro, SS in FS + Si with TW, SS in FS + Pro with Si, and SS in FS + TW with Pro are all used as SS or FS. Indicators of yield (such as the number of grains per row and the number of rows per ear), photosynthetic pigments (such as chlorophyll a and b, carotenoids), and photosynthetic efficiency (such as transpiration rate, net photosynthetic rate, and stomatal conductance) all rose with the height of the plant. Similar patterns are seen in the data for the 2021 and 2022 growing seasons (Table 2). The best therapy in FS + Si with Si was the integrative SS, followed by Si + FS with Pro and considerably outperforming other integrative treatments in both seasons.
RWC, MSI, EL, MDA, free praline, and total soluble sugars
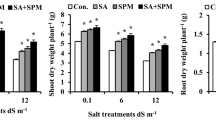
The SS in FS + TW with Si, SS in FS + TW with Pro, SS in FS + Si with TW, SS in FS + Pro with TW, SS in FS + Si with Si, and SS in Fs + Pro with Pro Treatments significantly (P ≤ 0.05) increased the RWC, MSI, free proline, and soluble sugars while significantly (P ≤ 0.05) decreased MDA and EL when compared to the SS in TW + FS with TW control treatment. The statistics show parallel patterns in the growth seasons of 2021 and 2022 (Fig. 1, Supplementary Table S1).
Seed soaking in and/or foliar spray by silicon or proline effects on relative water content (RWC), membrane stability index (MSI), electrolyte leakage (EL), malondialdehyde (MDA), proline content, and total soluble sugars content of salt-stressed maize plants in two growing seasons. Data are means (n = 5) ± SE. The same letters in each column indicate no significant differences according to the LSD test (p ≤ 0.05). TW tap water, Si silicon, Pro. proline.
When compared to other integrative treatments, the integrated Pro in Si + FS with Pretreatment and the integrated SS in FS + Si with Si generated the best outcomes.
Nutrient contents and K+/Na+ ratio
In contrast to the control (SS in TW plus FS with TW), the SS in Si or FS + Pro with Si or Pro treatment expanded the content of N, P, Ca, and K, as well as the K+/Na+ ratio. even though much decreasing Na+ content (P 0.05). Results show similar patterns for the 2021 and 2022 growing seasons (Fig. 2; Table 1), demonstrating because of the integrated SS in SS in Si + FS with Si therapy was the most successful and outperformed all other integrative therapies.
Seed soaking in and/or foliar spray by silicon or proline effects on nutrient content (i.e. N, P, K, Ca, and Na) and K+/Na+ ratio of salt-stressed maize plants in two growing seasons. Data are means (n = 5) ± SE. The same letters in each column indicate no significant differences according to the LSD test (p ≤ 0.05). TW tap water, Si silicon, Pro. proline.
Enzymes activities (CAT, POX, APX, SOD; and GR), non-enzymatic antioxidant compounds (AsA, GSH, and α-TOC), and oxidative stress markers (H2O2, and O2 ⋅−).
In contrast to the control (SS in TW plus FS with TW), every therapy of Si and/or Pro Used as SS or FS (i.e., SS in FS + TW with Si, SS in FS + TW with Pro, SS in Si + FS with TW, SS in FS + Pro with TW, SS in Si + FS with Si and SS in FS + Pro with Pro) strongly (P ≤ 0.05) raised GR, SOD, APX, POX, CAT, AsA, GSH and α-TOC while decreased H2O2 and O2⋅−.Similar patterns are seen in the data for the 2021 and 2022 growing seasons (Figs. 3, 4, Supplementary Table S2). The integrated SS in Si + FS with Si and the integrated Pro in Si + FS with Pro, both of which outperformed other integrative therapies, were the best therapies in both seasons.
Seed soaking in and/or foliar spray by silicon or proline effects on antioxidant enzymes of salt-stressed maize in two growing seasons. Data are means (n = 5) ± SE. The same letters in each column indicate no significant differences according to the LSD test (p ≤ 0.05). TW tap water, Si silicon, Pro. proline.
Seed soaking in and/or foliar spray by silicon or proline effects on hydrogen peroxide (H2O2), superoxide radical (O2⋅−), α-Tocopherol (α-TOC), Ascorbate (AsA) and glutathione (GSH)of salt-stressed maize plants in two growing seasons. The same letters in each column indicate no significant differences according to the LSD test (p ≤ 0.05). TW tap water, Si silicon, Pro proline.
Leaf anatomy
In the 2022 season, the combination of SS in Si or FS + Pro with Pro or Si therapy improved leaf structural aspects of salt-stressed maize plants vs the comparison (SS in FS + TW with TW) treatment (Table 3, Fig. 5).
