Abstract
Integrative taxonomy of Diamesus Hope, 1840 (Coleoptera: Silphinae) is presented. Adults of D. bimaculatus Portevin, 1914 (endemic to Taiwan) and D. osculans (Vigors, 1825) (widely distributed from northern India to Australia) are redescribed, keyed and figured, including characters of the male and female genitalia of both species. Variation in elytral maculation in D. osculans is discussed and illustrated. The absence of diagnostic differences of D. osculans var. reductus Pic, 1917 from D. osculans is discussed, and the former name is confirmed as a junior subjective synonym of D. osculans. Types of all three names available were studied; a lectotype and paralectotypes are designated for the name D. osculans var. bimaculatus Portevin, 1914. Molecular phylogenetic analysis confirms the genus Diamesus is sister group to the genus Necrodes Leach, 1815, and D. osculans and D. bimaculatus are two, well supported clades. Detailed data on the distribution of D. bimaculatus and D. osculans are presented and mapped. Species distribution models for both species were created and interpreted. Diamesus osculans is reported for the first time from India: Uttarakhand, China: Anhui, Hainan, Hunan, Jiangxi, Shaanxi and Zhejiang Provinces, and Australia: Victoria; it is also recently confirmed from Taiwan, being sympatric in distribution there with D. bimaculatus. Available data on the ecology and seasonality of both species of Diamesus are also discussed.
Similar content being viewed by others
Introduction
The subfamily Silphinae contains beetles with wide range of ecological and food strategies, which probably originated from necrophagy1. The necrophagous species belonging to Silphinae became the focus of many ecological studies in recent years, and as a result, it was found that different species react differently to the same stimuli. For example, values of lower developmental threshold (LDT) and accumulated degree days (ADD) can differ between species of the same genus2,3,4. Therefore, correct species delimitation is extremely important for ecology and many other fields of science including forensic entomology, which use LDT and ADD for predictions such as post-mortem interval (PMImin) or time of colonization (ToC)4. In this paper, we delimit the species of the genus Diamesus Hope, 1840, which can be further used for ToC prediction. Vigors5 described from “Indiâ Orientali” the species Necrodes osculans, and discussed its seemingly transitional evolutionary position between two genera of carrion beetles, Necrodes Leach, 1815 and Nicrophorus Fabricius, 1775. Hope6 created a separate genus Diamesus for this species. Portevin7 described a series of specimens from Taiwan: Kosempo with only bimaculate elytra as D. osculans var. bimaculatus. Later, Portevin8 elevated this variety to species status, based on its reduced elytral maculation and its shortened median crest of the pronotum and scutellum in comparison with D. osculans. Pic9 described a single specimen from Sumatra with reduced red elytral maculation as D. osculans var. reductus. Portevin10 provided a detailed redescription of both D. bimaculatus and D. osculans. Arnett11 also included a description and illustration of the female genitalia of D. osculans, and mentioned the virtual absence of differentiating characters between D. osculans and D. bimaculatus other than the reduced maculation in the latter. Peck12 reviewed the carrion beetles of Australia, and mapped the distribution of D. osculans in the Australian Region. King et al.1 studied the phylogenetic placement of Diamesus as sister to Necrodes Leach, 1815, and published sequences for Australian carrion beetles including D. osculans. Růžička and Schneider13 and Růžička14 reviewed the general distribution of both species of Diamesus through the Palaearctic Region. Zhang et al.15 published a complete mitochondrial genome of D. osculans.
Carrion beetles were traditionally classified as a separate family Silphidae, closely related to the megadiverse family Staphylinidae16,17. Recent molecular phylogenetic studies placed carrion beetles as an internal lineague within Staphylinidae18,19,20. Most recently, Cai et al.21 formally downgraded carrion beetles as a subfamily of Staphylinidae. This is also followed by Newton22. Traditionally, within Silphidae, two subfamilies were recognised, Nicrophorinae and Silphinae, the latter sometimes further divided into two tribes, Necrodini and Silphini17. Consequently, to the changes proposed by Cai et al.21, these lineagues should be downgraded to two tribes, Nicrophorini and Silphini, the latter with two subtribes, Necrodina and Silphina. This classification is followed further in the text.
Diamesus and Necrodes (the latter with three species, two Palaearctic and one Nearctic in distribution) were traditionally classified in the subtribe Necrodina, with other Silphini in Silphina Portevin10. This classification was followed by Peck12 and King et al.1. However, a preliminary phylogenetic analysis of Dobler and Müller23, which was later corroborated by King et al.1 showed that the Silphina is paraphyletic with respect to Necrodina. Also, Newton and Thayer24 listed Necrodina as a synonym of Silphini. This classification is followed in most recent papers, including Sikes17,25, Růžička14 and Newton22.
The aim of this paper is to employ an integrative taxonomy approach for delimitation of the species of Diamesus. Classical morphology is applied to redescribe adults of both species of Diamesus, focusing on details of external morphology (including characters of male and female genitalia), to demonstrate the validity of both taxa, which were mostly differentiated based on elytral colour pattern. We infer the phylogenetic placement of Diamesus within the subfamily Silphinae and compare differences between both species of Diamesus, based on three concatenated genes—two mitochondrial (COI, protein coding; 16S rDNA, ribosomal) and one nuclear (28S, ribosomal DNA). We also summarize and provide maps of the detailed distributions of both species as well as species distribution models based on the occurrence data (Figs. 1, 2).
Phylogenetic tree of Silphinae based on the topology calculated by Bayesian interference. Numbers next to branches show the posterior probability and bootstrap values of maximum likelihood (ML)/maximal parsimony (MP)/Bayesian interference (BI). Hadesia asamo Perreau & Pavićević, 2008 (Leiodidae) was selected as outgroup (* = 100, − < 50).
Results
Diamesus Hope, 1840 and Necrodes Leach, 1815 are two genera similar in appearance1. Adults of both can be characterized by a combination of the following characters: (1) body distinctly flattened dorsally, subtrapezoidal in shape in dorsal view, expanding posteriorly; (2) eyes large, prominent laterally; (3) pronotal postcoxal lobe narrow and prolonged posteriorly in lateral view; and (4) trochanter in large males flattened, extended or with the mesofemora in large males distinctly expanded, with small spine or spines along posterior apical portion10. The two genera can be separated using the following identification key:
-
1.
Pronotum subconical, widest before posterior angles. Antenna black, with ultimate antennomere orange (Fig. 5b). Body large, body length 22–49 mm (Eastern Palaearctic, Oriental to Australian). … Diamesus Hope, 1840
-
2.
Pronotum orbicular, widest toward middle. Antenna completely black, or with the last three antennomeres orange (Fig. 5a). Body generally smaller, body length 14–30 mm (Holarctic). … Necrodes Leach, 1815
Diamesus Hope, 1840
(Figs. 3a–m, 4a,c–n, 5b,c–e,g–h,j–k,m–r, 6a–j, 7a–d)
Diamesus bimaculatus Portevin, 1914, habitus of adults: (a–c) lectotype of Diamesus osculans var. bimaculatus, large male, dorsal, ventral, and lateral view; (d) paralectotype, small male, dorsal view; (e) paralectotype, female, dorsal view. D. osculans (Vigors, 1825), habitus of adults: (f–h) large male (Laos: Phu Pane Mt.), dorsal, ventral, and lateral view; (i) female (Laos: Nam Ha), dorsal view; (j) small male (Malaysia: Batu 25–26), dorsal view; (k) holotype of Necrodes osculans, small male, dorsal view. Labels: (l) holotype of Necrodes osculans; (m) lectotype of D. osculans var. bimaculatus.
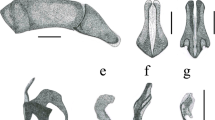
Morphological details of Diamesus Hope, 1840 and Necrodes Leach, 1815. Hind wings: (a) D. osculans (Vigors, 1825) (Vietnam: Alona forest), female; (b) N. littoralis (Linnaeus, 1758) (Czech Republic: Ladná env.), female. Diamesus osculans, head: (c) dorsal view; (d) ventral view. Variation in elytral maculation, dorsal view, schematized: (e–k) D. osculans; (l) D. bimaculatus Portevin, 1914. Female genitalia in dorsal view, D. osculans (Laos: 51 km N Sekong), SEM: (m) tergum 10, distal gonocoxite and gonostylus, arrangement of setae; (n) detail of gonostylus. Abbreviations: c clypeus, dg distal gonocoxite, ds digitiform setae, g gula, gs gonostylus, la labrum, ls long setae, mt mentum, ss small setae, t10 tergum 10.
Morphological details of Diamesus Hope, 1840 and Necrodes Leach, 1815. Right antenna in dorsal view: (a) N. littoralis (Linnaeus, 1758) (Czech Republic: Praha-Háje); (b) D. osculans (Vigors, 1825) (Laos: Phu Pane Mt.). Male mesotarsus in dorsolateral view: (c) D. osculans (Laos: Phu Pane Mt.). Male metatibia in outer lateral view: (d) D. osculans (Indonesia: Tanah Labang env.), large male; (e) D. osculans (Indonesia: Mt. Talagaranu), medium male; (f) N. littoralis (Czech Republic: Praha-Háje), large male. Male mesotibia: (g) D. osculans (Indonesia: Mt. Talagaranu), outer lateral view, medium male; (h) the same, inner lateral view; (i) N. littoralis (Czech Republic: Praha-Háje), outer lateral view, large male. Male metafemur in outer lateral view: (j) D. osculans (Indonesia: Tanah Labang env.), large male; (k) D. osculans (Indonesia: Mt. Talagaranu), medium male; (l) N. littoralis (Czech Republic: Praha-Háje), large male. Pronotum of D. osculans in dorsal view, detail of surface: (m) large male (Laos: Phu Pane Mt.); (n) small male (Malaysia: Batu 25–26); (o) large female (Laos: Nam Ha). Elytra of D. osculans in dorsal view, detail of surface: (p) large male (Malaysia: Batu 25–26); (q) large female (Laos: Nam Ha). Detail of elytral apex and abdominal tergites 3 and 4 of D. osculans in postero-dorsal view: (r) large female (Laos: Nam Ha). Abbreviations: t3 tergite 3, t4 tergite 4.
Male genitalia of Diamesus Hope, 1840: (a–c) D. bimaculatus Portevin, 1914 (Taiwan: Baling), aedeagus in dorsal, lateral and ventral view; (d–e) D. bimaculatus (Taiwan: Baling), male segments 9–10 in dorsal and ventral view; (f–h) D. osculans (Vigors, 1825) (Indonesia: Mt. Talagaranu), aedeagus in dorsal, lateral and ventral view; (i–j) D. osculans (Indonesia: Mt. Talagaranu), male segments 9–10 in dorsal and ventral view. Abbreviations: ml median lobe, pa paramere, sg spiculum gastrale, t9 tergite 9, t10 tergite 10, v9 ventrite 9.
