Abstract
Cisplatin (CP) is one of the most frequently used chemotherapy agents. The objective of this design was to determine the ameliorative effect of lycopene (LP) and/or N-acetylcysteine (NAC) in rats with hepatic and renal toxicity induced by CP. Rats were divided randomly into 7 groups (7 rats/group): control vehicle group (saline only), the LP group (10 mg/kg, orally), the NAC group (150 mg/kg, orally), the CP group (7.5 mg/kg, IP on day 27), the LP-CP group, the NAC-CP group, and the LP-NAC-CP group. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (APK), and levels of urea, creatinine, and lipids (cholesterol, triglycerides, and low-density lipoprotein-cholesterol) increased after CP injection in the serum. Moreover, CP decreased levels of protein, albumin, and HDL cholesterol. Meanwhile, malondialdehyde significantly increased with a decrease in reduced glutathione, superoxide dismutase, and catalase in the liver and kidney tissues. CP also induced some pathological lesions and increased the expression of caspase-3 in the liver and kidney tissues. Administration of LP and NAC alone or in combinations ameliorated hepatorenal toxicity and apoptosis induced by CP.
Similar content being viewed by others
Introduction
Cisplatin (CP) is one of the most frequently used chemotherapeutic drugs. Although cisplatin has well-known antineoplastic activity against multiple malignancies, adverse effects, mainly due to increased oxidative and cell apoptotic effects in different tissues, limit its administration1,2. CP is a highly active cytotoxic agent in cancer treatment3; however, nephrotoxicity limits its use4. CP administration leads to hepatic damage due to lipid peroxidation and oxidation leading to changes in liver biomarkers and antioxidant enzymes5. Many studies have reported CP-induced hepatotoxicity6,7,8,9 and nephrotoxicity10,11,12,13,14.
Lycopene (LP) is an acyclic carotenoid (a vitamin A derivative) with powerful and effective free-radical scavenging activity and anti-inflammatory, immunostimulant, antibiotic and anti-mutagenic effects15,16. LP is a red pigment that presents in high abundance in tomatoes and other red fruits. The chemical structure of lycopene contains many double bonds that have a major role in the scavenging reactive oxygen species (ROS)17. Consumption of tomatoes or tomato products is often associated with increased circulating lycopene levels and decreased oxidative damage to lipids, proteins, and DNA18. LP, a natural antioxidant, has antioxidant activity against several oxidative stress-mediated tissue injuries19. In vitro lycopene, antioxidant efficacy is up to 100 times more potent than vitamin E. In addition, LP has a chemo-preventive activity against certain forms of cancers20. The protective activity of LP against chemotherapeutic-induced hepatorenal damage has attracted considerable research activity in recent years.
N-acetylcysteine (NAC) is a medicinal and dietary supplement commonly used as a mucolytic agent to treat paracetamol overdose21. NAC is a pro-drug of L-cysteine, a precursor to glutathione (GSH). The oxidant-antioxidant balance can be modulated by NAC regulation of GSH levels in cells, inhibiting lipid peroxidation, and scavenging ROS22,23. NAC is capable of restoring the pro-oxidant/antioxidant balance and has been commonly used as an efficient antioxidant against oxidative stress both in vivo and in vitro24,25. CP has been used clinically for many diseases as a heavy metal chelator to protect against oxidative stress and prevent cell injury26. NAC has beneficial medicinal properties, including inhibition of carcinogenesis, tumorigenesis, mutagenesis, and tumor growth and metastases27.
The target of this design was to determine the protective effects of LP and/or NAC against hepatic and renal toxicity induced by CP in rats by exploring the biochemical, oxidative stress markers, and expression of caspase-3.
Materials and methods
Chemicals
CP (CAS No: 15663–27-1), (50 mg/ml parenteral administration) was bought from EIMC Pharmaceuticals Company (Cairo, Egypt). LP (CAS No: 502–65-8) was bought from Sigma Aldrich Company (Saint Louis, MO, USA). NAC (CAS No: 616–91-1) was bought from the South Egypt Drug Industries Company (SEDICO) (6 October City, Egypt). The analytical kits were bought from Bio-diagnostics Company (Giza, Egypt).