Transverse sections in the flag leaf blade on the main maize stem as affected by foliar application of silicon or proline under salt stress. Tw + TW seed soaking in and foliar spray with tap water, TW + Si seed soaking in tap water and foliar spray with silicon, TW + Pro seed soaking in tap water and foliar spray with proline, Si + Tw seed soaking in silicon and foliar spray with tap water, Pro + Tw seed soaking in proline and foliar spray with tap water, Si + SI seed soaking in silicon and foliar spray with silicon, Pro + Pro seed soaking in proline and foliar spray with proline, Mid V.B. midrib vascular bund.
When compared to controls, this treatment dramatically enhanced mesophyll tissue thickness, blade thickness, midrib length, midrib breadth, midrib vascular bund length, midrib vascular bund width, and vessel average diameter.
Discussion
Salinity in the soil made it difficult for plant roots to get water, which had bad results on the water quality. High respiration rates are linked to metabolic disorders because they hurt meristematic activity and cell elongation, which hinder plant growth and productivity in salty soil56,57.
Plants produce ROS, such as superoxide (O2⋅−), H2O2, and hydroxyl radicals (OH−), in response to salinity stress3,58,59,60. Because ROS damage protein, DNA, membrane functions, and chlorophyll, they produce oxidative stress in plants61,62,63. The plants have developed a sophisticated antioxidant defense mechanism to address and mitigate the negative effects brought on by ROS64,65. Proline, carotenoids, and ascorbic acid are only a few of the low-molecular-weight compounds found in plant antioxidant systems66,67. According to the structural data available from this study, the negative effect of soil salinity produced a reduction in plant development and yield. This is attributed to osmotic pressure's impact induced by salt stress, which increases growth blockers and decreases growth stimulators68. Salinity harms photosynthesis, stomatal closure, ionic and gas exchange imbalances, toxic ion buildup, and growth suppression11,69. The current research discovered that using silicon and proline as FS and/or SS significantly enhanced salt-stressed (EC = 7.14 dS m−1) maize plant development and output (Table 2), physio-biochemical characteristics (Table 2, Fig. 1), antioxidant enzyme activities (Figs. 3, 4), and leaf anatomical features (Table 3, Fig. 5), when vs the comparison (FS + SS with TW). The integrated Si or Pro SS + FS therapy outperformed all other therapies, consisting of the control (SS in FS + TW with Si or Pro, SS in Si or Pro + FS with TW, and FS + SS with TW).
According to a prior study, the addition of Si significantly improves development characteristics under stressful conditions70. Si enhances the growth and productivity of a range of plants by increasing their ability to withstand abiotic stress71. Exogenous Si's ability to change the metabolism of cell walls by increasing tissue extensibility and enhancing cell physio-biochemical process activities was discovered to improve bean plant dry weight under various stress conditions by Alzahrani et al.26. Si may also improve leaf rigidity by roughening the texture of the leaf71. Si is essential for plant growth because of its positive effects on mechanical strength and mineral nutrition, which increases plant resilience to abiotic stressors72.
According to Tahir et al.73 and other reports74,75, exogenous Si has been shown to improve bean plant dry weight under various stress conditions because of its ability to alter cell wall metabolism by boosting cell physio-biochemical process activities and increasing tissue extensibility. Si may also cause a leaf's texture to become rougher, increasing the rigidity of the leaf72. According to earlier studies76,77, Pro increases growth and keeps nutritional equilibrium in bean plants under stress enhancing P, N, and K absorption.
Many abiotically stressed plants exhibit pro accumulation, a normal physiological response that shows the plant can tolerate the stress. It protects plants from stress by stabilizing membranes, proteins, mitochondrial electron transport complex II, and Rubisco-like enzymes77,78. While providing photoassimilates from sappy leaves, maintaining the number of leaves per plant and the amount of green leaf area boosted leaf photosynthesis and increased sink capacity. The results match those published by Thomas and Howarth79 exactly.
According to Rios et al.80, salt stress causes osmotic stress, which results in nutritional imbalance, toxicity, and oxidative damage, which hinders cellular physiological processes, including photosynthesis. Stress causes the chlorophyllase enzyme to break down chlorophylls, which significantly reduces the amount of photosynthetic pigments81.
PSII functional activity can be used to estimate a plant's ability to photosynthesize. According to the study, lower photosynthetic production occurs under salt stress due to functional and structural damage to electron carriers and photosystems. The structural integrity of PSII is harmed by prolonged exposure to high salt stress82. In contrast, Si and/or Pro increased the quantity of chlorophyll and the photosynthetic activity in maize plants that were experiencing salt stress in this research (Table 2). In research by Fadzilla and Burdon83, Si enhances leaf turgidity by holding the leaf with greater horizontality, which slows down leaf aging, increases chlorophyll content, and increases ribulose-bisphosphate carboxylase activity. Additionally, it improves leaf uprightness to enable enhanced water consumption efficiency (WUE) and leaf water potential as well as light penetration for increased photosynthesis84. Si also expands the surface area of the leaf and prevents chlorophyll degradation, allowing for more light to reach the leaves and for photosynthetic activity to take place85.