Female genitalia of Diamesus Hope, 1840: (a–b) D. bimaculatus Portevin, 1914 (Taiwan: Baling), dorsal and ventral view; (c–d) D. osculans (Vigors, 1825) (Indonesia: Mt. Talagaranu), dorsal and ventral view. Abbreviations: ap apodeme, dg distal gonocoxite, gs gonostylus, pg proximal gonocoxite, t8 tergite 8, t9 tergite 9, t10 tergite 10, v8 ventrite 8, vs ventral sclerite.
Diamesus Hope, 1840: 149 (description by indication, type species Necrodes osculans Vigors, 1825, by original designation).
Diamesus Hope: Kraatz 1876: 355 (redescription).
Diamesus Kraatz: Portevin 1926: 167 (redescription).
Diagnostic description
Body large, robust, dorsolaterally flattened. Body length very variable, between 22 and 49 mm (48 specimens measured), generally considered to be the largest species of Silphinae15.
Colouration
Body brown to dark brown/black (pale brown in teneral specimens), with orange ultimate antennomere and orange spots or bands of various extent on elytra (Fig. 3a–k), two pairs of orange spots on tergum 3 and 4 (Fig. 5r) are usually covered by elytra, exposed probably only during flight.
Head
Clypeus with regularly rounded anterior margin (Fig. 4c, c). Antenna inserted in front of eyes, with elongate depression going to mid-length of eyes (Fig. 4c). Labrum anteriorly with wide medial emargination (Fig. 4c, la). Eyes large, prominent (Fig. 4c,d). Ventrally, head with large, flat, heavily sclerotized mentum, subtrapezoid in shape (Fig. 4d, mt), and narrow, hour-glass shaped gula (Fig. 4d, g).
Antenna
With 11 antennomeres, scapus large, long as three subsequent antennomeres (Fig. 5b). Antennal club loosely formed, antennomeres 6–8 transverse, 9–11 more robust; antennomeres 1–8 glossy, 9–11 with opaque surface; antenna black, antennomeres 9–10 sometimes greyish, only ultimate antennomere contrastingly coloured, orange (Fig. 5b).
Pronotum
Subconical, narrow anteriad and regularly extending posteriad, suddenly narrowing posteriorly (Fig. 3f); surface weakly, regularly vaulted; posterolaterally sometimes with a shallow, transverse pair of depressions. Anterior half sometimes in males with loosely indicated carina along medial line (Fig. 3a,d). Surface with distinct granulation in large males (Fig. 5m), or with coarse punctation in small males and females, very fine and superficial medially (Fig. 5n–o). Anterior margin bordered by dense, long, orange setation (Fig. 4c).
Scutellum
Very large (ca. 0.5 as wide as pronotum), triangular, with longitudinal, elevated keel along medial line, better indicated in males (Fig. 3i).
Elytra
Apically truncate, usually exposing abdominal tergites 5–8 in rest position (Fig. 3a,d–f,i–k). Outer costa raised, two inner costae flat (Figs. 3f, 5r). Surface finely granulate in large males (Fig. 5p) or coarsely punctate in small males and in females (Fig. 5p,q).
Hind wings
Well developed, ca. 1.7 times as long as elytra. Veins MP3+4 and MP3 more elongate than in Necrodes (Fig. 4a,b). Anal field broader, vein AP3+4 more elongated than in Necrodes (Fig. 4a,b).
Legs
Generally large and robust (Fig. 3a–k). Protibia straight, with two combs formed by large setae along inner and outer lateral margin. Protarsi with expanded tarsomeres 1–4 in males (Fig. 3a–d,f–h,j,k), unmodified in females (Fig. 3e,i). Mesotibia dorsoventrally flattened in basal part, suddenly expanded apically (Fig. 5g,h). External lateral surface of mesotibia with a longitudinal carina of small denticles (Fig. 5g), internal lateral surface with a comb of long setae along its length, and another transverse comb apically (Fig. 5h). Dorsal surface in apical part granulated, granules combined with robust, short setae (Fig. 5g,h). Large males with mesotarsomeres 1–4 expanded, flattened, with long, orange lateral and ventral setation (Figs. 3b,g, 5c), almost unmodified in smaller males and in females (Fig. 3d,e,i–k). Metatrochanter of large males flattened, apically broadened (Fig. 5j), in smaller males only with a small lateral, subapical denticle (Fig. 5k), in females unmodified. Metafemora greatly expanded in large males (Figs. 3a–b,f–g, 5j), less expanded in medium-sized males (Fig. 5k), unmodified in small males and females (Fig. 3d–e,i–k). Inner margin of metafemora with a single, subapical denticle in large and medium-sized males (Fig. 5j–k), unmodified, simple in small males and in all females. Metatibia expanded apically and distinctly bent in large males (Fig. 5d); only slightly expanded, straight, with proximal inner denticle in medium-sized males (Fig. 5e); unmodified, straight, in small males and in all females (Fig. 3d–e,i–k).
Abdomen
Dorsally, with distinctly developed lateral lobes, especially in large males (Fig. 3a,f). Tergum 3 and 4 each with a pair of orange spots (Fig. 5r). Ventrally, medially with a path of long, erected, orange to brown setae (Fig. 3b,g). Surface finely granulate in large males, or coarsely punctate in small males and in females.
Male genitalia
Tergum 9 separated into two separate, lateral sclerites, which are largely desclerotized on inner portion in dorsal view (Fig. 6d,i, t9). Basal part ventrally prolonged into long, anteriorly facing, V-shaped spiculum gastrale (Fig. 6e,j, sg). Ventrite 9 small, triangular, lateral parts at base covered by tergite 9 in ventral view (Fig. 6e,j, v9). Tergite 10 large, oval, narrowing basally and widely emarginate medially on posterior margin (Fig. 6d,i, t10). Aedeagus with short, broad, dorsoventrally slightly flattened median lobe (Fig. 6a–c,f–h, ml). Median lobe unsclerotized medially (in different extent, completely or only basally) in dorsal view (Fig. 6a,f). Apex of median lobe narrowly or more broadly desclerotized laterally in ventral view (Fig. 6c,h). Paramere robust, slightly longer than median lobe, apex slightly expanded (Fig. 6a–c,f–h, pa).
Female genitalia
Tergite 8 not or only weakly emarginate anteriorly; and very widely, distinctly emarginate medially on posterior margin (Fig. 7a,b, t8). Tergum 9 separate into two lateral sclerites (Fig. 7a–d, t9). Ventrally, tergite 9 with more sclerotized, almost longitudinal apodeme (Fig. 7b,d, ap). Tergite 10 oval, heart-shaped, laterally weakly sclerotized or partly unsclerotized, emarginate posteriorly in dorsal view (Fig. 7a,b, t10). Paired proximal gonocoxites developed mostly ventrally (Fig. 7a–d, pg), medially separated by narrow, longitudinal ventral sclerite (Fig. 7b,d, vs). Distal gonocoxites expanded into lateral lobes, with its posterior part flattened, desclerotized, bearing long setae on ventral margin and also centrally, and numerous short, digitiform setae (Fig. 4m, dg). Gonostylus with apical part expanded into elliptic, asymmetrical plate with extended external part (Figs. 4m, gs, 7a–d, gs). Its posterior part bears oval, desclerotized sensory field. Arrangement of setae consists of an outer circle of small, pointed setae (Fig. 4n, ss); numerous digitiform setae covering the sensory field (Fig. 4n, ds), and several long setae, located in the middle (Fig. 4n, ls).
Variability and sexual dimorphism
Extremely variable species in body size and proportions. Large males with granulate surface (Fig. 5m,p), small males and all females with coarse punctures on surface (Fig. 5n,o,q). Large males also with more developed lateral lobes on abdomen (Fig. 3a,f). Large males with expanded, flattened mesotarsomeres bearing long, yellow setation (Fig. 5c) and with extremely expanded metafemora (Figs. 3a–b,f–g, 5j); medium-sized males with less expanded metafemora (Fig. 5k); small males and all females with unmodified mesotarsomeres and simple metafemora. Also, metatibia is expanded and bent in large males (Fig. 5d), slightly expanded but straight in medium-sized males (Fig. 5e) and unmodified in small males and in all females (Fig. 3d,e,i–k).
Morphometry
We found significant differences between pronotum width to length ratio for males and females of D. bimaculatus (t value = 2.555, p value = 0.0144). Partial significance was found in elytra width to length ratio at species level (t value = 1.798, p value = 0.0796), but not between males and females (t value = 0.18, p value = 0.8578). For body length and scutellum length to width ratio we did not find any statistically significant difference at species level (t value = 0.74, p value = 0.463; t value = − 0.31, p value = 0.758 respectively) nor sex (t value = 0.603, p value = 0.55; t value = − 0.198, p value = 0.844 respectively). Therefore, our results do not support the observation of Portevin10 that the two species can be differentiated based on scutellum length to width ratio. For a graphical summary of the morphometric analysis see SM4 (a–d).
Taxonomy
Hope6 published the name Diamesus in a table, and labelled “Nec.[rodes] osculans, Vigors”, the only included species, as “typical species”. Although this is the only included species name in Diamesus, according to Article 68.2 of the Code, Necrodes osculans was designated as the type species of Diamesus by original designation, not by monotypy26. As Hope6 provided no formal description of Diamesus, Kraatz27 provided its formal description. This also led Portevin10 to the conclusion that Diamesus Hope, 1840 is a nomen nudum, and he attributed this generic name to Kraatz. However, for a new genus-group name published before 1931, its publication in combination with available specific name is treated as an ‘indication’ and makes Diamesus Hope, 1840 available, according to Article 12.2.5 of the Code26.
Phylogenetic analysis
In total, 37 specimens were used for the phylogenetic analysis, including outgroup (Table 1). Concatenated sequences resulted into an alignment of a total length of 1702 bp (COI 727 bp, 16S 451 bp, and 28S 524 bp). The monophyly of the two tribes within the subfamily Silphinae, Silphini (ML: Bootstrap value 94/MP: Bootstrap value 100/BI: Posterior probability 100) and Nicrophorini was supported (100/100/100) (Fig. 1). The two genera forming the sister clade to remaining members of the of the tribe Silphini—Ptomaphila Kirby & Spence, 1828 and Oxelytrum Gistel, 1848—were strongly supported (99/94/100) as well as the well-established genera Thanatophilus Leach, 1815 and Oiceoptoma Leach, 1815 (Fig. 1). The focal genus of this study—Diamesus—was represented by 3 individuals of D. bimaculatus (all from Taiwan) and 7 individuals of D. osculans (sampled throughout Asia and Australia, incl. Taiwan). The clade consisting of these two species was strongly supported (100/100/100) and the two species were reciprocally monophyletic with strong support (both 100/100/100). The support of the lineage containing both sister genera, Necrodes and Diamesus, was 59/54/94 (Fig. 1).