Experimental rats and design
Forty-nine Wister Albino male rats with a weight of 190 ± 10 g were derived from the Egyptian Organization for Biological Products and Vaccines. All animals were housed at 25 ± 2° C, exposed to a 12:12 h light / dark cycle and given free access to water and commercial pellet, and left for 7 days for acclimatization prior to experiment. The experimental rats complied with the guidelines for the care and use of laboratory animals that were ethically approved by the Research Ethical Committee of the Faculty of Veterinary Medicine, Benha University, Egypt (Approval No. BUFVTM 03–03-21). Rats were divided randomly into seven groups (7 rats/group) as follows: the vehicle control group was given saline only once daily; the LP group received 10 mg/kg LP orally once daily28; the NAC group received 150 mg/kg NAC orally once daily29; the CP toxic control group received saline orally once daily and a single 7.5 mg/kg IP dose of CP on the 27th day of the experiment30; the LP + CP, NAC + CP, and LP + NAC + CP groups received LP, NAC, and/or CP as described above. Saline, LP, and NAC were administered for 30 days at 10 AM.
Sampling and processing
Rats were anesthetized by isoflurane 1 day after the last treatment. Blood samples were collected from the retro-orbital plexus for separation of the serum (centrifugation at 1200 g for 15 min). The separated samples were stored at − 20 °C for further biochemical analysis. The biochemical parameters were Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, total protein, urea, creatinine, total cholesterol, HDL-cholesterol, triglycerides, and low-density lipoprotein (LDL). The previous biochemical tests were measured according data protocol provided by using commercial kits obtained from Biodiagnostic Company, Giza, Egypt. Finally, all dead rats (49) and remnants of samples were buried in the strict hygienically controlled properly constructed burial pit.
The livers and kidneys were quickly excised, washed with saline (0.9% NaCl in distilled water), and perfused with ice-cold 50 mmol/L sodium phosphate-buffered saline (100 mmol/L Na2HPO4/NaH2PO4, pH 7.4) containing 0.1 mmol/L EDTA. The tissue samples were stored at -80 °C until used. The tissue samples were homogenized on ice using an electrical homogenizer where 1 g tissue was homogenized with 5 ml phosphate buffer pH 7.4. N-ethylmaleimide was added directly after homogenization to prevent oxidation of GSH. After homogenization, the homogenates have been centrifuged at 1200 × g for 20 min at 4 °C for separation of supernatants. These supernatants were used for the detection of biomarkers of oxidative stress. The measured oxidative stress biomarkers were malondialdehyde (MDA; Biodiagnostic, # MD 2529, Egypt), catalase (CAT, Biodiagnostic, # CA 2517, Egypt), superoxide dismutase (SOD; Biodiagnostic, # SD 2521, Egypt), and reduced glutathione (GSH; Biodiagnostic, # SD 2511, Egypt).
For histological and immunohistochemical analysis the remaining liver and kidney tissues were immediately preserved with ten percent neutral buffered formalin for 24 h. After that, the tap water was used for washing the samples then the samples immersion in ethyl alcohol serial dilutions. Then the specimens were embedded in paraffin and cut into Sects. (4 μm thickness). The cut sections were stained with hematoxylin and eosin for histopathological examination under a light microscope31. For immunostaining, sections of the liver and kidney tissues were deparaffinized and dehydrated sequentially in graded ethyl alcohol. Then, sections were autoclaved (121 °C for 5 min) in distilled water for achieving antigen retrieval. After that, the slides were immersed in 3% H2O2 for inactivation of the endogenous peroxidase. For reducing nonspecific reactions, the slides were blocked in 5% bovine serum albumin blocking reagent for 20 min after washing 3 times in PBS. The blocking slides were incubated with polyclonal anti-caspase 3 antibodies (Invitrogen, Cat# PA5-77,887, dilution 1/100) for overnight at 4 °C followed by incubation with avidin–biotin complex (ABC kit, Vector Laboratories) at 37 °C for 45 min. The reaction product was visualized by treatment with 3,3-diaminobenzidine tetrahydrochloride (DAB) and the slides were counterstained with Mayer’s hematoxylin.
Statistical analysis
Data are represented as the mean ± SE. Data were analyzed by one-way ANOVA followed by Duncan’s post hoc test for multiple group comparisons using the statistical software package SPSS for Windows (Version 21.0; SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at P < 0.05.
Ethical approval
The current study was approved by the Ethical Committee for the live animals sampling at the Faculty of Veterinary Medicine, Benha University, Egypt.