Furthermore, exogenous Si strengthens plant resilience to water stress, according to Ouzounidou et al.71. This is because Si paves the path to a steady providing assimilates to developing tissues by maintaining water for developing plant leaf chlorophyll and carotenoids. Si improves water efficiency by contributing significantly to photosynthesis in a variety of abiotic stress circumstances26. The reduction of stress-induced damage may be associated with an improvement in the photosynthetic process. According to Ming et al.86, Si addition strengthens antioxidant defenses, which lessens oxidative damage to photosynthetic enzymes.
RWC brought on by stress reduces water flow from roots to shoots, which is bad for maize plants. However, water consumption increased when Si or Pro were added to saline soil. Si or Pro increased relative water content, which matched changes in water consumption. It is possible to sustain metabolic activity by making osmotic adjustments and other adaptations to salinity and/or heavy metals when RWC in cells and tissues is in great condition87.
Salinized soil in this case reduced RWC, however Si or Pro supplementation may have reduced water stress by increasing WUE. By boosting K translocation and absorption to stomatal protection cells, where K affects the conductivity of the stomata, as well as by decreasing transpiration rate through Si or Pro deposition in leaf and stem epidermis cells, the researchers found that Si or Pro treatments increased RWC under stressful conditions88. Si or Pro can advance plant water content when exposed to salt stress, according to research showing that salt stress decreased tomato leaves' osmotic potential (making it more negative) and raised their turgor pressure89.
In this research, the RWC of maize plants that have been treated with Si-NPs is superior to that of the control plants. This result is compatible with past findings showing that adding Si or Pro nutrition completely restored RWC to non-stress levels and RWC in wheat plants was much higher than decreased in challenging circumstances73.
According to the current research, saline soil stress dramatically decreased MSI. In agreement with Merwad et al.'s70 findings, we found results that indicated stress decreased MSI. Si treatment decreased the permeability of the leaf cells' plasma membrane, resulting in improved MSI. An assistive mechanism may be provided by increased MSI after Si therapy because of increased antioxidant activity. According to Agarie et al.85, Si enhanced plant growth and production by preserving the membrane integrity and functions of plants under stress from a water shortage. Si enhanced the structural and functional integrity of cell membranes in plants under water deprivation stress, which may have reduced the structural and functional degeneration of cell membranes in stressed plants.
Another mechanism of Si for stress tolerance was suggested by Liu et al.75, who found that Si application increased water uptake and transport in sorghum seedlings under stress through the increase of root hydraulic conductance, which attributed to Si-mediated up-regulating the transcription of some aquaporin genes. Additionally, it has been proposed that Si deposition in rice leaves may limit transpiration via the cuticle to preserve plant water content under stressful circumstances, but Si application in maize drastically reduces stomatal transpiration. The mechanical/physical barrier created by Si deposition on these plant surfaces may have had a direct or indirect impact on the plants' capacity to withstand water shortages74,90. The higher plant sections were more hydrated as a result of Si treatment, which increased plants' ability to absorb and retain more water.
MDA concentration, which was used to measure lipid peroxidation, is thought to be a good biochemical indication of stress sensitivity and tolerance91. When maize seedlings were exposed to salt stress, the MDA concentration increased and was related to oxidative stress (O2− and H2O2). Electrolyte leakage (EL), membrane integrity, and cellular water content are all negatively correlated with higher MDA and oxidative stress19. These opposed qualities have a deleterious impact on metabolic activities and, as a result, plant biomass output. In stressed maize plants, the rate of photosynthesis is lowered by shutting the stomata, resulting in a drop in CO2 fixation but electron exchange and light response normally continue. Additionally, NADP's ability to absorb electrons is diminished, and oxygen has the potential to serve as an electron receptor. More ROS, such as the 1O2, O2⋅−, H2O2, and OH⋅ radicals that cause cell membrane peroxidation and increase EL, are therefore created92.
MDA levels were significantly lowered in stressed maize that had been given Si or Pro treatment. El also comprises. This undesirable outcome was addressed by the interaction of Si and Pro, which offered a superior membrane state than either Si or Pro alone. MDA, a lipid peroxidation byproduct that is found in plant tissue and is assumed to represent the impacts of stress, is reduced by Si. In accordance with the findings of Coskun et al.93, Si may therefore help to maintain cell membrane safety while lowering their permeability. The interaction between Si and Pro supports the finding in this study that Si decreases the permeability of the leaf cell plasma membrane by increasing MSI and lowering EL. This positive outcome could be attributed to notable increases in antioxidant activity, which are important defense mechanisms against a range of environmental stressors. Soylemezoglu et al.94 found that Si reduces (MDA) concentration and EL as a result of lipid peroxidation in maize and grapevine rootstock.
