Abstract
Salicylic acid (SA) and spermine (SPM) elicit particular responses in response to various environmental stressors. However, there is little known about the underlying mechanism of their combination treatments' mediating effect on salt stress tolerance. In this investigation, the potential impact of 100 mg L−1 SA and/or 30 mg L−1 SPM in avoiding salt damage at saline environments of 6.0 and 12.0 dS m–1 in wheat was examined. Results showed that by increasing mineral acquisition, chlorophyll a and b contents, antioxidant enzymes activity, osmolytes accumulation, leaf water content, grains carbohydrate and protein content as well as reducing Na+ accumulation, membrane electrolyte leakage, malondialdehyde and hydrogen peroxide contents, exogenously applied SA and/or SPM significantly reduced the detrimental impacts of soil salinization and increased wheat growth and productivity. The best outcomes came from combining the SA and SPM treatments. Overall, this combined treatment enhanced mineral homeostasis, osmolytes accumulation, and antioxidant response, pointing to a potential role for it in minimizing the negative consequences of salt stress. Therefore, combining SA and SPM in a sustainable agricultural system can be viewed as a successful technique for reducing salt damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinization is among the most widespread ecological concerns that significantly harms agriculture (Talaat and Shawky 2012; Morton et al. 2019). Globally, around 950 million hectares of cultivated land are impacted (Zörb et al. 2019). Osmotic stress, particular ionic effects, and nutritional imbalances are all results of a complex abiotic stress. Additionally, it results in an excess of reactive oxygen species (ROS) burst that cause cellular oxidative stress (Moghaieb et al. 2011; Talaat 2019; Hasanuzzaman et al. 2020). To protect themselves, plants either increase the production of osmolytes, antioxidants, and phytohormones, or they decrease the entry of salt ions into the cytoplasm (Talaat 2015; Es-sbihi et al. 2021; Shamili et al. 2021). Osmolyte production is essential for maintaining osmotic potential, metabolic activity, and water uptake under saline circumstances (Rady et al. 2019; Talaat and Shawky 2022). Antioxidant enzymes also aid plants in preventing excessive ROS generation (Shamili et al. 2021; Shopova et al. 2021; Talaat et al. 2022a). Moreover, ionic balance within the plant cell is essential for salt tolerance because excess salt ions in the cytoplasm disrupt ion homeostasis and impair plant development (Talaat 2019; Es-sbihi et al. 2021).
Salicylic acid (SA) is crucial for improving plant stress resistance (Sharma et al. 2020; Talaat and Shawky 2022). By controlling a number of physiological activities like antioxidant capacity, radical scavenging activity, cellular signaling, photosynthetic reactions, cellular respiration, and mineral absorption, exogenously applied SA enhances plant salt tolerance (Bukhat et al. 2020; Hoang et al. 2020; Kamran et al. 2020; Kaya et al. 2020; Shamili et al. 2021; Talaat 2021; Talaat and Shawky 2022; Talaat and Todorova 2022). Despite this, little is known about the main routes via which SA mediates plant salt tolerance.
Spermine (SPM) is a tetraamine that contributes to plant resistance to abiotic stress (Igarashi and Kashiwagi 2019; Talaat 2020). When applied exogenously, SPM can act as a potent plant defense activator (Todorova et al. 2016; Seifi and Shelp 2019; Talaat et al. 2022a). By scavenging ROS radicals, defending the structure and operation of the photosynthetic apparatus, maintaining cationic–anionic stability, lowering ethylene production, increasing protein content, modifying endogenous phytohormone levels, and inducing the accumulation of organic solutes, exogenously applied SPM lessens the detrimental impacts of salinity on plant development (Nahar et al. 2016; Ahangera et al. 2019; Islam et al. 2020; Xu et al. 2020; Geng et al. 2021). Nevertheless, its potential functions under saline conditions are not entirely known.
Around the world, wheat is a significant grain crop. For a sizable section of the world's population, it offers essential sustenance. Its production is reduced by salt stress, endangering the safety of the world's food supply (Talaat 2019). The most cost-effective tactic is considered to be improving its ability to survive in saline environments. Foliage application of biostimulants like SA and SPM is gaining popularity as new approaches for enhancing plant resistance to salt toxicity. Due to the lack of knowledge regarding the influence of combining SA and SPM on wheat salt tolerance, the goal of the current study was to ascertain if SA and/or SPM may aid wheat plants in withstanding in saline soils by measuring growth, productivity, chlorophyll a and b concentrations, nutrient acquisition, osmoprotector solutes accumulation, antioxidant enzymes activity, grains carbohydrate and protein content, electrolyte leakage, leaf water content, as well as malondialdehyde and hydrogen peroxide contents. The results will advance knowledge of the function of SA and/or SPM on ameliorating salt toxicity.