Consent for publication
Not applicable.
Results
Cisplatin made an induction for hepatoxicity and nephrotoxicity that indicated by the elevated serum levels of the liver and kidney biomarkers (Table 1). AST, ALT, and ALP activities and concentration of creatinine, urea, cholesterol, triglycerides, and LDL cholesterol were substantially increased as a result of CP treatment compared to those of the control rats. Also, CP reduced the serum concentrations of total protein, albumin, and HDL cholesterol. On the other side, the case is different where these parameters were significantly reduced in the CP treated rats with LP, NAC, or combination treatment (LP and NAC) compared to the CP group. Notably, these parameters were significantly decreased when CP-intoxicated rats were treated with both LP and NAC relative to treatment with LP or NAC alone. The values were significantly lower compared to the controls. Thus, a combination of LP and NAC indicated better protection from hepatorenal damage caused by CP than either alone.
The effects of CP intoxication and treatment with LP, NAC, and their combination on MDA, reduced glutathione, and antioxidant enzymes in the liver and kidney tissues are shown in Tables 2 and 3, respectively. MDA levels increased significantly and CAT, SOD, and GSH levels in the liver decreased significantly in CP-intoxicated rats compared to control rats. LP and NAC treatments attenuated the effects of CP on MDA in the liver tissue, CAT, SOD, and GSH, but these values were still significantly different from control values. Combined LP and NAC treatment significantly improved oxidative damage caused by CP in hepatic and renal tissues compared to the LP or NAC treatments alone. These results were confirmed by the result of histopathology.
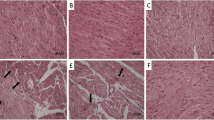
Histolopathological findings showed normal organized hepatocytes forming radiating hepatic cords around the central vein, with normal hepatic tissues including blood sinusoids and portal structures that observed in tissue sections from control saline, LP, and NAC-treated rats. In contrast, we observed severe alterative changes in the hepatic parenchyma, including loss of normal arrangement of hepatic cords, congested central vein and sinusoids, a foamy vacuolated cytoplasm and marked degenerative changes associated with severe nuclear pyknosis that noticed in sections of CP treated rats. Interestingly, LP, NAC, and their combination notably restored the usual hepatic architecture (Fig. 1).
Histopathological changes in liver sections. (a)–(c) Normal organization of the hepatic cords (thick arrow), blood sinusoid (thin arrows), and central vein (CV) of the liver in the control (a), LP (b) and NAC (c) groups. CP-induced changes in the liver include severe degenerative changes, such as loss of normal hepatic cords arrangement, vacuolation (thin arrows), foamy appearance (thick arrow), and pycnotic nuclei of some hepatocytes (S). (e) CP-induced loss of normal cellular architecture of hepatic cords with severe hepatic vacuolation (thick arrow), dilated blood sinusoid (thin arrow), and pycnotic nuclei of hepatocytes (short arrow). (f) histological changes in the liver in the LP + CP group. Moderate effects in the liver include congested blood vessels (CV), dilated blood sinusoid (thick arrow), and condensation of nuclear chromatin of hepatocytes (thin arrow). (g) Histological changes in the liver in the NAC + CP group. Moderate effects in the liver include congested CV, hydropic degeneration (thin arrows), and degranulated cytoplasm of hepatocytes (thick arrow). (h) Histological changes in the liver in the LP + NAC + CP group. Mild effects in the liver include dilated blood vessels (CV) and blood sinusoid (arrows). Scale bar = 50 μm.
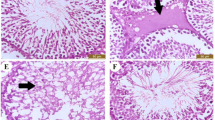
On the other side, Fig. 2 showed the normal architecture of the renal cortex, renal corpuscle, glomerulus, proximal convoluted tubules, and distal convoluted tubules of rats from the control, LP, and NAC groups. In contrast, CP-intoxicated rats exhibited severe nephrotic lesions associated with marked degenerative changes within the renal tubular epithelial lining, congested inter-renal blood vessels, hydropic degeneration, pyknotic nuclei in epithelial cells, and hyaline cast materials in the lumen of most tubules. Treatment with LP, NAC, or their combination prevented the histopathological kidney changes induced by CP.