With or without Si or Pro treatment, the amount of pro and soluble sugars significantly increased in salty soil, acting as an osmoprotective mechanism. A non-enzymatic antioxidant called proline, which rises in concentration in response to stress strengthens plant antioxidant defenses while simultaneously making up for lost plant energy16,66. Pro is crucial for the osmotic correction of stressed plant cells95. It decreases ROS damage and raises plant tolerance by reducing the detoxification of ROS brought on by salt stress96.
Similar to this, the buildup of soluble sugars preserves the proportionality of the proline-like vacuoles and the osmotic properties of the cytosol47. A more effective strategy that aids in preserving the status of plant cell water may be the accumulation of suitable solutes under stressful environments. Pro aggregation in salt stress circumstances may be accompanied by a reduction in the rate of proline's oxidative conversion to glutamate, a rise in the proline's enzymatic production, and an increase in its utilization in protein synthesis97.
The increase in proline content in plants treated with Si or Pro compared to the control could be ascribed to the proline biosynthesis pathway being upregulated to maintain high levels26. The increase in soluble sugar concentration after Si or Pro administration suggests that these compounds have positive effects on the enzymes responsible for carbohydrate metabolism. Additionally, during osmotic stress, Si or Pro enhances the proline pool size and accumulates total free amino acids70. According to Rizwan et al.98, exogenously administered Si may enhance plant tolerance to drought stress by boosting osmolyte levels and modifying osmotic potential.
In plants growing in salty soil, Si or Pro treatment increased the accumulation of Na + ions and lowered the levels of N, P, Ca, and K ions as well as the K+/Na+ ratio. This favorable nutritional state was mostly due to silicon's role in improving food absorption. In our investigation, high salt concentrations (EC = 7.11–7.17 dS m−1) affect food intake as well as the synthesis and operation of cell membranes. The degree of membrane vector activity, which is involved in the transportation of ions from the soil to the plant and subsequently controls their distribution inside and across plant cells, is a crucial element in defining the nutritional homeostasis of plants99.
Due to the Na+ ion's antagonistic behavior towards nutrients (i.e. N, K, Ca, and P), membranes can trigger chemical changes in stressed plant cells that represent deficient symptoms in plants100. The Si or Pro application, which corrected the K+ imbalance found in cells' cytosols on the act of salt stress, positively updated the results of the present study by keeping the cytosolic K+ concentrations high and the K+/Na+ ratio in the proper range as an underlying defense against salt stress in plants, according to Rios et al.80. We discovered that treatment with Si or Pro significantly increased the maize's selectivity for nutrient translocation and absorption of N, P, K, and Ca. Supplemental Si or Pro significantly increased the N, P, K, and Ca levels in cucumber plant shoots under salt stress. Si and Pro decreased the osmotic pressure of soil solution that stressors might cause, increasing plant roots' capacity to absorb water and nutrients19.
In this research, salt stress-induced oxidative stress led to increased GR activities, SOD, APX, POX, and CAT. High enzymatic activity levels were accelerated and drove them to their peak activity by the interaction between Pro and Si to lessen cellular ROS while under the act of stress. SOD converts stress-related O2⋅− generated in plant tissues into H2O2, which is a potent oxidant because it is produced by SOD canalization. It is forbidden by the glutathione (GSH) and ascorbate (AsA) cycles. The oxidant OH− is also dangerous and extremely reactive. Without restriction, it may respond with any macromolecule. By integrating their behaviors, SOD and CAT can stop or lessen the production of OH−101,102.
Gong et al.84 assert that Si increases CAT and SOD activity. In a different experiment, wheat leaves treated with Si under salt stress exhibited much higher CAT and SOD activity. Because peroxidases (POXs) act by consuming H2O2 during physiological processes, they can regulate the levels of ROS. POXs have a higher affinity for H2O2 than CAT, but they can also help oxidize other molecules, such as NADPH, to create H2O2103. Additionally, Li et al.104 discovered that POX activity was elevated in salt-stressed Glycyrrhiza uralensis seedlings with 6 mM Si. Si also inhibits the production of ROS and increases its scavenging by antioxidants, both non-enzymatic and enzymatic80.
In the current study, salt stress led to an increase in POX activity. By successfully utilizing Si in metabolic pathways that scavenge ROS, Si can therefore improve the safety and integrity of cell membranes at the cellular level and alleviate stress-induced oxidative stress. Additionally, by adding Si and/or Pro to the leaves, maize tolerance to salt stress is boosted, increasing the activity of both enzymatic and non-enzymatic antioxidant mechanisms. In contrast to antioxidant defense systems created with individual Si or Pro application, those given by the interaction between Si and Pro improved maize growth, yield, photosynthetic efficiency, and nutritional homeostasis.
Under typical circumstances, defense mechanisms in plants, such as the buildup of antioxidant molecules, regulate the number of free radicals. To protect stressed plants from oxidative stress caused by salt, soluble sugar, proline, and antioxidant-related chemicals such phenolic, α-TOC, GSH, AsA, and enzymatic antioxidants are biosynthesized and accumulate under salt stress105. According to the results of the current study, the integrative application of Si or Pro increased the levels of AsA, α-TOC, and GSH in salt-stressed maize plants, pointing to an improved AsA-GSH cycle, which is crucial for wheat tolerance and the detoxification of ROS106.
