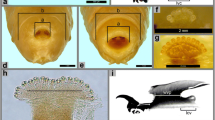
Abstract
Determination of insect species and their instars, occurring on human remains, is important information that allows us to use insects for estimation of postmortem interval and detect possible manipulation with the body. However, larvae of many common species can be identified only by molecular methods, which is not always possible. The instar determination is even more challenging, and qualitative characters that would allow a more precise identification are mostly unknown. Thanatophilus rugosus (Linnaeus, 1758) is a common necrophagous beetle in the whole Palaearctic region from Europe to Japan. The species is often encountered on corpses of large vertebrates including humans, and its potential to become a useful bioindicator for forensic entomology is therefore high. Adults can be easily distinguished from other species; however, larvae were never thoroughly described to allow species and instar identification. The aim of this study was to provide reliable morphological characters that would allow species and instar identification of T. rugosus larvae. The material for morphological study was obtained from rearing under controlled conditions (20 °C and 12:12 h of light/dark period), and specimens that were not studied morphologically were allowed to complete their development. Quantitative and qualitative morphological characters for instar and species identification are described and illustrated. Additionally, we report observations of biology and developmental length for all stages of the species.











Similar content being viewed by others
References
Midgley JM, Richards CS, Villet MH (2010) The utility of Coleoptera in forensic investigations. In: Amendt J, Goff ML, Campobasso CP, Grassberger M (eds) Curr. Concepts Forensic Entomol. Springer Netherlands, Dordrecht, pp 57–68
Midgley JM, Villet MH (2009) Development of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) at constant temperatures. Int J Legal Med 123(4):285–292. https://doi.org/10.1007/s00414-008-0280-0
Bourel B, Tournel G, Hédouin V, Lee Goff ML, Gosset D (2001) Determination of drug levels in two species of necrophagous Coleoptera reared on substrates containing morphine. J Forensic Sci 46(3):600–603. https://doi.org/10.1520/JFS15010J
Matuszewski S, Szafałowicz M, Jarmusz M (2013) Insects colonising carcasses in open and forest habitats of Central Europe: search for indicators of corpse relocation. Forensic Sci Int 231(1-3):234–239. https://doi.org/10.1016/j.forsciint.2013.05.018
Ridgeway JA, Midgley JM, Collett IJ, Villet MH (2014) Advantages of using development models of the carrion beetles Thanatophilus micans (Fabricius) and T. mutilatus (Castelneau) (Coleoptera: Silphidae) for estimating minimum post mortem intervals, verified with case data. Int J Legal Med 128(1):207–220. https://doi.org/10.1007/s00414-013-0865-0
Charabidze D, Vincent B, Pasquerault T, Hedouin V (2016) The biology and ecology of Necrodes littoralis, a species of forensic interest in Europe. Int J Legal Med 130(1):273–280. https://doi.org/10.1007/s00414-015-1253-8
Goff LM (2010) Early postmortem changes and stages of decomposition. In: Amendt J, Goff ML, Campobasso CP, Grssberger M (eds) Curr. Concepts Forensic Entomol. Springer, Dordrecht, pp 1–24
Rivers D, Dahlem G (2014) The science of forensic entomology. John Wiley & Sons, Inc., Chichester
Richards CS, Villet MH (2008) Factors affecting accuracy and precision of thermal summation models of insect development used to estimate post-mortem intervals. Int J Legal Med 122(5):401–408. https://doi.org/10.1007/s00414-008-0243-5
Amendt J, Krettek R, Zehner R (2004) Forensic entomology. Naturwissenschaften 91(2):51–65. https://doi.org/10.1007/s00114-003-0493-5
Wells JD, Sperling FAH (2001) DNA-based identification of forensically important Chrysomyinae (Diptera: Calliphoridae). Forensic Sci Int 120(1-2):110–115. https://doi.org/10.1016/S0379-0738(01)00414-5