Histopathological changes in kidney sections. (a)–(c) Normal architecture of the renal cortex, renal corpuscle (arrow), glomerulus (G), proximal convoluted tubules (P), and distal convoluted tubules (D) in the control (a), LP (1b), and NAC (d) groups. (d) CP-induced severe degenerative changes 1in the renal tubule epithelial lining (f), congested inter-renal blood vessels (I), hydropic degeneration (thin arrow), and pycnotic nuclei of epithelial cells (thick arrow). (e) CP-induced loss of normal architecture in the renal tubules, with hyaline cast materials in the lumen of most tubules (thick arrows), degranulated cytoplasm in some epithelial cells (d), and desquamated cells (thin arrow). (f) Histological changes in the kidney in the LP + CP group. Moderate effects in the renal tubules and degenerated and sloughed epithelial cells (thick arrow). (g) Histological changes in the kidney in the NAC + CP group. Moderate effects include congested inter-renal blood vessels (C), hyaline cast materials in the lumen of some tubules (h), and necrosis in some epithelial cells (e). (h) Histological changes in the kidney in the LP + NAC + CP group. Mild effects include proteinaceous materials in the lumen of some tubules (thick arrows). Scale bar = 50 μm.
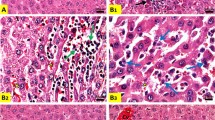
Regarding results of the immunohistochemical, there was a dramatic up-regulation of caspase-3 either cytoplasmic or nuclear expression in the hepatic and renal tissues caused by CP (Fig. 3 and 4, respectively). On the other side, caspase-3 expressions slightly up-regulated in contrast to the control group were found in the LP + CP and NAC + CP groups. Moreover, the CP-mediated caspase-3 up-regulation reduced sharply by combined treatment of LP and NAC.
Changes in hepatic caspase-3 expression. (a)–(c) showed the negative immunostaining reactions in the Control (a), LP (b), and NAC (c) groups. (d) and (e) CP-induced changes showing severe immunostaining reaction. (f) Caspase staining in the LP + CP group showing moderate immunostaining. (g) Caspase staining in the NAC + CP group showing moderate immunostaining. (h) Caspase staining in the LP + NAC + CP group showing mild immunostaining. Scale bar = 50 μm.
Changes in renal caspase-3 expression. (a)–(c) showed the negative immunostaining reactions. (a). Control group showing very mild caspase-3 immunostaining. Caspase-3 staining in the LP (b) and NAC (C) groups showing the negative immunostaining reaction. (d) and (e) treated group with CP showed severe immunostaining reaction. (f) Caspase staining in the LP + CP group showing moderate immunostaining. (g) Caspase staining in the NAC + CP group showing moderate immunostaining. (h) Caspase staining in the LP + NAC + CP group showing mild immunostaining. Scale bar = 50 μm.
Discussion
CP elicits anticancer effects by interacting with DNA and inducing programmed cell death. Multiple in vitro studies have demonstrated the cytotoxic effects of CP in different cell lines, but only a few in vivo studies have been performed4,5,32,33,34,35,36. Our findings are consistent with the in vivo results of other studies, including the involvement of oxidative stress and apoptotic mechanisms in CP-induced hepatorenal damage and the potential use of LP and NAC as protective agents against CP-induced injury.
Elevated activities of liver enzymes indicate cellular leakage and loss of functional hepatocyte integrity; the liver enzymes are released into the bloodstream when hepatocyte plasma membranes are impaired1. In this study, CP-induced hepatotoxicity was evidenced by significant alternations in serum liver enzymes (AST, ALT, and ALP). CP is taken up by the liver and accumulates in hepatocytes, causing cellular damage that eventually leads to increases circulating liver enzymes8. In addition, CP elevated creatinine and urea levels, in agreement with previous studies1,17,37. Elevated creatinine and urea levels are caused by reduced glomerular filtration rate. Moreover, Cayir et al.38 attributed the toxicity of the liver and kidney caused by CP to free radicals that generate in the cells of the liver and kidney, resulting in peroxidation of the lipid and consequently leads to oxidative stress that damage cells.
CP administration induced significant decreases in total circulating protein and albumin. This result was in agreement with Abuzinadah and Ahmad39. Following liver damage, CP intoxication reduces protein synthesis and alters the functional integrity of the kidney, leading to proteinuria and, eventually, to decreased circulating protein levels40.