Additionally, elevated GSH levels support the formation of complexes with phytochelatin, which aid in phytochelatin production and Na ion sequestration in the vacuole107. In the present research, Si or Pro treatment enhanced the salt-stressed maize plants' leaf morphological characteristics (Fig. 1), proving that Si or Pro therapy mitigates the negative salt stress's impact on the architecture of the leaf. In the act of challenging salt stress conditions, these enhancements in the anatomical features of the leaf made by Si or Pro enabled a decent relocation of assimilated nutrients entering cells, as well as nutrients for use in various metabolic activities, which are indicative of healthy development and acceptable production. By promoting the development of protective tissue, Si or Pro can be utilized to improve a maize leaf's resistance to dehydration.
Conclusion
The Si or Pro can be utilized as an effective natural bio-stimulant in maize plants growing under the act of salt stress (EC = 7.14 dS m−1) to increase salt tolerance. Si or Pro can be applied as seed soaking to supplement seeds with extra bioactive ingredients (such as AsA, GSH, α-TOC, mineral nutrients, and sugar) for quick and powerful germination. For seedlings to grow quickly and strongly and to promote plant salt tolerance, Si or Pro can also be applied as a foliar spray (integrative application). This can aid plants in surviving in salty soil. The application of Si or Pro, particularly as seed soaking combined with foliar spray and as the best remedy, is found to be effective in reducing salt stress in maize because of the enhanced antioxidant systems; enzymatic antioxidants and non-enzymatic (i.e. GR, APX, SOD, POD, CAT, carotenoids and free proline) and the decreased ROS; O2⋅− radicals and H2O2. The effect caused by Si or Pro is sometimes referred to in this study as the "stay green effect" since its bioactive components enhance the antioxidant defense system in plants under the act of salt stress.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
References
El-Sappah, A. H., & Rather, S. A. Genomics approaches to study abiotic stress tolerance in plants. Plant Abiotic Stress Physiol. 2, 25–46 (2022).
Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250 (2002).
El-Sappah, A. H., et al. Genome-wide identification and expression analysis of metal tolerance protein gene family in Medicago truncatula under a broad range of heavy metal stress. Front. Genet. 12, 713224. https://doi.org/10.3389/fgene.2021.713224 (2021).
El-Sappah A. H., et al. Interplay of silymarin and clove fruit extract effectively enhances cadmium stress tolerance in wheat (Triticum aestivum). Front. Plant Sci. 14(14), 1144319. https://doi.org/10.3389/fpls.2023.1144319 (2023).
El-Sappah A. H., et al. Genome-wide identification and expression analysis of metal tolerance protein (MTP) gene family in soybean (Glycine max) under heavy metal stress. Mol Biol Rep. 50(4), 2975–2990. https://doi.org/10.1007/s11033-022-08100-x (2023).
Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410 (2002).
Foyer, C. H. & Noctor, G. Oxygen processing in photosynthesis: A molecular approach. New Phytol. 146, 359–388 (2000).
Marschner, H. Mineral nutrition of higher plants, 2nd Ed., Academic Press, London ISBN 01247 35436 (1995).
Rady, M. M., Desoky, E. M., Elrys, A. S. & Boghdady, M. S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants?. S. Afr. J. Bot. 121, 294–305 (2019).
Bethke, P. C. & Drew, M. C. Stomatal and nonstomatal components to inhibition of photosynthesis in leaves of Capsicum annum during progressive exposure to NaCl salinity. Plant Physiol. 99, 219–226 (1992).
Rady, M. M. et al. E. M. Spirulina platensis extract improves the production and defenses of the common bean grown in a heavy metals-contaminated saline soil. J. Environ. Sci. 129, 240–257 (2003).
Kahrizi, S., Sedghi, M. & Sofalian, O. Effect of salt stress on proline and activity of antioxidant enzymes in ten durum wheat cultivars. Ann. Biol. Res. 3, 3870–3874 (2012).
Sitohy, M. Z., Desoky, E. M., Osman, A. & Rady, M. M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 271, 109495 (2020).
FAO. Food and Agriculture Organization of the United Nations; Statistical Database; FAO Rome (2020).
Chinnusamy, V., Jagendorf, A. & Zhu, J. K. Understanding and improving salt tolerance in plants. Crop Sci. 45, 437–448 (2005).
Desoky, E. M., Mansour, E., Yasin, M. A. T., El-Sobky, E. E. A. & Rady, M. M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2, e0802 (2020).
Shirinbayan, S., Khosravi, H. & Malakouti, M. J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 133, 138–145 (2019).
Ashraf, M. & Akram, N. A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 27, 744–752 (2009).