Materials and methods
Plant material and experimental design
A pot experiment was carried out in the greenhouse of Cairo University's Faculty of Agriculture's Plant Physiology Department. The experiment was repeated twice, on September 10th, 2019 and 2020. Wheat (Triticum aestivum L. cv. Shandawel 1) grains were kindly supplied by the Wheat Research Department of the Agriculture's Research Center. The pots were 30 cm in diameter and 35 cm in height and contained 15 kg of clay loamy soil (sand 37%, silt 28%, clay 35%). NPK fertilization was performed in accordance with Ministry of Agriculture recommendations. Table 1 displays the results of the soil chemical analysis carried out according to the procedures of Cottenie et al. (1982). Each pot contained ten grains, which were later thinned to six after germination.
Before sowing, pots were divided into three groups. The first group served as a control (non-saline; 0.1 dS m–1) and the other two as two salinity treatments (6.0 and 12.0 dS m–1). To create saline environments, a mixture of NaCl, CaCl2, and MgSO4 in the molar ratio 2:2:1, respectively, was added to the soil. According to reference crop evapotranspiration (ET0) values, pots were irrigated with tap water at the proper intervals. There were no excessive water losses below the root zone since the pots were closed at the bottom.
According to preliminary investigations, the most effective concentrations were 100 mg L−1 SA and 30 mg L−1 SPM out of a possible range of concentrations of 0 to 150 mg L−1 for SA and 0 to 50 mg L−1 for SPM. When the plants were 50 days old (vegetative stage) and 100 days old (grain filling stage), they were sprayed with 0.00 (distilled water; DW), 100 mg L−1 SA, 30 mg L−1 SPM, and 100 mg L−1 SA + 30 mg L−1 SPM. Salicylic acid and spermine were purchased from Sigma (USA) and dissolved properly in ethanol. Tween-20 (0.05%) was utilized as a surfactant at the time of treatment.
The experimental design was completely randomized and included two factors: three salt treatments [0.1 dS m–1 (non-saline), 6.0 and 12.0 dS m–1], and four foliage applications [0.00 (distilled water; DW), 100 mgL−1 SA, 30 mgL−1 SPM, and 100 mgL−1 SA + 30 mgL−1 SPM]. There were four replicates of each treatment. The plants were sampled after 75 days of sowing to assess the total leaf area (using a portable leaf area meter LI-COR 3000, Lambda Instruments Corporation, Lincoln, Nebraska, USA), and dry weights for the shoots and roots. After maturation, grain number and grain yield were estimated. Data were collected from four replicates, and each replicate includes six plants gathered from the same pot.
The following physiological and biochemical traits were determined in 75-day-old (after 25 days of SA and/or SPM first applications) wheat leaves. Data were collected from four replicates, each of which contained six plants gathered from the same pot.
Chlorophyll estimation
Fresh leaves were used to extract chlorophyll a and chlorophyll b, which were then spectrophotometrically measured using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan) in accordance with Lichtenthaler and Buschmann's (2001) instructions.
Determination of mineral element concentrations
A transparent solution was produced after digesting 0.5 g of dried grains in a mixture of boiling perchloric acid and hydrogen peroxide for 8 h. In line with Pregl (1945), the modified micro-Kjeldahl method was used to determine nitrogen concentration. The vanadomolybdophosphoric technique was used to estimate the concentration of phosphorus (Kacar and Inal 2008). A flame photometer (ELE UK) was used to measure the amount of sodium and potassium. An atomic-absorption spectrophotometer (Unicam 989-AA Spectrometer-UK) was used to determine the concentrations of calcium, magnesium, iron, zinc, and copper.
Estimation of compatible solutes buildup
According to Irigoyen et al. (1992), Moore and Stein (1954), and Grieve and Grattan (1983), respectively, the amounts of total soluble sugars, total free amino acids, and glycinebetaine were measured in dried ground leaves. The procedure described by Bates et al. (1973) was used to estimate the proline concentration in samples of fresh leaves.