Administration of CP resulted in significantly increased circulating cholesterol, triglycerides, and LDL-cholesterol, and decreased HDL-cholesterol. The liver plays a vital role in regulating plasma cholesterol levels. Thus, when hepatic dysfunction is induced by drug treatment, serum total cholesterol (TC) and LDL-cholesterol levels are increased41. The substantial rise in serum levels of TC, triglycerides (TG), and LDL-cholesterol following exposure of rats to cisplatin is likely due to the adverse effects of CP, leading to hepatocellular dysfunction and impaired lipid metabolism, in agreement with the findings of Akindele et al.42. The liver synthesizes TG and transforms TG into very-low-density lipoprotein (VLDL) cholesterol for transport to peripheral tissues and impairment of VLDL-C synthesis results in elevated TG levels43. A marked recovery from CP damage was observed in the LP and NAC-treated groups. In addition, NAC and LP have hypolipidemic effects28,44.
Concerning the oxidative stress/antioxidant parameters, MDA levels (increased lipid peroxidation) were significantly increased and antioxidants (CAT, SOD, and GSH) were significantly decreased in liver and kidney tissues after CP treatment. These results are in agreement with Abd El-Kader and Taha11, Abdel-Razek et al.12, and Elkomy et al.1. The ROS generation such as superoxide anions and hydroxyl radicals results in the mediation of oxidative stress and depletion of plasma antioxidants39.
LP inhibits lipid peroxidation by reacting directly with various ROS and by preventing mitochondrial damage induced by CP. Our results suggest that LP interferes with the oxidation of mitochondrial membrane lipids. LP inhibits lipid peroxidation as a chain breaker, free radical scavenger, and antioxidant modulator45.
The beneficial effects of the NAC are attributed to its role as a strong free radical scavenger. The free sulfhydryl group of NAC can react directly with electrophilic compounds, such as free radicals46. NAC also stimulates GSH synthesis and, thus, stimulates endogenous antioxidant activity22. The direct antioxidant activity and stimulation of endogenous antioxidant activity explain the ability of NAC to restore oxidative homeostasis in the liver and kidney in our study. The free radical scavenging of NAC can prevent disrupted renal blood flow after inferior vena cava occlusion47 and prevent the reduced renal vascular resistance caused by CP48. The effect of NAC on renal blood flow and vascular resistance in CP-intoxicated rats can explain the improvement in renal function observed in this study.
In addition to the ROS scavenging activity, LP and NAC restored the activities of SOD and CAT and the levels of GSH in CP-intoxicated rats. Therefore, we strongly suggest that LP and/or NAC-mediated improvements in the serum biochemical parameters tested in this study were mediated by ROS suppression and the up-regulation of antioxidant mechanisms against CP-induced oxidative injury.
LP is composed of carbon and hydrogen atoms (C40H56) with many double bonds that reduce the energy required for the delocalization of electrons providing a good source of hydrogen atom donation required to stabilize free radicals. Since LP is lipophilic, it is integrated with the lipid bilayer of the cell membrane allowing to abstract H atom from LP instead of unsaturated fatty acids halting CP-induced lipid peroxidation seen by a drastic reduction in MDA49.
NAC is a thiol donor with antioxidant properties. It is an excellent source of sulfhydryl groups and is converted in vivo into metabolites that stimulate glutathione (GSH) production, thereby maintaining intracellular GSH levels, enhancing detoxification, and acting directly as a free-radical scavenger50.
The histological and immunohistochemical observations of the current study were in harmony and confirmed the alterations of the biochemical and oxidant/antioxidant parameters among the experimental groups. CP treatment showed marked nephrotoxic effects, including cytoplasmic vacuolization in tubular epithelial cells and apoptosis as reported by Alhoshani et al.4. Severe degenerative changes in the renal tubules occurred after CP treatment, including hydropic degeneration, pycnotic nuclei, increased cytoplasmic vesicles, cytoplasmic vacuolization, necrosis, and apoptosis of tubular cells, and desquamation of necrotic epithelial cells, filling the tubular lumens and forming hyaline casts. These results are in agreement with the results of Perše and Večerić-Haler51. CP treatment induced expression of caspases-3, suggesting the occurrence of tubular epithelial cell apoptosis. These results confirm the results of Liu et al.52 and Miller et al.53, who demonstrated that CP was metabolically converted to a more potent toxin, which caused DNA injury and mitochondrial DNA and respiration damage. These toxin-induced changes lead to the activation of apoptotic pathways and the initiation of inflammatory responses. The CP-induced hepatotoxicity, indicated by sinusoidal dilation, congestion of blood vessels, and disorganized architecture of hepatic lobules, are consistent with the CP-induced effects reported by Elkomy et al.1.