Rady, M. M., Elrys, A. S., Abo El-Maati, M. F. & Desoky, E. M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 139, 558–568 (2019).
Qirat, M., Shahbaz, M. & Perveen, S. Beneficial role of foliar-applied proline on carrot (Daucus carota L.) under saline conditions. Pak. J. Bot. 50(5), 1735–1744 (2018).
Yang, S. L., Lan, S. S. & Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 166, 1694–1699 (2009).
Ihtisham M, et al. Primary plant nutrients modulate the reactive oxygen species metabolism and mitigate the impact of cold stress in overseeded perennial ryegrass. Front Plant Sci. 31(14), 1149832. https://doi.org/10.3389/fpls.2023.1149832 (2023).
Yildiz, M. & Terz, H. Effect of NaCl stress on chlorophyll biosynthesis, proline, lipid peroxidation and antioxidative enzymes in leaves of salt-tolerant and salt sensitive barley cultivars. J. Agric. Sci. 19, 79–88 (2013).
Singh, M., Kumar, J., Singh, V.P., Prasad, S.M. Proline and salinity tolerance in plants. Biochem. Pharmacol. 3, 6. https://doi.org/10.4172/2167-0501.1000e170 (2014).
Aslam, M. et al. Specific role of proline against heavy metals toxicity in plants. Int. J. Pure App. Biosci. 5(6), 27–34 (2017).
Alzahrani, Y., Kuşvuran, A., Alharby, H. F., Kuşvuran, S. & Rady, M. M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 154, 187–196 (2018).
Parveen, N. & Ashraf, M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak. J. Bot. 42(3), 1675–1684 (2010).
Shi, Y. et al. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 7, 196 (2016).
Hajiboland, R., Cherghvareh, L. & Dashtebani, F. Effect of silicon supplementation on wheat plants under salt stress. J. Plant Process Funct. 5(18), 1–12 (2016).
Zhang, C.H., Wang, L., Nie, Q., Zhang, W., & Zhang, F. Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ. Exp. Bot. 62, 300–307 (2008).
Black, C. A. Soil plant relationships 2nd edn. (John Wiley and Sons, 1968).
Jackson, M. L. Soil Chemical Analysis (Prentice Hall, 1973).
Dahnke, W. C., & Whitney, D. A. Measurement of soil salinity. In Dahnke, W.C. (Ed.), Recommended Chemical Soil Test Procedures for the North Central Region, 499. North Central Regional Publication 221. North Dakota Agricultural Experiment Station Bulletin, pp. 32–34 (1988).
Avron, M. Photophosphorylation by swiss-chard chloroplasts. Biochim. Biophys. Acta BBA—Ioenerg. 40, 257–272 (1960).
Barrs, H. D. & Weatherley, P. E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 24, 519–570 (1962).
Premchandra, G. S., Saneoka, H. & Ogata, S. Cell membrane stability, an indicator of drought tolerance as affected by applied nitrogen in soybean. J. Agric. Sci. 115, 63–66 (1990).
Heath, R. L. & Packer, L. Photo peroxidation isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968).
Irigoyen, J. J., Emerich, D. W. & Sanchez-Diaz, M. Water stress induced changes in the concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Plant Physiol. 8, 455–460 (1992).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water stress studies. Plant Soil. 39, 205–207 (1973).
Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 13, 1035–1059 (1982).
Lachica, M., Aguilar, A. & Yanez, J. Analisis foliar: Métodosutilizadosen la Estacion Experimental del Zaidin. An. Edafol. Agrobiol. 32, 1033–1047 (1973).
Chapman, H. D. & Pratt, F. P. Determination of minerals by titration method: Methods of analysis for soils, plants and water 2nd edn, 169–170 (Agriculture Division, 1982).
Watanabe, F. S. & Olsen, S. R. Test of ascorbic acid method for determine phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. Proc. 29, 677–678 (1965).
Vitoria, A. P., Lea, P. J. & Azevado, R. A. Antioxidant enzymes responses to cadmium in radish tissues. Phytochem. 57, 701–710 (2001).
Chance, B. & Maehly, A. C. Assay of catalase and peroxidase. Methods Enzymol. 2, 764–775 (1955).
Fielding, J. L. & Hall, J. L. A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum. J. Expt. Bot. 29, 969–981 (1978).
Sairam, R. K., Rao, K. V. & Srivastava, G. C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163, 1037–1046 (2002).
Rao, M. V., Paliyath, G. & Ormrod, D. P. Ultraviolet-B radiation and ozone-induced biochemical changes in the antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 110, 125–136 (1996).
Kampfenkel, K. & Van Montagu, M. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 225, 165–167 (1995).
Griffth, O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2 vinyl pyridine. Anal. Biochem. 106, 207–212 (1980).
Konings, E. J., Roomans, H. H. & Beljaars, P. R. Liquid chromatographic determination of tocopherols and tocotrienols in margarine, infant foods, and vegetables. J. AOAC Int. 79(4), 902–906 (1996).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151, 59–66 (2000).