Leaf water content and electrolyte leakage estimation
To assess the relative water content, the process outlined by Hayat et al. (2007) was used. According to Li et al. (2015), an electrical conductivity meter was used to measure electrolyte leakage.
Hydrogen peroxide (H2O2) and malondialdehyde (MDA) measurement
To estimate H2O2 and MDA, 0.1 g of fresh wheat leaves were ground with 900 L buffer in a mortar, according to the instructions in the H2O2 and MDA kits, using the approach outlined by Nawaz et al (2018). The contents of H2O2 and MDA were measured at 405 nm and 532 nm, respectively.
Extraction and measurement of antioxidant-defense enzymes
Fresh wheat leaves (0.5 g) were homogenized in 5 mL of ice-cold 100 mM phosphate buffer (pH 7.4) with 1% polyvinylpyrrolidine and 1 mM EDTA before centrifugation at 15,000×g for 10 min at 25 °C. The supernatant was gathered and used to assess the activity of the antioxidant enzymes. Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured at 560 nm by measuring the reduction of nitroblue tetrazolium, a marker for the generation of superoxide anion (Becana et al. 1986). At 240 nm, the activity of catalase (CAT, EC 1.11.1.6) was measured according to the method of Chance and Maehly (1955). The activity of peroxidase (POD, EC 1.11.1.7) was measured at 470 nm with guaiacol as a substrate (Hemeda and Klein 1990).
Estimation of carbohydrate and protein content of grains
Methods of Yih and Clark (1965) and Lowry et al. (1951) were used to determine the carbohydrate and protein content of dry ground wheat grains, respectively.
Statistical evaluation of data
Four replicates of each treatment were employed in a completely randomized design. A combined analysis was performed since the results of the two growth seasons had a similar tendency. SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for a two-way analysis of variance (ANOVA). At a significance level of p < 0.05, the Duncan test was performed to analyze differences between the treatments. The data are displayed as means ± standard error (SE).
Results
Foliage applications of SA and/or SPM reduce the negative effects of salinity on wheat growth and yield
Salt stress inhibited wheat development, resulting in significant decreases in leaf area, shoot and root dry weights, grain yield, and grains number. In contrast, SA and/or SPM foliar sprays greatly mitigated such reductions when compared to the untreated salt-stressed plants (Figs. 1, 2, 3, 4 and Table 2). The best outcomes came from combining the SA and SPM treatments. Combination treatment significantly (p < 0.05) increased the leaf area (42.2% and 55.0%), shoot dry weight (36.4% and 49.8%), root dry weight (38.0% and 50.0%), grains number (49.6% and 60.0%), and grain yield (47.5% and 59.5%) as compared to measurements of untreated ones at salinity treatments of 6.0 and 12.0 dS m–1, respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the growth attributes of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the grains of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
SA and/or SPM applications enhance chlorophyll a and b concentrations in salt-stressed plants
Chlorophyll a/b ratio increased while chlorophyll a and b concentrations were significantly reduced by soil salinization. However, foliage applications of SA and/or SPM counteracted the negative effects of saline environments and boosted the chlorophylls level in both the absence and presence of salt stress (Fig. 5). Co-application of SA and SPM had the greatest ameliorative effect. It raised the concentration of chlorophyll a (26.0% and 62.8%) and chlorophyll b (48.9% and 114.9%) when compared to untreated plants at salinity treatments of 6.0 and 12.0 dS m−1, respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the chlorophylls content in leaves of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Spraying of SA and/or SPM regulate mineral acquisition in salt-stressed plants
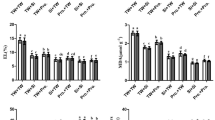
The N, P, K+, Fe, Zn, and Cu levels were negatively impacted by salinity, and this effect was most prominent at high salinity level. Conversely, raising the salt doses in the soil resulted in noticeably higher Na+, Ca2+, and Mg+2 concentrations. Applications of exogenous SA and/or SPM lessened the harmful effects of saline environments on mineral acquisition, which was associated with an increase in N, P, K+, Fe, Zn, Cu acquisition and a decrease in Na+ accumulation (Fig. 6). The best results came from combining SA and SPM, which increased the concentrations of N (34.9% and 53.9%), P (42.1% and 53.3%), K+ (50.0% and 60.0%), Fe (35.8% and 47.6%), Zn (34.5% and 47.8%), and Cu (28.0% and 37.5%) as well as reduced the concentration of Na+ (22.0% and 31.3%) in grains of wheat plants subjected to salinity treatments of 6.0 and 12.0 dS m−1, respectively, in comparison to untreated plants.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the nutrients concentration in grains of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