Estimation of the divergence time
An alignment of 36 specimens was used for the estimation of the divergence time. Using three nodal priors resulted in an estimated median age of 81.5 mya for the clade consisting of the genera Necrodes and Diamesus (95% mean posterior interval age estimation of 62.6–103.0 mya) and estimated median age of 40.3 mya for the two species of Diamesus—Diamesus osculans and Diamesus bimaculatus (95% mean posterior interval age estimation of 27.0–56.1 mya).
Species distribution models
We used 40 observations of D. bimaculatus and 513 observations of D. osculans to model their distribution. Both models of D. bimaculatus and D. osculans showed high scores of area under curve (AUC) values (0.9887 and 0.8797 respectively). The maps were reclassified to binomial values (predicted presence/absence) using maximum training sensitivity plus specificity Cloglog thresholds, which were 0.2357 and 0.4005 respectively (see Fig. 8a,b). The maps of predicted presence behaved well in the case of D. bimaculatus and presence was not predicted outside of Taiwan (Fig. 8d). However, in the case of D. osculans (Fig. 8c), the model seemed to behave conservatively for some parts of China and Australia, where some localities were not considered probable by the model. Although, in other regions (e.g., Japan, New Caledonia) the model predicted the presence of this species was possible despite the separation of these regions from the rest of the distribution by large water masses and lack of specimen evidence.
Distribution of Diamesus bimaculatus Portevin, 1914 and D. osculans (Vigors, 1825). (a) general distribution; (b) detailed distribution of both species in Taiwan, produced by ESRI ArcMap 10.8.1 of ArcGIS Desktop 10.8.1 suite (https://www.esri.com/en-us/arcgis). Species distribution models of D. osculans (c) and D. bimaculatus (d) were created using MaxEnt v.3.4.4, https://biodiversityinformatics.amnh.org/open_source/maxent/.
Key to adults of Diamesus Hope, 1840
-
1.
Body dark brown to black (pale brown in teneral specimens), each elytron only with a single small orange spot near apex (Figs. 3a–e, 4l). Male: tergite 10 subtrapezoidal, weakly and widely emarginate on posterior margin (Fig. 6d, t10). Median lobe of aedeagus shorter, sclerotized parts in dorsal view 1.4 times as long as wide; in dorsal view broadly unsclerotized medially (Fig. 6a). Female: tergite 10 more elongate, 1.3 times as long as wide posteriorly (Fig. 7a, t10). (Endemic to Taiwan, Fig. 8a,b). … D. bimaculatus Portevin, 1914
-
2.
Body brown (pale brown in teneral specimens), each elytron with two orange bands, variable in size and shape, bands sometimes split into 2–3 isolated spots (Figs. 3f–k, 4e–k). Male: tergite 10 oval, distinctly narrower in anterior part, deeply and narrowly emarginate on posterior margin (Fig. 6i, t10). Median lobe of aedeagus longer, sclerotized parts in dorsal view 1.6 times as long as wide; in dorsal view narrowly unsclerotized medially only in anterior part, sclerotization medially fused in central part (Fig. 6f). Female: tergite 10 less elongate, only 1.1 times as long as wide posteriorly (Fig. 7c, t10). (Widely distributed from India to Australia, incl. Taiwan, Fig. 8a,b). … D. osculans (Vigors, 1825)
Diamesus bimaculatus Portevin, 1914
(Figs. 3a–e,m, 4l, 6a–e, 7a–b, 8a–b)
Diamesus osculans var. bimaculatus Portevin, 1914: 6 (description, type locality: Kosempo).
Diamesus bimaculatus: Portevin 1922: 3 (elevated to species).
Type material examined
LT ♂ (SDEI) (here designated), labelled (Fig. 3m) “Kosempo [= Chiahsien or Jiaxian, Kaohsiung hsien county, ca. 23°05′N 120°35′E]/Formosa [= Taiwan]/H. Sauter [leg.] [p] VII [19]11 [hw]//Syntypus [p, red label]//Diamesus/osculans/v. bimaculatus/mihi [hw, Portevin’s MS]//LECTOTYPE ♂/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”; PLT 12 ♂♂, 5 ♀♀ (SDEI), “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//Syntypus [p, red label]//Portevin det. [hw]//PARALECTOTYPE ♂ [or ♀]/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”; PLT 1 ♀ (SDEI), labelled “Portevin det. [p]//Syntypus [p, red label]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”; PLT 1 ♀ (SDEI), labelled “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//Syntypus [p, red label]//Diamesus/osculans Vig./v. bimaculatus mihi [hw, Portevin’s MS]//Portevin det. [hw]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 2 ♂♂ (MNHN), labelled “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//TYPE [p, red label]//Muséum Paris/Coll. M. Pic [p]//bimaculatus/var. tibialis/m. [hw, Portevin’s MS, manuscript name, ony under first ♂]//PARALECTOTYPE ♂/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 1 ♀ (MNHN), labelled “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//Muséum Paris [p]/G. Portevin 1920 [hw]//TYPE [p, red characters on white label]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 1 ♀ (NMNH), labelled “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//PARATYPUS [p, red label]//Diamesus/osculans var/bimaculatus Port [hw]//Loan from/USNMNH/2026393 [p]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 1 ♀ (NMNH), labelled “Kosempo/Formosa/H. Sauter [leg.] [p] VII [19]11 [hw]//COTYPUS [p, red label]//Carl F Baker/Collection [p]//Diamesus os-/culans v. bima-/culatus Port. [hw]//Diamesus/osculans Vigors/v. bimaculatus/Port. [hw, red label with black frame]//Loan from/USNMNH/2026393 [p]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 1 ♀ (NMNH), without locality label, labelled only “COTYPUS [p, red label]//Carl F Baker/Collection [p]//Loan from/USNMNH/2026393 [p]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”;
PLT 1 ♀ (BMNH), labelled: “Co-/type [p, round label with yellow margin]//PARATYPUS [p, pink label]//Formosa./H. Sauter. [leg.]/Brit. Mus./1923-61. [p]//Kosempo/Formosa/H. Sauter [leg.] [p] VII. [19]11 [hw]//Diamesus/osculans/bimacula-/tus Portev. [hw, Portevin’s MS?]//PARALECTOTYPE ♀/Diamesus/osculans var. bimaculatus/Portevin, 1914/Jan Růžička design. 2021 [p, red label]//Diamesus/bimaculatus/Portevin, 1914/Jan Růžička det. 2021 [p]”.
Description
Measurements
Body length: male 27–44 mm (median 40 mm), female 33–38 mm (median 37 mm). Pronotum width to length ratio: male 1.19–1.35 (median 1.27), female 1.18–1.28 (median 1.20). Scutellum length to width ratio: male 1.02–1.29 (median 1.19), female 1.09–1.24 (median 1.16). Elytra width to length ratio: male 1.15–1.27 (median 1.22), female 1.15–1.29 (median 1.20).
External morphology
Body dark brown to black (pale brown in teneral specimens), each elytron only with a single small orange spot near apex (Figs. 3a–e, 4l).
Male genitalia
Ventrite 9 triangular, extending into slender posterior apex (Fig. 6e, v9). Tergite 10 subtrapezoidal, weakly and widely emarginate on posterior margin (Fig. 6d, t10). Median lobe shorter, sclerotized parts in dorsal view 1.4 times as long as wide. Median lobe in dorsal view broadly unsclerotized medially (Fig. 6a). Apex of median lobe widely sclerotized along median line (Fig. 6c).
Female genitalia. Tergite 8 with weak emargination anteriorly and distinct, deep emargination posteriorly (Fig. 7a, t8). Tergite 10 more elongate, 1.3 times as long as wide posteriorly (Fig. 7a, t10).
Variability
Body length varies between 27 and 44 mm. Elytral colouration uniform (Fig. 3a–e).
Bionomics
Necrophagous species, also frequently collected using UV and mercury-vapour light traps (see SM1). Label data indicate that adults have been collected at elevations from 260 to 1600 m (SM1). Seasonality: available data confirm occurrence in majority of the year (except February, November, and December), with a peak in July, declining until September (Table 2).
References on distribution
Taiwan: 六亀 [= Liugui District, ca. 23°00′N 120°38′E] (Nishikawa 2014)28; Kosempo [= Chiahsien or Jiaxian, Kaohsiung hsien county, ca. 23°05'N 120°35'E] (Portevin 1914)7; 日月澤 [= 日月潭 (Riyuedan), ca. 23°51′N 120°54′E] (Nishikawa 2014)28; Hori [= Puli, ca. 23°58′N 120°58′E] (Nishikawa 2014)28; Wushe [霧社, ca. 24°02′N 121°07′E] (Nishikawa 2014)28; ムシヤ [霧社 (Wushe), ca. 24°02′N 121°07′E]) (Nishikawa 2014)28; Taihoku [= Taipei, ca. 25°04′N 121°31′E] (Miwa 1931)29.
Distribution. Endemic species to Taiwan (Fig. 8a–b).
Diamesus osculans (Vigors, 1825)
(Figs. 3f–l, 4a,c–k,m–n, 5b–e,g,h,j,k,m–r, 6f–j, 7c,d, 8a,b).
Necrodes osculans Vigors, 1825: 537 (description, type locality: Indiâ Orientali).
Necrodes bifasciatus Dejean, 1833: 118 (unavailable, no diagnosis, locality: Java).
Diamesus osculans: Hope 1840: 149 (new combination).
Diamesus osculans var. reductus Pic, 1917: 2 (description, type locality: Sumatra).
Diamesus osculans ab. diffusus Portevin, 1926: 172 (unavailable, infrasubspecific name).
Dimesus osculans var. reductus: Růžička & Schneider 2004: 230 (synonymy with D. osculans).
Type material examined
HT ♂ of Necrodes osculans (BMNH), labelled (Fig. 3l) “Type [p, round label with red border]//Madras. [ca. 13°05′N 080°16′E]/Major Sale. [p]//59.57/Vigors’ Coll. [p]//osculans. V. [hw]//Type. figured &/described by Vigors/in Zool. Journal [hw]”.
HT ♀ of Diamesus osculans var. reductus (MNHN), labelled “Palembang [Sumatera Selatan province, ca. 03°00′S 104°45′E]/Sumatra [p]//420 [p]//v. reductus/Pic [hw, Pic’s MS]//TYPE [p, red label]//type [hw, Pic’s MS]//Muséum Paris/Coll. M. Pic [p]//Diamesus/osculans/(Vigors, 1825)/Jan Růžička det. 2002”.