NAC had no side effects in the liver or kidney, as manifested by the normal histology in these tissues. Our data confirmed that treatment with NAC had a protective effect against nephrotoxicity and hepatotoxicity, indicated by attenuation of the CP-induced degenerative changes in the liver and kidney. Treatment of rats with LP reduced the nephrotoxic and hepatotoxic effects of CP, as indicated by moderate histopathological findings in the liver and kidney. A combination of LP and NAC had a great prophylactic effect against CP-induced hepatotoxicity and nephrotoxicity. The rats treated with LP and NAC before CP treatment had only mild histopathological lesions of the liver and kidney and mild expression of caspase 3, indicating a lowered level of apoptosis. These results are consistent with the findings of Jiang et al.54, who considered LP an antioxidant drug that protects the liver and kidney from oxidative damage induced by CP. These results are also in agreement with Abdel-Wahab et al.46, who demonstrated that NAC attenuated CP-induced nephrotoxicity and restored proper kidney functioning, and with De Vries55, who indicated that NAC promoted liver detoxification. NAC may also reduce CP concentration in the kidney by increasing its renal excretion and/or preventing its accumulation in the renal tissue, as reported by Appenroth56. Zhao and Shichi57 demonstrated that the free sulfhydryl group can react directly with electrophilic compounds, such as free radicals, and Yalcin22 demonstrated that NAC can act as an antioxidant drug.
Pretreatment with LP and/or NAC protected against CP-induced hepatorenal toxicity, as shown by the improved biochemical parameters, oxidative stress markers, histopathology, and caspase-3 expression. A dietary combination of LP and NAC may enhance ROS scavenging capacity17. Our data revealed that co-administration of LP and NAC elicits a better protective effect against CP insult than their individual supplementations.
Also, other natural products protected against hepatorenal toxicity induced by CP as Citrullus colocynthis30, garlic oil9, thymoquinone39, and L-carnitine1.
Conclusion
Overall, our data suggest that CP causes significant tissue damage in the liver and kidney due to oxidative stress and apoptotic mechanisms as evidenced by altered biochemical parameters and histopathological lesions. The combination of LP and NAC exhibits protective effects against CP-mediated damage in the liver and kidney. Moreover, LP and NAC, both individually or in combination, provided pronounced hepatorenal protection against CP-induced oxidative stress and apoptosis.
Data availability
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
References
Elkomy, A. et al. L-Carnitine mitigates oxidative stress and disorganization of cytoskeleton intermediate filaments in cisplatin-induced hepato-renal toxicity in rats. Front. Pharmacol. 11, 574441 (2020).
Sallam, A. O. et al. The ameliorative effects of L-carnitine against Cisplatin induced gonadal toxicity in rats. Pak Vet. 41(1), 147–151 (2021).
Antunes, L. M. G., Darin, J. D. A. C. & Bianchi, M. D. L. P. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol. Res. 41(4), 405–411 (2000).
Alhoshani, A. R. et al. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrol. 18(1), 194 (2017).
Karale, S. & Kamath, J. V. Effect of daidzein on cisplatin-induced hematotoxicity and hepatotoxicity in experimental rats. Indian J. Pharmacol. 49(1), 49–54 (2017).
Boroja, T. et al. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem Toxicol. 118, 252–263 (2018).
Neamatallah, T., El-Shitany, N. A., Abbas, A. T., Ali, S. S. & Eid, B. G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 9(7), 3743–3754 (2018).
Mohamed, H. E. & Badawy, M. M. M. Modulatory effect of zingerone against cisplatin or γ-irradiation induced hepatotoxicity by molecular targeting regulation. Appl Radiat Isot. 154, 108891 (2019).
Abdel-Daim, M. M. et al. Impact of garlic (Allium sativum) oil on cisplatin-induced hepatorenal biochemical and histopathological alterations in rats. Total Sci. Environ. 710, 136338 (2020).