SA and/or SPM treatments improve organic solutes accumulation under salt stress
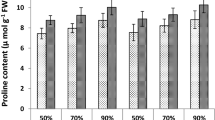
Exogenous SA and/or SAM treatments as well as salinity treatments caused a rapid increase in the accumulation of organic solutes (Fig. 7). The greatest values were recorded in plants that had been sprayed with SA and SPM and were under saline environments. In comparison to unsprayed plants, the combination treatment under saline treatments of 6.0 and 12.0 dS m−1 increased the concentration of total soluble sugars (14.9% and 17.3%), total free amino acids (12.5% and 20.3%), proline (21.2% and 32.2%), and glycinebetaine (32.0% and 44.0%), respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the organic solutes concentration in leaves of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Foliar applications of SA and/or SPM improve water content in salt-stressed plants
Under saline conditions, leaf water content was significantly decreased. However, exogenous SA and/or SPM applications dramatically reduced this reduction (Fig. 8). In comparison to unsprayed plants, combined treatment under saline treatments of 6.0 and 12.0 dS m−1 significantly (p < 0.05) raised RWC values by 36.0% and 59.0%, respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the relative water content (%) in leaves of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Foliage applications of SA and/or SPM enhance the antioxidant response in plants grown in salty environments
Plants grown in salty soils had much greater amount of H2O2 and activity of antioxidant enzymes than the unstressed ones. On the contrary, SA and/or SPM foliar applications lessen the toxicity of salt stress by reducing H2O2 generation and boosting antioxidant enzymes activity (Fig. 9). The combination application enhanced the activity of SOD (35.9% and 44.4%), CAT (22.5% and 51.8%), and POD (50.0% and 100.0%) as well as reduced the content of H2O2 (25.2% and 39.7%) at saline treatments of 6.0 and 12.0 dS m−1, respectively, in comparison to untreated plants.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the H2O2 content and the superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) activity in leaves of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Foliage applications of SA and/or SPM maintain the integrity of cell membrane under salt stress
The MDA and EL values were greatly elevated in the leaves of plants stressed by salt, but they were significantly reduced after the salt-stressed plants were sprayed with SA and/or SPM (Fig. 10). The combination of SA and SPM had the greatest positive effect. In comparison to unsprayed plants, combined treatment under saline treatments of 6.0 and 12.0 dS m−1 dramatically decreased EL by 17.5% and 25.7% and MDA content by 22.7% and 40.8%, respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the lipid peroxidation and electrolyte leakage (%) in leaves of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Spraying of SA and/or SPM enhance grains carbohydrate and protein content under saline conditions
Compared to unstressed plants, salt-stressed plants' grains contained substantially less amount of carbohydrate and protein. On the contrary, SA and/or SPM treatments significantly (p < 0.05) improved their contents in both saline and non-saline circumstances (Fig. 11). The SA and SPM combined treatment produced the greatest impact. When compared to untreated plants, it increased the content of protein (34.9% and 53.9%) and carbohydrate (31.4% and 48.2%) at salinity treatments of 6.0 and 12.0 dS m−1, respectively.
Effect of salicylic acid (SA, 100 mg L−1) and/or spermine (SPM, 30 mg L−1) foliage applications on the carbohydrate and protein content in grains of wheat plants exposed to various salt treatments. The results are mean of four replicates (n = 4) and the bars represent the standard error (± SE). Asterisks denote the significant difference among treatments (p < 0.05), according to Duncan test
Discussion
By interfering with a number of processes, including photosynthesis, protein synthesis, antioxidant response, osmolyte buildup, and ion homeostasis, saline circumstances restricts plant development (Talaat 2019; Hasanuzzaman et al. 2020; Talaat and Shawky 2022). Exogenous application of SA or SPM has been demonstrated to improve plant salt stress resistance (Ahangera et al. 2019; Xu et al. 2020; Shamili et al. 2021; Talaat 2021; Talaat and Todorova 2022). However, current knowledge of the processes underlying how their combination treatment affected salt tolerance is limited. Therefore, in the current study, we looked at how exogenously applied SA and/or SPM affected nutritional homeostasis, the production of osmoprotectants, and ROS detoxification in wheat grown in salty environments. Our findings clearly show that combining SA and SPM treatment may lessen the negative impacts of saline circumstances on wheat development by enhancing mineral uptake, encouraging osmolyte buildup, and regulating antioxidant response.