Description
Measurements
Body length: male 22–49 mm (median 38 mm), female 28–44 mm (median 41 mm). Pronotum width to length ratio: male 1.18–1.38 (median 1.24), female 1.16–1.28 (median 1.21). Scutellum length to width ratio: male 0.89–1.20 (median 1.14), female 1.05–1.33 (median 1.13). Elytra width to length ratio: male 1.14–1.54 (median 1.29), female 1.17–1.32 (median 1.26).
External morphology
Body brown (pale brown in teneral specimens), each elytron with two orange bands, variable in size and shape, bands sometimes split into 2–3 isolated spots (Figs. 3f–k, 4e–k), rarely also the surface of elytron between bands is light brown to orange, probably mostly in subteneral specimens (Fig. 3k).
Male genitalia
Ventrite 9 triangular, extending into broad, regularly rounded posterior apex (Fig. 6j, v9). Tergite 10 oval, distinctly narrower in anterior part, deeply and narrowly emarginate on posterior margin (Fig. 6i, t10). Median lobe longer, sclerotized parts in dorsal view 1.6 times as long as wide. Median lobe in dorsal view narrowly unsclerotized medially only in anterior part, sclerotization medially fused in central part (Fig. 6f). Apex of median lobe narrowly sclerotized along median line (Fig. 6h).
Female genitalia
Tergite 8 without emargination anteriorly and with shallow emargination posteriorly (Fig. 7c, t8). Tergite 10 less elongate, only 1.1 times as long as wide posteriorly (Fig. 7c, t10).
Variability
Body length varies between 22 and 49 mm. Elytra with variable colour pattern (Fig. 4e–k), as described above. Colour and size variation sometimes widely variable even in individuals from the same population. Similar variation in elytral pattern of Necrodes surinamensis (Fabricius, 1775) was described by Ratcliffe30.
Taxonomy
The female holotype of D. osculans var. reductus falls within the intraspecific variation of D. osculans. The maculation on the elytra is less easily observed because the specimen is generally darkened, but the extent of the orange spot is similar to typical specimens. Consequently, we follow Růžička and Schneider13 who considered D. osculans var. reductus Pic, 1917 as a junior subjective synonym of D. osculans (Vigors, 1825).
Necrodes bifasciatus was only listed by Dejean31 atributed to Spinola and reported from Java. Following Bousquet and Bouchard32, all newly introduced species names in this work are unavailable, as they are only listed without any description.
Diamesus osculans ab. diffusus was introduced by Portevin10 to describe an individual variation in elytra colouration, when median part of elytron between anterior and posterior macula is brownish orange. This situation is generally present in subteneral adults. This name is not available, as it describes only individual colour aberration, and should be considered infrasubspecific.
Bionomics
Necrophagous species, frequently collected on large carrion, with reported forensic significance (see below). However, adults of Necrodes feed primarily on fly larvae during the active decay stage of a carcass30,33, and the situation is probably similar for Diamesus. Most frequently collected using UV and mercury-vapour light traps12 (see SM1). According to Peck12, preferred habitats are rainforest, but also some disturbed or secondary open or closed canopy forest sites. Label data confirm the information from Peck12, that adults have been collected at elevations from sea level up to 1500 m in Papua New Guinea; we have seen records from altitude around 800 m in Australia: Queensland, 1500 m in Malaysia: Pahang, 1700 m in China: Guangdong and Thailand, 1800 m in Indonesia: Sumatra, over 1900 m in northern Vietnam, and even a single record at 2800 m in Nepal (see SM1). Seasonality: as the species is widely distributed from India to Australia, we select abundant available data from Bishop Museum Field Station, Wau, Papua New Guinea (ca. 07°19′S), to summarize the seasonality: available data confirm occurrence of adults in all months of the year, with peaks in March–April and August–October, with teneral adults evenly distributed throughout the year (Table 2). This is similar to pattern observed in Australia, again with the main peak in February–April and a smaller peak also in September–December34.
Defensive behaviour
Adult D. osculans in a laboratory colony were observed showing defensive behaviour—rapid movement, combined with ejection of a malodorous fluid from the mouth and anus, and even spraying it from the abdomen for a distance of several centimetres (J. Růžička & P. Jakubec, unpubl.). This is similar to the behaviour pattern described for Necrodes surinamensis by Ratcliffe30 and Eisner and Meinwald35. The chemical substances secreted by N. surinamensis were later identified as α-and β-necrodols, and their repellence was tested on ants and some other insects36. In Necrodes, these substances are probably used to monopolize the carrion and also to provide heat to increase beetle fitness37. The chemical composition of defensive substances in Diamesus is probably similar.
The orange colouration on the apices of antennae, elytra and abdominal tergites 3–4 in combination with dark brown to black body colour probably play a role as an aposematic signal warning predators that Diamesus is defended. As the colour pattern on tergites 3–4 is normally covered by the elytra in the resting position, we speculated that it is only exposed during flight. A similar bicoloured pattern of body dorsum was mentioned for other carrion beetles by Jones38 for Nicrophorus Fabricius, 1775 and Fisher and Tuckerman39 for Necrophila Kirby & Spence, 1828. No information is available for Diamesus. Future studies should also focus on this phenomenon of aposematic signaling.
Pollination
Two series of specimens from Indonesia (Sumatera Barat province: Palupuh and Bukittinggi) were found to be associated with Titan arum (Amorphophallus titanum (Becc.) Becc. ex Arcangeli (Araceae)). Titan arum is known to emit a unique rotting animal-like odour from its inflorescence that attracts insects for pollination; the odor consists of several sulphur-containing volatile organic compounds including dimethyl trisulfide (DMTS)40. DMTS is part of the odour of carrion and was also found to be attractive to two species of Central European Nicrophorus (Coleoptera: Staphylinidae: Silphinae) using electroantennography41. Diamesus is also mentioned as a pollinator of Titan arum in the review by Davis et al.42, where this association of carrion beetles and flies with Titan arum is called sapromyophily.
Phoresy
Halliday43 reported Macrocheles agilis Halliday, 2000 (Acari: Gamasida: Macrochelidae) as phoretic mite on Diamesus osculans in Papua New Guinea and Australia.
Forensic significance
Wang et al.44, Eddie et al.45 and Magni et al.46 reported D. osculans on pig carrion in southern China, Malaysia and Western Australia, and briefly pointed out its forensic significance. Zhang et al.15 also mentioned D. osculans as a forensically important species in southern China. However, the larva of Diamesus is not described (J. Růžička et al. in prep.), and thermal summation models for its developmental stages are currently not available.
References on distribution
India: “Indiâ Orientali” (Vigors 1825)5, South India (Arrow 1909)47; Nepal: Annapurna mts, Poon Hill, 28°34′N 083°50′E (Schawaller 2003)48; Cambodia: R’leak Korng Cherng village, 11°46.731′N 103°46.592′E (Sin et al. 2021)49; Prey Lang Wildlife Sanctuary, 13°14.705′N, 105°37.278′E (Sin et al. 2021)49; China: Guangdong province: Zhongshan, 22°31′N 113°22′E (Wang et al. 2008)44; Chaozhou [ca. 23°39′N 116°37′E] (Zhang et al. 2020)15; Chongqing province: Chongqing (Růžička et al. 2002)50; list of provinces, without precise localities (Ji 2012)51; Taiwan: Kôshun [= Pingtung County, Hengchun, ca. 22°00′N 120°45′E] (Miwa 1931)29, “Taiwan” (Ji 2012)51; Thailand: Chiang Mai, Doi Suthep [ca. 18°48′N 98°53′E] (Nishikawa 2014)28; Japan: Yaeyama Islands (Kurosawa 1974; Matoba 1975; Shoyama 2020)52,53,54; Philippines: Luzon Island: Imugan, Nueva Vicaya (Arnett 1950)11; Cebú Island: Bugó [= Bogo, ca. 11°03′N 124°00′E] (Arnett 1950)11; Malaysia: Sarawak (Arrow 1909)47; Sabah: Tombongon [ca. 06°00′N 116°14′E] (Eddie et al. 2016)45; Indonesia: Papua Barat and Papua provinces [6 unnamed localities] (Peck 200112: fig. 11); Papua New Guinea: Woodlark Is. [= Muyua Island] (Arrow 1909)47; New Britain Island: Gazelle Peninsula, Yalom [ca. 04°25′S 151°45′E] (Mroczkowski 1966)55; Wau [ca. 07°20′S 146°43′E] (Halliday 2000)43; Amboin [ca. 04°37′S 143°29′E] (Halliday 2000)43; Erap [ca. 06°32′S 146°42′E] (Halliday 2000)43; [12 unnamed localities] (Peck 200112: fig. 11), Australia: Queensland (Arrow 1909)47; Northern Territory, Mudginberri [ca. 12°37′S 132°52′E] (Halliday 2000)43; Northern Territory, Cape Crawford, 16°34′S 135°41′E (Halliday 2000)43; Queensland, Kirrama Ra. [ca. 18°06′S 145°42′E] (Halliday 2000)43; Queensland, 15 km S Biloela [ca. 24°32′S 150°31′E] (Halliday 2000)43; Queensland, Binna Burra [ca. 28°11′S 153°11′E] (Halliday 2000)43; Queensland, Ingham [ca. 18°39′S 146°09′E] (Halliday 2000)43; New South Wales, Huonbrook [ca. 28°32′S 153°23′E] (Nishikawa 2014)28; New South Wales, Bonville [ca. 30°23′S 153°04′E] (Halliday 2000)43; [51 unnamed localities] (Peck 200112: fig. 11).
Distribution
Widely distributed species, known from India (Assam, Karnataka, Kerala, Meghalaya, Sikkim, Tamil Nadu, Uttarakhand and West Bengal), Sri Lanka, Nepal, Bhutan, China (Anhui, Chongqing Municipality, Fujian, Guangdong, Guangxi Autonomous Region, Hainan, Hunan, Jiangxi, Shaanxi, Xizang (Tibet) Autonomous Region, Yunnan and Zhejiang), Taiwan, Japan (Ryukyu Islands: Iriomote-jima Is. and Ishigaki-jima Is.), Myanmar, Thailand, Laos, Cambodia, Vietnam, Philippines, Malaysia (Johor, Kedah, Malacca, Pahang, Perak, Sabah and Sarawak), Brunei, Indonesia (Aceh, Bali, Bengkulu, Jakarta, Jawa Barat, Lampung, Maluku, Nusa Tenggara Barat, Nusa Tenggara Timur, Papua, Papua Barat, Riau, Sulawesi Selatan, Sulawesi Tengah, Sulawesi Utara, Sumatera Barat, Sumatera Selatan and Sumatera Utara), Papua New Guinea, Solomon Islands and Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, Victoria and Western Australia). First records from India: Uttarakhand State, China: Jiangxi and Shaanxi Provinces and Xizang (Tibet) Autonomous Region, and Australia: Victoria (Fig. 8a,b).