Kubis, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 165, 397–406 (2008).
Paolillo, D. J. & Zobel, R. W. The formation of adventitious roots on root axes is a widespread occurrence in field-grown dicotyledonous plants. Am. J. Bot. 89, 1361–1372 (2002).
Johansen, D. A. Plant Microtechnique (McGraw-Hill Book Company, 1940).
Abdul Qados, A. M. S. Effects of salicylic acid on growth, yield and chemical contents of pepper (Capsicum annuum L.) plants grown under salt stress conditions. Int. J. Agric. Crop Sci. 8(2), 107–113 (2015).
Kaydan, D. & Okut, M. Y. Effects of salicylic acid on the growth and some physiological characters in salt stressed wheat (Triticum aestivum L.). TarimBİlimleriDergisi 13(2), 114–119 (2007).
El-Sappah, A. H., et al. Comprehensive genome wide identification and expression analysis of MTP gene family in tomato (Solanum lycopersicum) under multiple heavy metal stress. Saudi J. Biol. Sci. 28, 6946–6956. https://doi.org/10.1016/j.sjbs.2021.07.073 (2021).
Lin, C. C. & Kao, C. H. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil 230, 135–143 (2001).
Tsai, Y. C., Hong, C. Y., Liu, L. F. & Kao, C. H. Relative importance of Na+ and Cl− in NaCl-induced antioxidant systems in roots of rice seedlings. Physiol. Plant 122, 86–94 (2004).
Abbas M, et al. Genome-wide analysis and expression profiling of the SlHsp70 gene family in Solanum lycopersicum revealed higher expression of SlHsp70–11 in roots under Cd(2+) stress. Front. Biosci. (Landmark edition) 27, 186 (2022).
Cheeseman, J. Hydrogen peroxide and plant stress: A challenging relationship. Plant Stress 1, 4–15 (2007).
El-Sappah, A. H. et al. Natural resistance of tomato plants to Tomato yellow leaf curl virus. Front. Plant Sci. 13, 1081549. https://doi.org/10.3389/fpls.2022.1081549 (2022).
Halliwell, B. & Gutteridge, M. J. C. Free radicals in biology and medicine (Oxford University Press, 2007).
El-Sappah, A. H. et al. Heat Stress-Mediated Constraints in Maize (Zea mays) Production: Challenges and Solutions. Front. Plant Sci. 13, 879366. https://doi.org/10.3389/fpls.2022.879366 (2022).
Desoky, E. M., Elrys, A. S. & Rady, M. M. Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals contaminated saline soil. Ecotoxicol. Environ. Saf. 169, 50–60 (2019).
Schutzendubel, A. & Polle, A. Plant responses to abiotic stresses: Heavy metal induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53, 1351–1365 (2002).
Desoky, et al. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 272, 109576 (2020).
El-Saadony, M. T., et al. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 106, 1–14 (2021).
Merwad, A. M. A., Desoky, E. M. & Rady, M. M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 228, 132–144 (2018).
Ouzounidou, G., Giannakoula, A., Ilias, I. & Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz. J. Bot. 39(2), 531–539 (2016).
Hamayun, M., Sohn, E. Y., Khan, S. A., Shinwari, Z. K., Khan, A. L., Lee, I. J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak. J. Bot. 42(3), 1713–1722 (2010).
Tahir, M.A., et al.. Beneficial effects of silicon in wheat (Triticum aestivum L.) under salinity stress. Pak. J. Bot. 38(5), 1715–1722 (2006).
Gao, X., Zou, C., Wang, L. & Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 29(9), 1637–1647 (2006).
Liu, P. et al. Aquaporin mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 65, 4747–4756 (2014).
Ali, Q., Ashraf, M., Shahbaz, M. & Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 40, 211–219 (2008).
Vicente, O., AlHassan, M. & Boscaiu, M. Contribution of osmolyte accumulation to abiotic stress tolerance in wild plants adapted to different stressful environments. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies (eds Iqbal, N. et al.) 13–25 (Springer, 2016).
McNeil, S. D., Nuccio, M. L. & Hanson, A. D. Betaines and related osmoprotectants: Targets for metabolic engineering of stress resistance. Plant Physiol. 120, 945–950 (1999).
Thomas, H. & Howarth, C. J. Five ways to stay green. J. Exp. Bot. 51, 329–337 (2000).
Rios, J. J., Martínez-Ballesta, M. C., Ruiz, J. M., Blasco, B. & Carvajal, M. Silicon mediated improvement in plant salinity tolerance: the role of aquaporins. Front. Plant Sci. 8, 948 (2017).
Reddy, M. P. & Vora, A. B. Changes in pigment composition, hill reaction activity and saccharide metabolism in bajra (Pennisetum typhoides S&H) leaves under NaCl salinity. Photosynthetica 20, 50–55 (1986).