Soil salinization has detrimental impacts on plant development, reducing crop yields (Kaya et al. 2020; Talaat and Shawky 2022; Talaat et al. 2022b). Our results revealed that salt stress significantly (p < 0.05) hindered wheat development (Figs. 1, 2, 3, 4) by reducing the chlorophyll a and b content (Fig. 5), essential nutrient availability (Fig. 6), water content (Fig. 8), membrane stability (Fig. 10), and the protein and carbohydrate content of grains (Fig. 11). However, in agreement with previous studies (Nahar et al. 2016; Fariduddin et al. 2018; Ahangera et al. 2019; Bukhat et al. 2020; Xu et al. 2020; Es-sbihi et al. 2021; Talaat 2021), exogenous SA and/or SPM treatments counteracted the inhibition caused by salt treatments on wheat growth and production (Figs. 1, 2, 3, 4). Our findings clearly demonstrate that the application of SA and SPM can efficiently contribute to wheat stress tolerance by enhancing chlorophyll concentration (Fig. 5), maintaining optimal mineral nutrition (Fig. 6), increasing osmoprotectant accumulation (Fig. 7), improving tissue water content (Fig. 8), bolstering antioxidant response (Fig. 9), and protecting cell membrane integrity (Fig. 10), which subsequently improve adequate food synthesis, storage, and translocation (Fig. 11). This study's findings lead us to draw the conclusion that SA and SPM may function as potential regulators to increase wheat growth and development under saline conditions by promoting osmolytes accumulation, modifying antioxidant response, and enhancing nutritional intake (Fig. 12).
Chlorophyll content is an important factor controlling plant growth and photosynthetic effectiveness (Talaat 2019). Reduced chlorophyll concentration caused growth inhibition in salt-stressed plants (Singh and Gautam 2013). In this investigation, we displayed that saline treatments drastically reduced the chlorophyll a and b levels (Fig. 5). This change in chlorophylls content may be caused by excessive H2O2 generation (Fig. 9). Previous studies have demonstrated that in response to salt stress, the thylakoid and cell membranes can build up more ROS, resulting in lipid peroxidation and a change in membrane permeability (Talaat 2019; Hasanuzzaman et al. 2020). However, as shown in Fig. 5, exogenous SA and/or SPM treatments were able to stop the decline in chlorophylls content. Evidences suggest that SA and SPM may boost the performance of enzymes participated in the manufacture of chlorophyll or reduce the malfunction of the photosynthetic system, hence reducing chlorophyll breakdown (Nahar et al. 2016; Fariduddin et al. 2018; Ahangera et al. 2019; Bukhat et al. 2020; Kaya et al. 2020; Islam et al. 2020; Es-sbihi et al. 2021). According to our data, exogenous SA and SPM were effective in boosting chlorophylls level and reducing stress-related damage in saline conditions.
In a salty soil, ion homeostasis is crucial for plant survival (Talaat 2019; Hasanuzzaman et al. 2020). Due to the low water content, excessive salinity interferes with selective ion absorption, which affects the nutrient accessibility (Geng et al. 2021; Talaat and Shawky 2022). In the present study, SA and/or SPM foliar sprays significantly elevated the acquisition of essential elements in wheat grains and minimizing the negative consequences of salt treatments (Fig. 6). This could be because treated plants have the ability to improve mineral nutrition uptake, accumulation, and translocation while reducing ions leakage by retaining membrane properties (Fig. 10). Reduction of Na+ uptake and modulation of ion uptake in stressed SA- or SPM-treated plants were also reported by Nahar et al. (2016), Ahangera et al. (2019), Kaya et al. (2020), Xu et al. (2020), and Geng et al. (2021). By promoting H+-ATPase activity, SA has been demonstrated to be important in ion transport (Inada et al. 2005; Talaat and Shawky 2022). SA can also prevent salt-induced K+ leakage (Gharbi et al. 2018). Thus, effective nutrient uptake in salt-stressed treated plants could be contributed to (i) maintaining cell membrane integrity, (ii) minimizing ions leakage, and (iii) selective ions uptake.