El-Kordy, E. A. Effect of suramin on renal proximal tubular cells damage induced by Cisplatin in rats (histological and immunohistochemical study). J. Microsc. Ultrastruct. 7(4), 153–164 (2019).
Abd El-Kader, M. & Taha, R. I. Comparative nephroprotective effects of curcumin and etoricoxib against cisplatin-induced acute kidney injury in rats. Acta Histochem. 122(4), 151534 (2020).
Abdel-Razek, E. A., Abo-Youssef, A. M. & Azouz, A. A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 243, 117272 (2020).
Barakat, L. A. A., Barakat, N., Zakaria, M. M. & Khirallah, S. M. Protective role of zinc oxide nanoparticles in kidney injury induced by cisplatin in rats. Life Sci. 262, 118503 (2020).
Sadeghi, H. et al. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem. 44(5), e13190 (2020).
Müller, L., Fröhlich, K. & Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 129, 139–148 (2011).
Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 61, 9534–9550 (2013).
Abdel-Daim, M. M. et al. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia. Oreochromis niloticus. Environ. Toxicol. Pharmacol. 69, 44–50 (2019).
Palabiyik, S. S. et al. Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp. Toxicol. Pathol. 65, 853–861 (2013).
Hu, J., Zhang, B., Du, L., Chen, J. & Lu, Q. Resveratrol ameliorates cadmium induced renal oxidative damage and inflammation. Int. J. Clin. Exp. Med. 10, 7563–7572 (2017).
Huang, C. S. & Hu, M. L. Lycopene inhibits DNA damage and reduces hMTH1 mRNA expression in the liver of Mongolian gerbils treated with ferric nitrilotriacetate. Food Chem. Toxicol. 49, 1381–1386 (2011).
Shimizu, M. H. et al. N-acetylcysteine protects against star fruit-induced acute kidney injury. Ren. Fail. 239(1), 193–202 (2017).
Yalcin, S., Bilgili, A., Onbasilar, I., Eraslan, G. & Ozdemir, M. Synergistic action of sodium selenite and N-acetylcysteine in acetaminophen induced liver damage. Hum. Exp Toxicol. 27(5), 425–429 (2008).
Samuni, Y., Goldstein, S., Dean, O. & Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 1830(8), 4117–4129 (2013).
Srivastava, R. K., Rahman, Q., Kashyap, M. P., Lohani, M. & Pant, A. B. Ameliorative effects of dimetylthiourea and N-acetylcysteine on nanoparticles induced cyto-genotoxicity in human lung cancer cells-A549. PLoS One. 6(9), e25767 (2011).
Campos, R. et al. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. Am. J. Physiol. Lung Cell Mol. Physiol. 302(7), L640–L650 (2012).
Kumamoto, M., Sonda, T., Nagayama, K. & Tabata, M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci. Biotechnol. Biochem. 65, 126–132 (2001).
Pendyala, L. & Creaven, P. J. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during phase 1 trial. Cancer Epidemiol. Biomarkers Prev. 4, 245–251 (1995).
Wang, Q., Wang, X., An, J., Wang, C. & Wang, F. Lycopene’s protective effect on oxidative damage of L02 cells and its mechanism. Wei Sheng Yan Jiu. 47(2), 281–306 (2018).
Feng, D. et al. Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’ testis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 792, 35–45 (2015).
Adeyemi, O.O., Ishola, I.O., Ajani, I.D. (2017). Citrullus colocynthis Linn. Fruit extract ameliorates cisplatin-induced hepato-renal toxicity in rats. J. Complement. Integr. Med. 15(1):/j/jcim.2018.15.issue-1/jcim-2017–0086/jcim-2017–0086.xml.
Bancroft, J., Stevens, A., Turner, D. (1996). Theory and practice of histological techniques: churchill livingstone New York. the text. p. 766
Li, C. Y. et al. Urinary metabolomics reveals the therapeutic effect of HuangQi Injections in cisplatin-induced nephrotoxic rats. Sci. Rep. 7(1), 1–12 (2017).
Zhu, X., Jiang, X., Li, A., Zhao, Z. & Li, S. S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients 9(2), 166 (2017).
Rjeibi, I. et al. Lycium europaeum extract: A new potential antioxidant source against cisplatin-induced liver and kidney injuries in mice. Oxid. Med. Cell Longev. 2018, 1630751 (2018).