Discussion
Forensic significance
From anecdotal evidence based on locality data it seems that the ecology of both species of the genus Diamesus is similar to their close relatives from the genus Necrodes, which breeds on dead bodies of larger vertebrates and can also be attracted to light33,56. This is in line with field observations and experiments where typically D. osculans was present (see12,46,49,57). Like other genera of the subfamily Silphinae e.g., Necrodes58,59, Necrophila Kirby & Spence, 182860, Oxelytrum61 or Thanatophilus4,62, species of the genus Diamesus, therefore might be used in forensic entomology to estimate time of colonization, which is crucial information for medico-legal casework63. This is further supported by observations of the species (D. osculans) feeding as adults and larvae on human corpses64.
Distribution
Peck12 summarized the distribution of D. osculans in Eastern Australia, and commented on the absence of records of this species from Australia in Portevin10 and speculations of introduction into northern Queensland in Tillyard65. He also mentioned an imprecisely localized specimen from “Australia” collected in 1896 and other specimens collected in 1909 (Queensland: Cairns) and before 1917 (Queensland: Normanton). The collection of J. Kořenský (NMPC) houses two additional specimens from Melbourne and “Australia” (see SM1). According to Kořenský66, he visited Australia and collected insects there in 1900–1901. All these findings support the idea of the autochthonous occurrence of D. osculans in Australia.
Diamesus osculans was already listed from Taiwan by Miwa29. These beetles may be based on specimens from the “Shiraki specimens”, which includes non-Taiwanese material, labelled with added fake Taiwanese localities67,68,69,70. This was ignored in recent catalogues13,14 along with a series of other clear misidentifications of carrion beetles reported from Taiwan by Miwa29. However, recent data on occurrence of both D. bimaculatus and D. osculans show that both species live in sympatry in Taiwan. Fang-Shuo Hu (pers. comm.) mentioned that D. osculans is rarely collected in Taiwan, compared to D. bimaculatus, which is much more abundant. Future research should study in detail possible competition between the two species of Diamesus, and examine if the reason for the relative rarity of D. osculans on Taiwan can be explained by repeated invasions or unintentional introductions of D. osculans from mainland Asia, or if there is an established population in Taiwan. The species is present on even quite remote and isolated islands in Indonesia, Papua New Guinea, and the Solomon Islands (Fig. 8a, SM1).
Similarly, there are only four collecting records (in three short communications) on D. osculans from two islands (Iriomote-jima Is. and Ishigaki-jima Is.) of Yaeyama Islands, in the most south-western part of Ryukyu Islands, Japan52,53,54. Most probably, D. osculans has not established stable population there, and these specimens collected on Yaeyama Islands were unintentionally introduced by human activities (S. Nomura, pers. comm.). However, the occurrence of D. osculans in this region is also predicted by the MaxEnt model. Similar case of a recent introduction of Cicindela batesi Fleutiaux, 1894 from Taiwan to Iriomote-jima Is. and C. chinensis okinawana Nakane, 1957 (both Coleoptera: Carabidae) from Okinawa-jima Is. to Ishigaki-jima Is. is described by Osozawa et al.71 Other exotic insect species from Philippines, Indochina or even New Guinea were accidentally found in the Yaeyama Islands, but these appear to have not established permanent populations (S. Nomura, pers. comm.).
Species distribution models
These models mostly agree with the published works of Portevin10 and Peck12. The model for D. bimaculatus did not find it plausible that the species would occur outside of Taiwan. On the other hand, the presence of D. osculans on Tasmania and New Caledonia was suggested. Both islands were investigated in the past by a number of entomologists without recording the species, and we think its presence there is very unlikely due to its size and tendency to occur in large numbers on cadavers and its ease of being attracted to lights, including light traps. The model simply suggest that suitable conditions are present on these islands without taking into consideration the past biogeography and distance from the nearest source population.
The species distribution model of D. osculans suggested a possible presence of the species in parts of the World where it was so far not reported, or the reports were not considered as likely. In the last category certainly belongs Japan, where the species was observed only on the south-west part of Ryukyu Islands, but the models suggest occurrence also on Yakushima Is. Other parts where we consider the presence of the species less likely is Vanuatu. This archipelago is separated by large water bodies and its fauna is relatively well known, so the presence of a large necrophagous beetle would surely be recorded. On other hand, presence of the beetle on Tasmania could be possible as well as on other small islands in the Maritime Southeast of Asia due to their position close to previously confirmed populations of the species.
Phylogeny
The molecular phylogeny strongly agrees with the previously published phylogenies of the subfamily Silphinae and the tribe Silphini1,23,72,73. However, this is the first analysis including both species of the genus Diamesus. The phylogenetic analysis confirmed the monophyly of the genus Diamesus and supports the genus as a sister lineage to Necrodes. The specimens of Diamesus osculans used for the phylogenetic analysis represent populations from a wide distribution area, as well as the specimens of D. bimaculatus collected in Taiwan, which remains its only known area of distribution.
Plate tectonics models suggest that colonization pathways from Asia to Australia could occur approximately only in the last 10 mya74. Also, according to these models, the occurrence of D. osculans on the islands of Sumatra, Java, Borneo and others might be relict from this time period as these islands were probably repeatedly interconnected with continental Asia74,75. Suggesting, that the clade consisting of the genera Necrodes and Diamesus originated in the northern hemisphere. This idea is further supported by the existence of a Beringian land bridge between Asia and North America approximately ca. 65.5 and ca. 58 mya when the colonization of North America by Necrodes might have occurred76.
Diamesus osculans show generally only small intraspecific variation in compared sequences, based on individuals collected throughout its range. This, in combination with phylogenetic analysis can suggest quite high historical genetic connectivity between separate populations within its range.
Estimation of the divergence time
The estimated split of both species of Diamesus (Palaeocene–Eocene, 26.9–54.1 mya) is much older than the proposed appearance of Taiwan as a continental island, emerging above sea level only at the Miocene-Pliocene boundary (ca. 5 mya), owing to the collision of the Philippine Sea plate and the Eurasian plate77. Obviously, endemic occurrence of D. bimaculatus on Taiwan can be relictual, and the original distribution range could have been much broader, with extirpation throughout its former range.
Material and methods
Specimen sampling, museum abbreviations
Specimens examined in this study were loaned from the following 52 museums and private collections (acronyms listed according to Arnett et al.78):
- AMNH::
-
American Museum of Natural History, New York, U.S.A. (L. Herman)
- BHHC::
-
collection of Bin-Hong Ho, Taipei, Taiwan
- BMNH::
-
Natural History Museum, London, United Kingdom (M.V.L. Barclay)
- BPBM::
-
Bernice Pauahi Bishop Museum, Honolulu, Hawaii, U.S.A. (†J. Boone, N. Evenhuis)
- CAS::
-
California Academy of Sciences, San Francisco, California, U.S.A. (D. Kavanaugh)
- CAU::
-
China Agricultural University, Beijing, China (Xin-Li Wang)
- CMNH::
-
The Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, U.S.A. (R.L. Davidson)
- CYTC::
-
collection of Cheng-Yan Tu, Taipei, Taiwan
- DSSC::
-
collection of Derek S. Sikes, Fairbanks, U.S.A.
- FMNH::
-
Field Museum of Natural History, Chicago, U.S.A. (A.F. Newton, M. Thayer)
- FSHC::
-
collection of Fang-Shuo Hu, Yilan, Taiwan
- HNHM::
-
Magyar Természettudományi Muzeum, Budapest, Hungary (†O. Merkl, Gy. Makranczy)
- IRSNB::
-
Institut royal des Sciences naturelles de Belgique, Bruxelles, Belgium (W. Dekoninck, A. Drumont)
- IZ-CAS::
-
Institute of Zoology, Chinese Academy of Sciences, Beijing, China (Hong-Zhang Zhou)
- JCLC::
-
collection of Jincheng Liu, Beijing, China
- JHAC::
-
collection of Jiří Háva, Prague, Czech Republic
- JRUC::
-
collection of Jan Růžička, Prague, Czech Republic
- JSCC::
-
collection of Jan Schneider, Prague, Czech Republic
- JVAC::
-
collection of Jiří Vávra, Ostrava, Czech Republic
- KHAC::
-
collection of Keitaro Harusawa, Osaka, Japan
- LDVC::
-
collection of Libor Dvořák, Tři Sekery, Czech Republic
- MHNG::
-
Muséum d’Histoire Naturelle, Genève, Switzerland (G. Cuccodoro)
- MNHN::
-
Museum national d’Histoire naturelle, Paris, France (Azadeh Taghavian)
- MNIC::
-
collection of Masaaki Nishikawa, Ebina, Japan
- MZLU::
-
Biological Museum, Lund University, Lund, Sweden (Ch. Fägerström)
- MZSP::
-
Museu de Zoologia da Universidade de São Paulo, São Paulo, Brasil (†U.R. Martins)
- NHMB::
-
Naturhistorisches Museum, Basel, Switzerland (Eva Sprecher-Uebersax, M. Borer)
- NHMW::
-
Naturhistorisches Museum, Wien, Austria (H. Schillhammer)
- NHRS::
-
Naturhistoriska riksmuseet, Stockholm, Sweden (J. Bergsten)
- NMNH::
-
National Museum of Natural History, Smithsonian Institution, Washington, U.S.A. (F. Shockley, G.F. Hevel)
- NMNS::
-
National Museum of Natural Science, Taichung City, Taiwan (Jing-Fu Tsai)
- NMPC::
-
Národní museum, Prague, Czech Republic (J. Hájek, M. Fikáček)
- NSMT::
-
National Museum of Nature and Science, Tsukuba, Japan (S. Nomura)
- RMNH::
-
Naturalis Biodiversity Center, Leiden, Netherlands (A. von Assen)
- ROM::
-
Royal Ontario Museum, Toronto, Canada (C. Darling)
- SDEI::
-
Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany (Marianna Simões, L. Behne)
- SEMC::
-
Shanghai Entomological Museum, Chinese Academy of Sciences, Shanghai, China (Hai-Sheng Yin)
- SHNU::
-
Department of Biology, Shanghai Normal University, China (Liang Tang, Zi-Wei Yin)
- SMFD::
-
Forschungsinstitut Senckenberg, Frankfurt am Main, Germany (Andrea Vesmanis, D. Kovac)
- SMNS::
-
Staatliches Museum für Naturkunde, Stuttgart, Germany (W. Schawaller, A. Faille)
- SMTD::
-
Museum für Tierkunde, Dresden, Germany (O. Jäger)
- SNUC::
-
Insect Collection of Shanghai Normal University, Shanghai, China (Liang Tang, Zi-Wei Yin)
- SYSU::
-
Institute of Entomology, Sun Yat-sen University, Guangzhou, China (Feng-Long Jia)
- UCDC::
-
The Bohart Museum of Entomology, University of California, Davis, U.S.A. (S. Heydon)
- WBAC::
-
collection of Wolfgang Barries, Wien, Austria (deposited in NHMW)
- YFTC::
-
Yunnan Forestry Technological College, Kunming, China (Guo-Feng Li)
- YJIC::
-
collection of Yun Ji, Beijing, China
- ZMAN::
-
Zoölogisch Museum Amsterdam, Amsterdam, the Netherlands (joined and transferred to RMNH)
- ZMAS::
-
Zoological Museum, Academy of Sciences, St. Petersburg, Russia (M.G. Volkovitsh)
- ZMHB::
-
Museum für Naturkunde, Berlin, Germany (J. Frisch, B. Jäger)
- ZMUC::
-
Zoological Museum, University of Copenhagen, Copenhagen, Denmark (A. Solodovnikov)
- ZYCC::
-
collection of Zhen-Yi Chen, Taipei, Taiwan
Exact label data are cited only for the type material, using the following set of abbreviations: coll. —collection of (not collector); leg.—collected by; MS—manuscript, HT—holotype, LT—lectotype, PLT—paralectotype(s). Authors’ remarks and addenda are enclosed in square brackets; [p]—the preceding data are printed; [hw]—preceding data are hand-written. Separate lines on labels are indicated (only for primary types) by “/”, separate labels by “//”. The lectotype and paralectotypes of D. osculans var. bimaculatus are designated in order to preserve stability of nomenclature in this group, according to the Article 74.7.3 of the Code26.