Plant cells accumulate suitable osmolytes to combat the deleterious effects of salinity (Kaya et al. 2020). In this investigation, we found that SA and/or SPM contributed to the osmoprotectants buildup (Fig. 7). The plants' coping mechanisms under salty environments are responsible for this accumulation. Several studies have shown that organic solutes production is influenced by the application of SA and SPM (Fariduddin et al. 2018; Ahangera et al. 2019; Kaya et al. 2020; Talaat 2020, 2021). According to earlier studies, SA causes the building of glycinebetaine by inhibiting the generation of ethylene (Khan et al. 2014) and causes the production of proline by boosting the activity of its biosynthesis enzymes (Khan et al. 2015). Our results imply that inducing organic solute accumulation via SA and SPM could be a plant's way of dealing with salt stress.
Soil salinization can also lower the potential of soil water, affecting plant root water absorption (Hasanuzzaman et al. 2020). Our findings clearly demonstrated that salt-stressed plants sprayed with SA and/or SPM had greater RWC levels than unsprayed ones (Fig. 8). This result can be attributed to SA and SPM's beneficial effect on the accumulation of organic solutes (Fig. 7). Our findings are in close agreement with Nahar et al. (2016), Ahangera et al. (2019), Kaya et al. (2020), and Xu et al. (2020). Considering our prior findings, the impact of SA and/or SPM on boosting growth (Figs. 1–4) could be attributed to increasing tissue water content by causing osmolytes buildup.
Overproduction and buildup of ROS, which result in oxidative damage, can be brought by salt stress (Talaat et al. 2022b). In this study, wheat leaves under salt stress produced an excessive amount of H2O2 (Fig. 9). However, when compared to untreated plants, wheat plants treated with SA and/or SPM had a lower H2O2 level. By promoting the antioxidant enzymes activity, plants may really defend their tissues from ROS damage (Talaat 2019; Hasanuzzaman et al. 2020; Shopova et al. 2021). In this investigation, we demonstrated that spraying SA and/or SPM on the salt-stressed plants boosted the antioxidant enzymes activity (Fig. 9). This observation is corroborated by other studies, which state that SA is crucial in altering the performance of antioxidant enzymes, giving cells a better chance of surviving damage brought by salinity (Bukhat et al., 2020; Kaya et al., 2020; Xu et al. 2020; Shamili et al. 2021). Additionally, studies have proven that in response to environmental challenges, SPM promotes the efficiency of antioxidant enzymes (Igarashi and Kashiwagi 2019; Talaat et al. 2022a). It is fair to assume that SA and SPM increase wheat's ability to withstand salt, possibly by making antioxidant enzymes more active.
In our study, we found that excessive H2O2 level may be to blame for salt-induced membrane damage because salt stress-induced H2O2 buildup was consistent with an increase in MDA and EL (Fig. 10). Foliage applications of SA and/or SPM, on the other hand, effectively decreased the elevated H2O2 level and protected cell membranes from oxidative damage. Due to their unique properties, SA and SPM can prevent membrane oxidation damage by acting directly as antioxidants to scavenge H2O2 and/or indirectly by activating antioxidant enzymes. These results support those made by Antonic et al. (2016), who showed that SA strengthens plant antioxidant defense and protects cell membranes from lipid peroxidation. Additionally, it has been noted that SPM interacts with negatively charged membranes to stabilize them in the face of extreme oxidative stress (Seifi and Shelp 2019). In the current study, stressed treated plants also accumulated a significant amount of proline and glycinebetaine (Fig. 7). This finding suggests that proline and glycinebetaine accumulations, which facilitate cellular osmotic adjustment, may also protect membrane integrity, and consequently ameliorate membrane damage brought by salt stress.
Considerable decreases in grain protein and carbohydrate content resulted from saline conditions (Fig. 11). Conversely, in both non-saline and saline circumstances, plants subjected to the combined treatment had a significant rise in their content (Fig. 11), which was accompanied by increases in photosynthetic pigment content (Fig. 5), nutrient acquisition (Fig. 6), and antioxidant response (Figs. 9 and 10). Our findings imply that SA and SPM can be used to increase grain protein and carbohydrate content, which may aid wheat plants in maintaining yield and grain nutritional quality under salt stress. Our approach revealed that the SA and SPM combination treatment enhances wheat plant productivity in both non-saline and saline environments, making it a promising candidate for crop protection and enhancement.