Kalaji, H. M. et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 38, 102 (2016).
Fadzilla, N. M. & Burdon, R. H. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J. Exp. Bot. 48, 325–331 (1997).
Gong, H., Chen, K., Chen, G., Wang, S. & Zhang, C. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 26(5), 1055–1063 (2003).
Agarie, S. et al. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.) monitored by electrolyte leakage. Plant Prod. Sci. 1, 96–103 (1998).
Ming, D. F., Pei, Z. F., Naeem, M. S., Gong, H. J. & Zhou, W. J. Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop Sci. 198(1), 14–26 (2012).
Slabbert, M. M. & Krüger, G. H. J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 95, 123–128 (2014).
Liang, Y., Nikolic, M., Bélanger, R., Gong, H., & Song, A. Silicon in agriculture: From theory to practice p. 235 (Springer, New York, NY, USA, 2015).
Romero-Aranda, M. R., Jurado, O. & Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 163, 847–855 (2006).
Matoh, T., Murata, S. & Takahashi, E. Effect of silicate application on photosynthesis of rice plants. Jpn. J. Soil Sci. Plant Nutr. 62, 248–251 (1991).
Hernández, J. A. & Almansa, M. S. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 115, 251–257 (2002).
Alireza, Y., Aboueshaghi, R. S., Dehnavi, M. M. & Balouchi, H. Effect of micronutrients foliar application on grain qualitative characteristics and some physiological traits of bean (Phaseolus vulgaris L.) under drought stress. Indian J. Fund. Appl. Life Sci. 4, 124–131 (2014).
Coskun, D., Britto, D. T., Huynh, W. Q. & Kronzucker, H. J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 7, 1072 (2016).
Soylemezoglu, G., Demir, K., Inal, A., & Gunes, A. Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci. Hortic. 123(2), 240–246 (2009).
Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 6, 66–71 (2001).
Howladar, S. M. A novel Moringa oleifera leaf extract can mitigate the stress effect of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 100, 69–75 (2014).
Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 168, 241–248 (2005).
Rizwan, M. et al. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Poll. Res. 22(20), 15416–15431 (2015).
Epstein, E., & Bloom, A. J. Mineral nutrition of plants, principles and perspectives. 2nd Edn. Sinauer Associates, Sunderland, MA ISBN 97808 78931e 729 (2005).
Grattan, S. R. & Grieve, C. M. Mineral nutrient acquisition and response of plants grown in saline environments. In Handbook of Plant and Crop Stress (ed. Pessarakli, M.) 203–229 (Marcel Dekker Press Inc, 1999).
Kusvuran, S., & Kiran, S. Antioxidant enzyme activities and abiotic stress tolerance relationship in vegetable crops. In Shanker, A. K. (Ed.), Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives. Intech, Chitra Shanker, pp. 481–506 (Chapter 21) (2016).
Li, J. et al. Zinc nanoparticles (ZnNPs): High-fidelity amelioration in Turnip (Brassica rapa L.) production under drought stress. Sustainability. 15(8), 6512. https://doi.org/10.3390/su15086512 (2023).
Ranieri, A. et al. Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol. Biochem. 43(1), 45–54 (2005).
Li, Y. T. et al. Silicon nutrition alleviates the lipid peroxidation and ion imbalance of Glycyrrhiza uralensis seedlings under salt stress. Acta Physiol. Plant. 38, 96–105 (2016).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 (2010).
Hasanuzzaman, M. et al. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8(9), 384 (2019).
Elrys, A. S. et al. Mitigate nitrate contamination in potato tubers and increase nitrogen recovery by combining dicyandiamide, moringa oil and zeolite with nitrogen fertilizer. Ecotoxicol. Environ. Saf. 209, 111839 (2022).
Funding
This work is supported by the Science and Technology Planning Project of Sichuan Province (NO. 2023NZZJ0010).
Author information
Authors and Affiliations
Contributions
Conceptualization: E.-S.D., J.L., M.R., C.G. and A.E.-S. Methodology: E.-S.D. Software: E.-S.D., C.G., and A.E.-S. Validation: R.S.M.E., E.-S.D. and C.G. Formal analysis, E.-S.D., C.G., L.W., and A.E.-S. Investigation: E.-S.D., A.E.-S., and M.M. Resources: E.-S.D., C.G., J.L. Writing—original draft preparation: E.-S.D. Writing—review, and editing: C.G., J.L., K.Y., M.R., H.H.E., Q.H., M.S., W.T., E.M.Y.A., M.I., E.-S.D. and A.E.-S. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gou, C., Huang, Q., Rady, M.M. et al. Integrative application of silicon and/or proline improves Sweet corn (Zea mays L. saccharata) production and antioxidant defense system under salt stress condition. Sci Rep 13, 18315 (2023). https://doi.org/10.1038/s41598-023-45003-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45003-8
- Springer Nature Limited