Wells JD, Pape T, Sperling FAH (2001) DNA-based identification and molecular systematics of forensically important Sarcophagidae (Diptera). J Forensic Sci 45(5):1098–1102. https://doi.org/10.1520/JFS15105J
Willows-Munro S, Schoeman MC (2015) Influence of killing method on Lepidoptera DNA barcode recovery. Mol Ecol Resour 15(3):613–618. https://doi.org/10.1111/1755-0998.12331
Cho S, Epstein SW, Mitter K, Hamilton CA, Plotkin D, Mitter C, Kawahara AY (2016) Preserving and vouchering butterflies and moths for large-scale museum-based molecular research. Peer J 4:e2160. https://doi.org/10.7717/peerj.2160
Dyar HG (1890) The number of molts of Lepidopterous larvae. Psyche A J Entomol 5:420–422
Klingenberg CP, Zimmermann M (1992) Dyar rule and multivariate allometric growth in 9 species of waterstriders (Heteroptera, Gerridae). J Zool 227(3):453–464. https://doi.org/10.1111/j.1469-7998.1992.tb04406.x
Midgley JM, Villet MH (2009) Effect of the killing method on post-mortem change in length of larvae of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) stored in 70% ethanol. Int J Legal Med 123(2):103–108. https://doi.org/10.1007/s00414-008-0260-4
Velásquez Y, Viloria AL (2010) Instar determination of the neotropical beetle Oxelytrum discicolle (Coleoptera: Silphidae). J Med Entomol 47(5):723–726. https://doi.org/10.1603/me09058
Fratczak K, Matuszewski S (2014) Instar determination in forensically useful beetles Necrodes littoralis (Silphidae) and Creophilus maxillosus (Staphylinidae). Forensic Sci Int 241:20–26. https://doi.org/10.1016/j.forsciint.2014.04.026
Frątczak K, Matuszewski S (2016) Classification of forensically-relevant larvae according to instar in a closely related species of carrion beetles (Coleoptera: Silphidae: Silphinae). Forensic Sci Med Pathol 12(2):193–197. https://doi.org/10.1007/s12024-016-9774-0
Jakubec P (2016) Thermal summation model and instar determination of all developmental stages of necrophagous beetle, Sciodrepoides watsoni (Spence) (Coleoptera: Leiodidae: Cholevinae). Peer J 4:e1944. https://doi.org/10.7717/peerj.1944
Stillwell RC, Fox CW (2009) Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: local adaptation versus phenotypic plasticity. Oikos 118(5):703–712. https://doi.org/10.1111/j.1600-0706.2008.17327.x
Anderson RS, Peck SB (1985) The insects and arachnids of Canada, part 13: the carrion beetles of Canada and Alaska (Coleoptera: Silphidae and Agyrtidae). Agriculture Canada, Ottawa
Navarette-Heredia JL (2009) Silphidae (Coleoptera) de México: diversidad y distribución. Universidad de Guadalajara, Guadalajara
Růžička J (2015) Silphidae. In: Löbl I, Löbl D (eds) Cat. Palaearct. Coleopt. Vol. 2/1. Hydrophiloidea–Staphylinoidea, Revis. Updat. Ed. Brill, Leiden & Boston, pp 291–304
Dobler S, Müller JK (2000) Resolving phylogeny at the family level by mitochondrial cytochrome oxidase sequences: phylogeny of carrion beetles (Coleoptera, Silphidae). Mol Phylogenet Evol 15:390–402. https://doi.org/10.1006/mpev.1999.0765
Sikes DS, Trumbo ST, Peck SB (2005) Silphinae. In: Tree Life Web Proj. http://tolweb.org/Silphinae/26994/2005.02.07. Accessed 26 Jul 2017
Schawaller W (1981) Taxonomie und Faunistik der Gattung Thanatophilus (Coleoptera: Silphidae). Stuttg Beitr Naturkunde, Ser A 351:1–21
Kozminykh VO (1994) A new species of carrion beetles of the genus Thanatophilus (Coleoptera, Silphidae) from the southern Urals. Zool Zhurnal 73:161–165
Růžička J (2002) Taxonomic and nomenclatorial notes on Palaearctic Silphinae (Coleoptera : Silphidae). Acta Soc Zool Bohemicae 66:303–320
Ji Y (2012) The carrion beetles of China (Coleoptera: Silphidae). China Forestry Publishing House, Beijing
Daniel CA, Midgley JM, Villet MH (2017) Determination of species and instars of the larvae of the afrotropical species of Thanatophilus Leach, 1817 (Coleoptera, Silphidae). African Invertebr 58(2):1–10. https://doi.org/10.3897/AfrInvertebr.58.12966