The following abbreviations are used for the determiners of the material: AFN—Alfred F. Newton, DSS—Derek S. Sikes, JH—Jiří Háva, JS—Jan Schneider, KH—Keitaro Harusawa, MN—Masaaki Nishikawa, SBP—Stewart B. Peck, WB—Wolfgang Barries, WS—Wolfgang Schawaller. If otherwise not mentioned, Jan Růžička determined or revised the material of adult beetles.
Morphological analysis
The morphological terminology used in this paper follows Lawrence and Ślipiński79. Hind wing venation was homologised following Kukalová-Peck and Lawrence80 and Lawrence et al.81. Male and female terminalia of Diamesus were studied after short clearing in a hot 10% solution of KOH and photographed submerged in alcohol, using a Canon macro photo lens MP-E 65 mm or EF-S 60 mm on a Canon 750D. Habitus pictures of adults (incl. types) and details of wings were based on photography of dry, manually cleaned specimens, using the same setup. For each structure, multiple layers of focus were combined in the Zerene Stacker 1.04 software (http://www.zerenesystems.com/cms/stacker). Other morphological characters were measured and documented using a Keyence VHX-6000 digital microscope.
To observe fine structures on female genitalia, we used a scanning electron microscope. Preparation of samples follows the methodology of Novák et al.62. Selected samples were dehydrated using a graded series of ethanol (75%, 80%, 90%, 95%, 100%) and left in each concentration for approximately 30 min. before transferring to acetone overnight. Dehydrated samples were dried using the critical point drying method. Dry samples were then attached to an aluminium disk target using copper foil tape and coated with gold in Bal-Tec Sputter Coater SCD 050. Samples were observed and documented with a JSM-6380LV (JEOL) scanning electron microscope.
All pictures were digitally enhanced using Adobe Photoshop CS4 or CorelPHOTO-PAINT 2018, plates were arranged in CorelDRAW 2018.
Measurements
Morphological characters were measured using a software available in Keyence VHX-6000 digital microscope. The following measurements were taken: body length, pronotum: median length and maximum width, scutellum: length and width, elytra: length (measured as length from posterior margin of scutellum to perpendicular distance of apex of both elytra) and combined width of both elytra. Body length was measured with precision of 1 mm, the other measurements with precision of 0.1 mm. In total, 11 males and 12 females of D. bimaculatus and 13 males and 12 females of D. osculans were measured, individuals were selected from dry mounted material to cover maximum variation in body size. For D. bimaculatus, mostly lectotype and paralectotypes were measured; in D. osculans, all non-type specimens are vouchers deposited in JRUC. Measured data are available in SM4.
We used linear regression with normal distribution of errors to model the effect of species identity and sex on four major morphological characteristics, body length, elytra width to length ratio, pronotum width to length ratio and scutellum length to width ratio. The significance level was set at 5%. Data management and all analyses were carried out using the R statistical program ver. 4.1.182. Graphical outputs were created using ggplot283.
Maps
Localities were interpreted from locality labels, unified (except primary types) and georeferenced with the help of several gazetteers and map sources: Microsoft Encarta Premium 200884, NGA GEOnet Names Server85, Google Earth Pro86 and during the initial phase, also Fuzzy Gazetteer87. For large cities without precise locality data, just centroids are reported in SM1 and used in distribution maps. These data were combined with published records of Peck12, from Papua, Papua New Guinea and Australia (in total, 68 unnamed localities). These were georeferenced from map 11, as published by Peck (2001: 97)12. Additionally, distribution data of Diamesus (119 observations, downloaded on 26 April, 2021) were mined from iNaturalist88, all records were verified by J.R., based on habitus pictures available for each record. Additional five observations of D. osculans from Taiwan were downloaded from iNaturalist on 23 October, 2022.
The distribution maps were produced by ESRI ArcMap 10.8.1 of ArcGIS Desktop 10.8.1 suite. For map layers, free level 0 data from Global Administrative Areas (http://www.gadm.org, ver. 2.8), Natural Earth (http://naturalearthdata.com, Natural Earth I with Shaded Relief, Water, and Drainages) (with 30% transparency and 15% brightness) over World Shaded Relief (https://www.arcgis.com/home/item.html?id=9c5370d0b54f4de1b48a3792d7377ff2) were used.
Species distribution models
Species distribution models (SDM) were established using the machine learning algorithm MaxEnt89 for both species (D. bimaculatus and D. osculans) based on presence only occurrence data. As explanatory variables we used values of four bioclimatic shapefiles (WorldClim 2.1)90 (annual mean temperature (Bio 1), mean diurnal range (Bio 2), temperature annual range (Bio 7), and precipitation of warmest quarter (Bio 18)) and map of terrestrial ecoregions of the World91. Due to large disparities between distribution ranges for both species, we used two resolutions of the explanatory shapefiles, 30 arc seconds for D. bimaculatus and 2.5 arc minutes for D. osculans. The quality of the models was assessed using area under curve (AUC) metric and resulting SDM models were binomially reclassified based on maximum training sensitivity plus specificity Cloglog thresholds.
Molecular analysis
DNA was extracted from hind-leg muscle tissue of specimen stored in 96% EtOH and dry specimens using commercial kit Geneaid Tissue & Blood Kit (Geneaid, New Taipei City, Taiwan) following provided protocol. Three genes were amplified—cytochrome oxidase I (COI, mtDNA), 16S (rDNA) and 28S (nDNA) using previously published C1-J-2183 (alias Jerry) & TL2-N-3014 (alias Pat)92, LR-J-12887 (alias 16Sbr) & LR-N-13398 (alias 16Sar)92, and Rd3.2a & Rd5b93. The PCR reactions were carried out at 25 µl based on provided PPP Master Mix protocol (Top Bio) (12.5 µl of 1 × PPP Master Mix, 9.5 µl PCR H2O, 0.4 μM of forward and 0.4 μM reverse primer) under the conditions shown in Table 3. PCR products were visualized by electrophoresis on 1% agarose gel and subsequently purified using ExoSAP-IT (Applied Biosystems) following the protocol provided, bi-directional sequencing was carried out in BIOCEV (Vestec, Czech Republic) using the Sanger sequencing method with the same primers as for the amplification.
Phylogenetic analysis
Obtained sequences for each gene were visualised in Chromas v2.6.6 (Technelysium Pty Ltd, Brisbane, Australia), visually compared, and trimmed. The newly generated sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov) and combined with previously published sequences from the GenBank database (http://www.ncbi.nlm.nih.gov) (Table 1). Alignment was generated using MAFFT94 using Guidance2 server95. Alignment was visualized, manually edited, and trimmed to an equal length in BioEdit v7.0.5.396. Prior to analysis, each gene alignment was checked for the best fitting model, using ModelFinder97 implemented in IQ-TREE webserver98 separately according to the Akaike Information Criterion—COI GTR + F + I + G4, 16S K3Pu + F + I + G4, and 28S GTR + F + I + G4. Subsequently, the alignments were concatenated using software MEGA-X v10.1.8.98. For phylogeny reconstruction the Maximum Likelihood (ML), Maximum Parsimony (MP), and Bayesian Inference (BI) methods were applied. The ML method was conducted in IQ-TREE webserver99 under 1000 Ultrafast bootstrap (UFBoot)100 iterations. The BI method was performed in MrBayes v3.2.7101 (mcmcp ngen = 10,000,000, samplefreq = 1000, printfreq = 1000, nchains = 4, savebrlens = yes, nst = 6 rates = invgamma), first 25% of trees were discarded. The MP method was conducted in PAUP* v4.0a169102 nreps = 10, swap = TBR nchuck = 100 chuckscore = 1, bootstrap nreps = 10,000, search = heuristic), results were visualised in FigTree v1.4.4103. The concatenated alignment and generated trees of each method are available at https://github.com/KarolinaMahlerova/Alignment-and-phylogenetic-trees-Diamesus_.
Estimation of the divergence time
The estimation of the divergence time of the selected genera of the subfamily Silphinae was conducted using BEAST2 software v2.6.6.0104. The concatenated alignment of mitochondrial genes COI and 16S of 36 individuals (excluding Necrodes surinamensis from the original dataset due to missing sequence of 16S) was used. Based on previously published divergence time estimates of the subfamily Silphinae105,106, the following parameters were set using BEAUti2 v2.6.6.0104: GTR substitutional model, frequencies: All Equal, Clock Model: Relaxed Clock Log Normal, Chain Length 10,000,000, and sampled every 1000 generations to generate .xml input file for estimation of the divergence time. Three priors with normal distribution were used for calibration of the model—Silphinae 175 mya (184.9–165.1; sigma 0.95)105,107, Nicrophorini 135 mya (144.9–125.1; sigma 0.95)105,108, and Necrodes 48.25 mya (53.0–47.5; sigma 0.95) (fossil from Green River formation; J. Růžička, unpublished), 25% of trees was discarded as burn-in. To assess the convergence Tracer v1.7.2 was used. TreeAnnotator v2.6.6.0 was used to generate maximum clade credibility tree, median ages and their 95% highest posterior density. The output file was visualised in FigTree v1.4.4103.
References
King, J. E., Riegler, M., Thomas, R. G. & Spooner-Hart, R. N. Phylogenetic placement of Australian carrion beetles (Coleoptera: Silphidae). Austral. Entomol. 54, 366–375 (2015).