Conclusions
Our results revealed that SA applied in conjunction with SPM seems to be a potential candidate for enhancing wheat production in saline environments. This combination treatment increased nutrient absorption effectiveness, encouraged osmolytes accumulation, and modulated antioxidant response in plants cultivated in salty soils. Through the application of SA and SPM, this work helps to build a useful technique for improving wheat's tolerance to salt stress.
Author contribution statement
NBT: conceptualized and coordinated the research, conceived the idea, designed and carried out the experiments, generated and analyzed the data, and wrote the manuscript. AWMM: contributed to measuring few parameters. AMAH: carried out the experiments and generated the data. All the authors read and approved the final manuscript.
Change history
03 December 2022
Communicated by update.
References
Ahangera MA, Qin C, Dong QM, Dong XX, Ahmad P, Abd-Allah EF, Zhang LX (2019) Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol Biochem 144:1–13
Antonic D, Milosevic S, Cingel A, Lojic M, Trifunovic-Momcilov M, Petric M, Subotic A, Simonovic A (2016) Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S Afr J Bot 105:226–233
Bates LS, Aldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Becana M, Aparicio-Tejo P, Irigoyan JJ, Sanchez-Diaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Bukhat S, Manzoor H, Athar H, Zafar ZU, Azeem F, Rasul S (2020) Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J Plant Growth Regul 39:809–822
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. Laboratory of analytical and agrochemistry. State University, Ghent, pp 14–24
Es-sbihi FZ, Hazzoumi Z, Aasfar A, Joutei KA (2021) Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem Biol Technol Agric 8:25
Fariduddin Q, Khan TA, Yusuf M, Aafaqee ST, Khalil RRAE (2018) Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica 56(3):750–762
Geng W, Qiu Y, Peng Y, Zhang Y, Li Z (2021) Water and oxidative homeostasis, Na+/K+ transport, and stress-defensive proteins associated with spermine-induced salt tolerance in creeping bentgrass. Environ Exp Bot 192:104659
Gharbi E, Lutts S, Dailly H, Quinet M (2018) Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanum lycopersicum) responses to salinity. Plant Signal Behav 13(5):e1469361
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Hemeda HM, Klein B (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hoang HL, de Guzman CC, Cadiz NM, Hoang TTH, Tran DH, Rehman H (2020) Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). J Soil Sci Plant Nutr 20:1759–1769
Igarashi K, Kashiwagi K (2019) The functional role of polyamines in eukaryotic cells. Int J Biochem Cell Biol 107:104e15
Inada M, Ueda A, Shi W, Takabe T (2005) A stress-inducible plasma membrane protein 3 (AcPMP3) in a monocotyledonous halophyte, Aneurolepidium chinense, regulates cellular Na+ and K+ accumulation under salt stress. Planta 220:395–402
Irigoyen JJ, Emerich DW, Sanchez-Dıaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Islam MA, Jin-huan P, Fan-wei M, Ya-wen L, Ning X, Chao Y, Jun L (2020) Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J Integr Agric 19(3):643–655
Kacar B, Inal A (2008) Plant analysis. Nobel publication No: 1241. Appl Sci 63:879
Kamran M, Xie K, Sun J, Wang D, Shi C, Lu Y, Gu W, Xu P (2020) Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol Environ Saf 188:09877
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol Biochem 147:10–20
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mung bean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462–494
Li X, Ahammed GJ, Zhang YQ, Zhang GQ, Sun ZH, Zhou J, Zhou YH, Xia XJ, Yu JQ, Shi K (2015) Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol 17:81–89
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protocols in Food Anal Chem 1:31–38
Lowry OH, Rosenbrough NJ, Aarr AL, Randaal RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Moghaieb REA, Abdel-Hadi AA, Talaat NB (2011) Molecular markers associated with salt tolerance in Egyptian wheats. Afr J Biotechnol 10(79):18092–18103
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Morton JL, Awila M, Al-Tamimi N, Saade S, Pailles Y, Negrão S, Tester M (2019) Salt stress under the scalpel-dissecting the genetics of salt tolerance. Plant J 97:148–163