von Lengerken H (1929) Studien über die Lebenserscheinungen der Silphini (Col.). XI-XIII. Thanatophilus sinuatus F., rugosus L. und dispar Hrbst. Z Morphol Ökol Tiere 14:654–666
von Lengerken H (1938) Beziehungen zwischen der Ernährungsweise und der Gestaltung der Mandibeln bei den Larven der Silphini (Coleopt.) Zool Anz 122:171–175
Dorsey CK (1940) A comparative study of the larvae of six species of Silpha (Coleoptera, Silphidae). Ann Entomol Soc Am 33(1):120–134. https://doi.org/10.1093/aesa/33.1.120
Paulian R (1941) Les premiers états des Staphylinoidea. Étude de morphologie comparée Mém Mus Natl Hist Nat (Nouv Ser) 15:1–361
Prins AJ (1984) Morphological and biological notes on some South African arthropods associated with decaying organic matter. Part 2. The predatory families Carabidae, Hydrophilidae, Histeridae, Staphylinidae and Silphidae (Coleoptera). Ann South African Mus 92:295–365
Xambeu PJV (1900) Moeurs et métamorphoses d’insectes, 9e mémoire, deuxieme partie. Rev Entomol Caen 19:1–56
Xambeu PJV (1892) Moeurs et métamorphoses d’insectes (Suite). Ann Soc Linn Lyon 39:137–194
Anderson RS (1987) Scientific note. The larva of Thanatophilus trituberculatus (Kirby) (Coleoptera: Silphidae). Coleopt Bull 41:34
Kočárek P (2003) Decomposition and Coleoptera succession on exposed carrion of small mammal in Opava, the Czech Republic. Eur J Soil Biol 39(1):31–45. https://doi.org/10.1016/S1164-5563(02)00007-9
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2010) Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: composition and residency patterns of carrion fauna. Forensic Sci Int 195(1-3):42–51. https://doi.org/10.1016/j.forsciint.2009.11.007
Dekeirsschieter J, Verheggen FJ, Haubruge E, Brostaux Y (2011) Carrion beetles visiting pig carcasses during early spring in urban, forest and agricultural biotopes of Western Europe. J Insect Sci 11:73. https://doi.org/10.1673/031.011.7301
Velásquez Y, Viloria AL (2009) Effects of temperature on the development of the Neotropical carrion beetle Oxelytrum discicolle (Brullé, 1840) (Coleoptera: Silphidae). Forensic Sci Int 185(1-3):107–109. https://doi.org/10.1016/j.forsciint.2008.12.020
Matuszewski S (2011) Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci Int 212(1-3):180–188. https://doi.org/10.1016/j.forsciint.2011.06.010
Novák M (2017) Redescription of immature stages of central European fireflies, part 1: Lampyris noctiluca (Linnaeus, 1758) larva, pupa and notes on its biology (Coleoptera: Lampyridae: Lampyrinae). Zootaxa 4247:429–444. https://doi.org/10.11646/zootaxa.4247.4.5
Lawrence JF, Slipinski SA (2013) Australian beetles. Volume 1: morphology, classification and keys. CSIRO Publishing, Collingwood
Kilian A, Mądra A (2015) Comments on the biology of Sciodrepoides watsoni watsoni (Spence, 1813) with descriptions of larvae and pupa (Coleoptera: Leiodidae: Cholevinae). Zootaxa 3955(1):45–61. https://doi.org/10.11646/zootaxa.3955.1.2 10.11646/zootaxa.3955.1.2
Acknowledgements
Thanks are due to Miroslav Hyliš (Praha, Czech Republic) for preparing our electron imaging samples and providing needed guidance at the SEM laboratory.
Funding
The project was supported by the Ministry of the Interior of the Czech Republic (grant no. VI20152018027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Šuláková is an employee of the Faculty of Environmental Sciences and Police of the Czech Republic.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Online Resource 1
(XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Novák, M., Jakubec, P., Qubaiová, J. et al. Revisited larval morphology of Thanatophilus rugosus (Coleoptera: Silphidae). Int J Legal Med 132, 939–954 (2018). https://doi.org/10.1007/s00414-017-1764-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1764-6