Montoya-Molina, S. et al. Developmental models of the forensically important carrion beetle, Thanatophilus sinuatus (Coleoptera: Silphidae). J. Med. Entomol. 58, 1041–1047 (2021).
Montoya-Molina, S. et al. Developmental models of the carrion beetle Thanatophilus rugosus (Linnaeus, 1758) (Coleoptera: Silphidae). Sci. Rep. 11, 19377 (2021).
Ridgeway, J. A., Midgley, J. M., Collett, I. J. & Villet, M. H. Advantages of using development models of the carrion beetles Thanatophilus micans (Fabricius) and T. mutilatus (Castelneau) (Coleoptera: Silphidae) for estimating minimum post mortem intervals, verified with case data. Int. J. Legal Med. 128, 207–220 (2014).
Vigors, N. A. Descriptions of some rare, interesting, or hitherto uncharacterized subjects of zoology. Zool. J. 1(409–418), 526–542 (1824).
Hope, F. W. The Coleopterist’s Manual, Part the Third, containing various Families, Genera, and Species of Beetles, recorded by Linnaeus and Fabricius. Also, Descriptions of Newly Discovered and Unpublished Insect. (J.C. Bridgewater and Bowdery & Kerby, 1840) https://doi.org/10.5962/bhl.title.9229.
Portevin, G. Révison des Silphides, Liodides et Clambides du Japon. Ann. la Société Entomol. Belgique 58, 212–236 (1914).
Portevin, G. Notes sur les Silphides (Col.). Misc. Entomol. 26, 1–4 (1922).
Pic, M. Descriptions abrégées diverses. Mélanges Exot. 26, 2–24 (1917).
Portevin, G. Les Grands Nécrophages du Globe. Silphini–Necrodini–Necrophorini. Encyclopédie Entomologique (Série A). 6, (Lechevalier, 1926).
Arnett, R. H. The Silphidae of the Philippine Islands. Proc. Entomol. Soc. Washingt. 52, 63–69 (1950).
Peck, S. B. Review of the carrion beetles of Australia and New Guinea (Coleoptera: Silphidae). Aust. J. Entomol. 40, 93–101 (2001).
Růžička, J. & Schneider, J. Family Silphidae Latreille, 1807. In Catalogue of Palaearctic Coleoptera, Vol. 2: Hydrophiloidea–Histeroidea–Staphylinoidea (eds Löbl, I. & Smetana, A.) 229–237 (Apollo Books, 2004).
Růžička, J. Silphidae. In Catalogue of Palaearctic Coleoptera Volume 2. Hydrophiloidea – Staphylinoidea, Revised and Updated Edition (eds Löbl, I. & Löbl, D.) 291–304 (Brill, 2015).
Zhang, X., Hou, Q., Zhang, L., Cai, J. & Meng, F. The complete mitochondrial genome of a potentially forensic related carrion beetle, Diamesus osculans (Vigors, 1825). Mitochondrial DNA Part B 5, 1423–1424 (2020).
Grebennikov, V. V. & Newton, A. F. Detecting the basal dichotomies in the monophylum of carrion and rove beetles (Insecta: Coleoptera: Silphidae and Staphylinidae) with emphasis on the Oxyteline group of subfamilies. Arthropod Systematics and Phyloge. Arthropod Syst. Phylogeny 70, 133–165 (2012).
Sikes, D. S. Silphidae. In Coleoptera, Beetles. Volume 1: Morphology and Systematics. Archostemata, Adephaga, Myxophaga, Polyphaga partim. 2nd edition. Arthropoda: Insecta, Handbook of Zoology. (eds Beutel, R. G. & Leschen, R. A. B.) 386–394 (de Gruyter, 2016).
McKenna, D. D. et al. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: Forest litter as a stepping stone for diversification of nonphytophagous beetles. Syst. Entomol. 40, 35–60 (2015).
McKenna, D. D. et al. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 40, 835–880 (2015).
Zhang, S.-Q. et al. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 9, 205 (2018).
Cai, C. et al. Integrated phylogenomics and fossil data illuminate the evolution of beetles. R. Soc. Open Sci. 9, 211771 (2022).
Newton, A. Staphyliniformia world catalog database. In Catalogue of Life Checklist (Aug 2022) (ed. Bánki, O. et al.) https://doi.org/10.48580/d4sl-3gk (2022).
Dobler, S. & Müller, J. K. Resolving phylogeny at the family level by mitochondrial cytochrome oxidase sequences: Phylogeny of carrion beetles (Coleoptera, Silphidae). Mol. Phylogenet. Evol. 15, 390–402 (2000).
Newton, A. F. & Thayer, M. K. Current classification and family-group names in Staphyliniformia (Coleoptera). Fieldiana Zool. New Series 67, iv + 1–92 (1992).
Sikes, D. S. Carrion Beetles (Coleoptera: Silphidae). In Encyclopedia of Entomology (ed. Capinera, J. L.) 749–758 (Springer, 2008). https://doi.org/10.1007/978-1-4020-6359-6.
ICZN. International Code of Zoological Nomenclature, Fourth Edition, adopted by the International Union of Biological Sciences. (International Trust for Zoological Nomenclature, 1999).
Kraatz, G. Über Systematik und geographische Verbreitung der Gattung Silpha L. und verwandten Genera. Dtsch. Entomol. Zeitschrift 20, 353–374 (1876).
Nishikawa, M. Family Silphidae. In Specimen list of Coleoptera in the insect collection of the Osaka Museum of Natural History (part 3) (ed. Shiyake, N.). Spec. Publ. from Osaka Museum Nat. Hist. vol. 46, 5–46 (2014).
Miwa, Y. A systematic catalogue of Formosan Coleoptera. Inst. Dep. Agric. Gov. Res. Institute, Formosa, Japan. Rep. 55, xi + A–B + 1–359 (1931).
Ratcliffe, B. C. The natural history of Necrodes surinamensis (Fabr.) (Coleoptera: Silphidae). Trans. Am. Entomol. Soc. 98, 359–410 (1972).
Dejean, P. F. M. A. Catalogue des coléoptères de la collection de M. le comte Dejean. [Livraisons 1–2]. (Mequignon-Marvis, 1833) https://doi.org/10.5962/bhl.title.8771.
Bousquet, Y. & Bouchard, P. The genera in the second catalogue (1833–1836) of Dejean’s Coleoptera collection. Zookeys 282, 1–219 (2013).
Ratcliffe, B. C. The carrion beetles (Coleoptera: Silphidae) of Nebraska. Bull. Univ. Nebraska State Museum 13, 1–100 (1996).
Atlas of Living Australia website. Atlas of Living Australia website. https://biocache.ala.org.au/occurrences/search?q=lsid:https://biodiversity.org.au/afd/taxa/e6da8a89-50de-4c8d-b5f7-01637d022393#tab_recordsView (Accessed 25 November 2021) (2001).
Eisner, T. & Meinwald, J. Defensive spray mechanism of a silphid beetle (Necrodes surinamensis). Psyche (Stuttg). 89, 357–367 (1982).
Eisner, T., Deyrup, M., Jacobs, R. & Meinwald, J. Necrodols: Anti-insectan terpenes from defensive secretion of carrion beetle (Necrodes surinamensis). J. Chem. Ecol. 12, 1407–1415 (1986).
Matuszewski, S. & Mądra-Bielewicz, A. Heat production in a feeding matrix formed on carrion by communally breeding beetles. Front. Zool. 18, 5 (2021).
Jones, F. M. Insect coloration and the relative acceptability of insects to birds. Trans. Entomol. Soc. Lond. 80, 345–386 (1932).
Fisher, R. M. & Tuckerman, R. D. Mimicry of bumble bees and cuckoo bumble bees by carrion beetles (Coleoptera: Silphidae). J. Kansas Entomol. Soc. 59, 20–25 (1986).
Shirasu, M. et al. Chemical identity of a rotting animal-like odor emitted from the inflorescence of the titan arum (Amorphophallus titanum). Biosci. Biotechnol. Biochem. 74, 2550–2554 (2010).
Kalinová, B., Podskalská, H., Růžička, J. & Hoskovec, M. Irresistible bouquet of death—how are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften 96, 889–899 (2009).
Davis, C. C., Endress, P. K. & Baum, D. A. The evolution of floral gigantism. Curr. Opin. Plant Biol. 11, 49–57 (2008).
Halliday, R. B. The Australian species of Macrocheles (Acarina: Macrochelidae). Invertebr. Taxon. 14, 273–326 (2000).
Wang, J., Li, Z., Chen, Y., Chen, Q. & Yin, X. The succession and development of insects on pig carcasses and their significances in estimating PMI in south China. Forensic Sci. Int. 179, 11–18 (2008).
Eddie, I., Chua, T. H. & Hiu, J. Diversity of forensically important insects in Sabah. Borneo J. Med. Sci. 10, 30–35 (2016).
Magni, P. A., Petersen, C., Georgy, J. & Dadour, I. R. The effect of suitcase concealment on insect colonization: A pilot study in Western Australia. Glob. J. Forensic Sci. Med. 1, 1–9 (2019).
Arrow, G. J. Systematic notes on Coleoptera of the Clavicorn families. Ann. Mag. Nat. Hist. Eight Ser. 4, 190–196 (1909).
Schawaller, W. New distributional data on Silphidae (Insecta: Coleoptera) from the Nepal Himalaya. in Biodiversität und Naturausstattung im Himalaya (eds. Hartmann, M. & Baumbach, H.) 201–203 (Verein der Freunde und Förderer des Naturkundemuseums, 2003).
Sin, S. et al. First record of the carrion beetle Diamesus osculans (Vigors, 1825) (Coleoptera: Silphidae) in Cambodia. Cambodian J. Nat. Hist. 1, 8–11 (2021).
Růžička, J., Schneider, J., Sikes, D. S. & Háva, J. Distributional records of carrion beetles (Coleoptera: Silphidae) from China, Part II. Klapalekiana 38, 227–253 (2002).
Ji, Y. The Carrion Beetles of China (Coleoptera: Silphidae) (China Forestry Publishing House, 2021).
Matoba, I. Additional records of Diamesus osculans from Ishigaki-jima Island, the Ryukyus. Coleopt. News 25/26, 9 (1975) (in Japanese).
Shoyama, M. Diamesus osculans collected on Iriomote-jima Island, the Ryukyus. Gekkan Mushi 589, 53–54 (2020) (in Japanese).
Kurosawa, Y. Diamesus osculans newly recorded from Japan. Coleopt. News 19/20, 6–7 (1974) (in Japanese).
Mroczkowski, M. Silphidae, Catopidae and Dermestidae (Col.) of the Noona Dan expedition to the Philippine and Bismark Islands. Entomol. Meddelelser 34, 325–328 (1966).