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamines confer salt tolerance in mung bean by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense and methylgyoxal detoxification systems. Front Plant Sci 7:1104
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtia M, Bie Z, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon on seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127
Pregl F (1945) Quantitative organic micro analysis, 4th edn. A. Churchill Ltd., London
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky EM (2019) Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J Hortic Sci Biotech 94:777–789
Seifi HS, Shelp BJ (2019) Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front Plant Sci 10:117
Shamili M, Ghalati RE, Samari F (2021) The impact of foliar salicylic acid in salt-exposed guava (Psidium Guajava L.) seedlings. Int J Fruit Sci 21:323–333
Sharma A, Sidhu GPS, Araniti F, Bali AS, Shahzad B, Tripathi DK, Brestic M, Skalicky M, Landi M (2020) The role of salicylic acid in plants exposed to heavy metals. Molecules 25:78
Shopova E, Katerova Z, Brankova L, Dimitrova L, Sergiev I, Todorova D, Talaat NB (2021) Modulation of physiological stress response of Triticum aestivum L. to glyphosate by brassinosteroid application. Life 11:1156
Singh PK, Gautam S (2013) Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol Plant 35:2345–2353
Talaat NB (2015) Effective microorganisms improve growth performance and modulate the ROS-scavenging system in common bean (Phaseolus vulgaris L.) plants exposed to salinity stress. J Plant Growth Regul 34:35–46
Talaat NB (2019) Role of reactive oxygen species signaling in plant growth and development. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Wiley, New York, pp 225–266
Talaat NB (2020) 24-Epibrassinolide and spermine combined treatment sustains maize (Zea mays L.) drought-tolerance by improving photosynthetic efficiency and altering phytohormones profile. J Soil Sci Plant Nutr 20:516–529
Talaat NB (2021) Polyamine and nitrogen metabolism regulation by melatonin and salicylic acid combined treatment as a repressor for salt toxicity in wheat (Triticum aestivum L.) plants. Plant Growth Regul 95:315–329
Talaat NB, Shawky BT (2012) Influence of arbuscular mycorrhizae on root colonization, growth and productivity of two wheat cultivars under salt stress. Arch Agron Soil Sci 58:85–100
Talaat NB, Shawky BT (2022) Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L.) plants by enhancing root H+-pump activity. Plants 11:416
Talaat NB, Todorova D (2022) Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J Soil Sci Plant Nutr 22:3527–3540
Talaat NB, Ibrahim AS, Shawky BT (2022a) Enhancement of the expression of ZmBZR1 and ZmBES1 regulatory genes and antioxidant defense genes triggers water stress mitigation in maize (Zea mays L.) plants treated with 24-epibrassinolide in combination with spermine. Agronomy 12:2517
Talaat NB, Mostafa AA, El-Rahman SNA (2022b) A novel plant growth-promoting agent mitigates salt toxicity in barley (Hordeum vulgare L.) by activating photosynthetic, antioxidant defense, and methylglyoxal detoxification machineries. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00993-8
Todorova D, Talaat NB, Katerova Z, Alexieva V, Shawky BT (2016) Polyamines and brassinosteroids in drought stress responses and tolerance in plants. In: Ahmad P (ed) Water stress and crop plants: A sustainable approach, vol 2. Wiley, NewYork, pp 608–627
Xu J, Yang J, Xu Z, Zhao D, Hu X (2020) Exogenous spermine-induced expression of SlSPMS gene improves salinity–alkalinity stress tolerance by regulating the antioxidant enzyme system and ion homeostasis in tomato. Plant Physiol Biochem 157:79–92
Yih RY, Clark HE (1965) Carbohydrate and protein content of boron deficient tomato root tips in relation to anatomy and growth. Plant Physiol 40:312–315
Zörb C, Geilffus CM, Dietz KJ (2019) Salinity and crop yield. Plant Biol 21:31–38
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talaat, N.B., Mahmoud, A.W.M. & Hanafy, A.M.A. Co-application of salicylic acid and spermine alleviates salt stress toxicity in wheat: growth, nutrient acquisition, osmolytes accumulation, and antioxidant response. Acta Physiol Plant 45, 1 (2023). https://doi.org/10.1007/s11738-022-03485-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03485-5