Charabidze, D., Vincent, B., Pasquerault, T. & Hedouin, V. The biology and ecology of Necrodes littoralis, a species of forensic interest in Europe. Int. J. Legal Med. 130, 273–280 (2016).
Geoff, W. Records of the carrion beetle Diamesus osculans Vigor (Silphidae: Coleoptera) from New South Wales. Aust. Entomol. 8, 47 (1981).
Frątczak, K. & Matuszewski, S. Classification of forensically relevant larvae according to instar in a closely related species of carrion beetles (Coleoptera: Silphidae: Silphinae). Forensic Sci. Med. Pathol. 12, 193–197 (2016).
Bajerlein, D., Taberski, D. & Matuszewski, S. Estimation of postmortem interval (PMI) based on empty puparia of Phormia regina (Meigen) (Diptera: Calliphoridae) and third larval stage of Necrodes littoralis (L.) (Coleoptera: Silphidae)—Advantages of using different PMI indicators. J. Forensic Leg. Med. 55, 95–98 (2018).
Jakubec, P., Qubaiová, J., Novák, M. & Růžička, J. Developmental biology of forensically important beetle, Necrophila (Calosilpha) brunnicollis (Coleoptera: Silphidae). J. Med. Entomol. 58, 64–70 (2021).
Lira, L. A., Barros-Cordeiro, K. B., Figueiredo, B., Galvão, M. F. & Frizzas, M. R. The carrion beetle Oxelytrum discicolle (Coleoptera: Silphidae) and the estimative of the minimum post-mortem interval in a forensic case in Brasília. Brazil. Rev. Bras. Entomol. 64, e201992 (2020).
Novák, M., Jakubec, P., Qubaiová, J., Šuláková, H. & Růžička, J. Revisited larval morphology of Thanatophilus rugosus (Coleoptera: Silphidae). Int. J. Legal Med. 132, 939–954 (2018).
Amendt, J., Richards, C. S., Campobasso, C. P., Zehner, R. & Hall, M. J. R. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 7, 379–392 (2011).
Levot, G. W. Insect fauna used to estimate the post-mortem interval of deceased persons. Gen. Appl. Entomol. 32, 31–39 (2003).
Tillyard, R. J. The Insects of Australia and New Zealand (Angus and Robertson, 1926).
Kořenský, J. K protinožcům, díl 1. [Travel to the antipodes, Part 1]. (J. Otto, 1904).
Kurosawa, Y. Chelidonium Memorandums (1). Coleopt. News 50, 7–13 (1980) (in Japanese).
Chu, Y. & Hsiao, M. L. On the so-called Shiraki collection. Chin. J. Entomol. 1, 26–32 (1981) (in Chinese).
Lee, C.-F. & Satô, M. The Helotidae of Taiwan (Coleoptera: Cucujoidea). Zool. Stud. 45, 529–552 (2006).
Chu, Y. Additional notes on the Shiraki collection. Taiwan Nat. Sci. 30, 40–47 (2011) (in Chinese).
Osozawa, S., Fukuda, H., Kwon, H.-Y. & Wakabayashi, J. Quaternary vicariance of tiger beetle, Cicindela chinensis, in Ryukyu, Japan, Taiwan and Korea-China. Entomol. Res. 46, 122–127 (2016).
Ikeda, H., Kubota, K., Cho, Y. B., Liang, H. & Sota, T. Different phylogeographic patterns in two Japanese Silpha species (Coleoptera: Silphidae) affected by climatic gradients and topography. Biol. J. Linn. Soc. 98, 452–467 (2009).
Mahlerová, K., Jakubec, P., Novák, M. & Růžička, J. Description of larval morphology and phylogenetic relationships of Heterotemna tenuicornis (Silphidae). Sci. Rep. 11, 16973 (2021).
Hall, R. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: Computer-based reconstructions, model and animations. J. Asian Earth Sci. 20, 353–431 (2002).
Parham, P. R. Late Cenozoic relative sea-level highstand record from Peninsular Malaysia and Malaysian Borneo: Implications for vertical crustal movements. Bull. Geol. Soc. Malaysia 62, 91–115 (2016).
Brikiatis, L. The De Geer, Thulean and Beringia routes: Key concepts for understanding early Cenozoic biogeography. J. Biogeogr. 41, 1036–1054 (2014).
He, J., Gao, Z., Su, Y., Lin, S. & Jiang, H. Geographical and temporal origins of terrestrial vertebrates endemic to Taiwan. J. Biogeogr. 45, 2458–2470 (2018).
Arnett, R. H. J., Samuelson, G. A. & Nishida, G. M. The Insect and Spider Collections of the World (Sandhill Crane Press, 1993).
Lawrence, J. & Ślipiński, A. Australian beetles Morphology, Classification and Keys Vol. 1 (CSIRO Publishing, 2013).
Kukalová-Peck, J. & Lawrence, J. F. Evolution of the hind wing in Coleoptera. Can. Entomol. 125, 181–258 (1993).
Lawrence, J. F., Zhou, Y.-L., Lemann, C., Sinclair, B. & Ślipiński, A. The hind wing of Coleoptera (Insecta): Morphology, nomenclature and phylogenetic significance. Part 1. general discussion and Archostemata–Elateroidea. In Ann. Zool. vol. 71, (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2009). https://doi.org/10.1007/978-0-387-98141-3.
Microsoft Corporation. Microsoft Encarta Premium 2008. (2007).
National Geospatial-intelligence Agency. NGA GEOnet Names Server. https://geonames.nga.mil/geonames/GNSHome/welcome.html. (Accessed 2 May 2021) (2021).
Google. Google Earth Pro. http://earth.google.com. (Accessed 24 Nov 2021) (2021).
Kohlschütter, C. Fuzzy Gazetteer. [presently non-functional!]. http://isodp.hof-university.de/fuzzyg/query/ (Accessed 26 Mar 2010) (2010).
iNaturalist. iNaturalist. https://www.inaturalist.org (Accessed 7 Feb 2021) (2021).
Phillips, S. J., Dudík, M. & Schapire, R. E. [Internet] Maxent software for modeling species niches and distributions (Version 3.4.1). (2022).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017).
Simon, C. et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87, 651–701 (1994).
Whiting, M. F. Mecoptera is paraphyletic: Multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scr. 31, 93–104 (2002).
Katoh, K. & Standley, D. MAFFT: Iterative refinement and additional methods. Methods Mol. Biol. 1079, 131–146 (2014).
Penn, O. et al. GUIDANCE: A web server for assessing alignment confidence scores. Nucleic Acids Res. 38, W23–W28 (2010).
Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. 41, 95–98 (1999).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A. & Minh, B. Q. IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44, 232–235 (2016).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Swofford, D. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0a. https://paup.phylosolutions.com/ (2002).
Rambaut, A. FigTree v1.4.4. (2018).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Sikes, D. S. & Venables, C. Molecular phylogeny of the burying beetles (Coleoptera: Silphidae: Nicrophorinae). Mol. Phylogenet. Evol. 69, 552–565 (2013).
Toussaint, E. F. A. & Condamine, F. L. To what extent do new fossil discoveries change our understanding of clade evolution? A cautionary tale from burying beetles (Coleoptera: Nicrophorus). Biol. J. Linn. Soc. 117, 686–704 (2016).
Huang, D. Y., Nel, A., Shen, Y. B., Selden, P. A. & Lin, Q. B. Discussions on the age of the Daohugou fauna—evidence from invertebrates. Prog. Nat. Sci. Mater. Int. 16, 308–312 (2006).
Cai, C.-Y. et al. Early origin of parental care in Mesozoic carrion beetles. Proc. Natl. Acad. Sci. 111, 14170–14174 (2014).
Acknowledgements
We are obliged to all colleagues and curators listed in the Material and Methods section for the possibility to study the collections under their care, and to Martin Fikáček (NMPC), Bin-Hong Ho (National Sun Yat-Sen University, Kaohsiung, Taiwan), Jan Horák (Prague, Czech Republic), Fang-Shuo Hu (FSHC), Stanislav Jakl (Prague, Czech Republic), Oto Nakládal (Czech University of Life Sciences Prague [CZU], Prague, Czech Republic), Enrico Ruzzier (Padova, Italy) and Petr Šípek (Faculty of Sciences, Charles University, Prague, Czech Republic) for providing additional dry or DNA-grade alcohol samples of Diamesus material for our study. Wolfgang Barries (WBAC), Zhen-Yi Chen (ZYCC), Keitaro Harusawa (KHAC), Jiří Háva (JHAC), Fang-Shuo Hu (FSHC), Yun Ji (YJIC), Alfred F. Newton (FMNH), Stewart B. Peck (Ottawa, Canada), Jan Schneider (JSCC), Derek S. Sikes (DSSC) and Cheng-Yan Tu (CYTC) kindly provided data on Diamesus from their collections. Martin Fikáček and Fang-Shuo Hu are thanked for sending data on Diamesus from NMNS, and Fang-Shuo Hu helped also with transliteration and interpretation of Taiwanese localities. Fang-Shuo Hu and Shûhei Nomura (NSMT) are thanked for discussions on the occurrence of D. osculans in Taiwan and Japan, respectively. Miroslav Hyliš (Charles University, Prague, Czech Republic) is acknowledged for preparing SEM samples and providing access to scanning electron microscope. Cheng-Bin Wang (Mianyang Normal University, Mianyang, Sichuan, China) helped with transliteration of Chinese localities and Shuran Zhao (CZU, Prague, Czech Republic) assisted with their georeferencing using the original names written in Chinese. Petra Šímová and Vítězslav Moudrý (both CZU, Prague, Czech Republic) kindly georeferenced records of D. osculans from unnamed points on a map published by Peck (2001). Karol Krak (CZU, Prague, Czech Republic) is thanked for assistance with phylogenetic analysis. Maxwell V. L. Barclay (Natural History Museum, London, U.K.) is thanked for comments and language corrections. This work was funded by the Internal Grant Agency of the Faculty of Environmental Sciences, University of Life Sciences Prague (no. 20194222).
Author information
Authors and Affiliations
Contributions
J.R. initiated the study. J.R., K.M. and P.J. wrote the initial manuscript. K.M. obtained the new sequences used in this study and conducted the phylogenetic analysis. J.R., P.J. and H.Š. analysed and photograph-documented morphological characters. J.R. and P.J. edited photographs and J.R. organized them into plates. J.R. and M.N. accumulated the distribution data for Diamesus, J.R. unified and georeferenced the records. P.J. performed the species distribution models. All authors discussed the results, edited, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Růžička, J., Jakubec, P., Mahlerová, K. et al. Integrative taxonomy and species distribution models of the genus Diamesus Hope, 1840 (Coleoptera: Staphylinidae: Silphinae). Sci Rep 13, 3192 (2023). https://doi.org/10.1038/s41598-023-30019-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30019-x
- Springer Nature Limited