Ahmad, S. et al. Quantification of berberine in berberis vulgaris l. root extract and its curative and prophylactic role in cisplatin-induced in vivo toxicity and in vitro cytotoxicity. Antioxidants 8(6), 185 (2019).
Kumburovic, I. et al. Antioxidant effects of Satureja hortensis L. attenuate the anxiogenic effect of cisplatin in rats. Oxid. Med. Cell Longev. 2019, 8307196 (2019).
Abo-Elmaaty, A. M. A., Behairy, A., El-Naseery, N. I. & Abdel-Daim, M. M. The protective efficacy of vitamin E and cod liver oil against cisplatin-induced acute kidney injury in rats. Environ. Sci. Pollut. Res. Int. 27(35), 44412–44426 (2020).
Cayir, K. et al. Protective effect of L-carnitine against cisplatin-induced liver and kidney oxidant injury in rats. Cent. Eur. J. Med. 4(2), 184–191 (2009).
Abuzinadah, M. F. & Ahmad, A. Pharmacological studies on the efficacy of a thymoquinone-containing novel polyherbal formulation against cisplatin-induced hepatorenal toxicity in rats. J. Food Biochem. 44(2), e13131 (2020).
Sen, S., De, B., Devanna, N. & Chakraborty, R. Cisplatin induced nephrotoxicity in mice: protective role of Leea asiatica leaves. Ren Fail. 35(10), 1412–1417 (2013).
Atawodi, S. E., Yakubu, O. E., Liman, M. L. & Iliemene, D. U. Effect of methanolic extract of Tetrapleura tetraptera (Schum and Thonn) Taub leaves on hyperglycemia and indices of diabetic complications in alloxan-induced diabetic rats. Asian Pac. J. Trop. Biomed. 4(4), 272–278 (2014).
Akindele, J. A., Iyamu, A. E., Dutt, P., Satti, K. N. & Adeyemi, O. O. Ameliorative effect of hydroethanolic leaf extract of Byrsocarpus coccineus in alcohol- and sucroseinduced hypertension in rats. J. Tradit. Complement Med. 4(3), 177–188 (2014).
Manninen, V. et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation 85, 37–45 (2002).
Lin, C. C., Yin, M. C., Hsu, C. C. & Lin, M. P. Effect of five cysteine-containing compounds on three lipogenic enzymes in Balb/cA mice consuming a high saturated fat diet. Lipids 39(9), 843–848 (2004).
Sheriff, S. A. et al. Lycopene prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide induced fulminant hepatic failure in albino rats. J. Proteome Res. 16(9), 3190–3199 (2017).
Abdel-Wahab, W. M., Moussa, F. I. & Saad, N. A. Synergistic protective effect of N-acetylcysteine and taurine against cisplatin-induced nephrotoxicity in rats. Drug Des. Devel. Ther. 11, 901–908 (2017).
Conesa, E. L. et al. N-acetylcysteine improves renal medullary hypoperfusion in acute renal failure. Am. J. Physiol. 281(3), R730–R737 (2001).
Abdelrahman, A. M. et al. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J. Appl. Toxicol. 30(1), 15–21 (2010).
Abdel-Daim, M. M. et al. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia. Oreochromis niloticus. Environ. Toxicol. Pharmacol. 69, 44–50 (2019).
Saha, L., Kaur, S. & Saha, P. K. N-acetyl cysteine in clomiphene citrate resistant polycystic ovary syndrome: a review of reported outcomes. J. Pharmacol. Pharmacother. 4, 187–191 (2013).
Perše, M. & Večerić-Haler, Ž. Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed. Res. Int. 2018, 1462802 (2018).
Liu, H. et al. Emodin ameliorates cisplatin-induced apoptosis of rat renal tubular cells in vitro by activating autophagy. Acta Pharmacol. Sin. 37(2), 235–245 (2016).
Miller, R. P., Tadagavadi, R. K., Ramesh, G. & Reeves, W. B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2(11), 2490–2518 (2010).
Jiang, W., Guo, Mh. & Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 22(46), 10180–10188 (2016).
De Vries, N. & De Flora, S. N-acetylcysteine. J. Cell Biochem. 17F, 270–277 (1993).
Appenroth, D., Winnefeld, K., Schroter, H. & Rost, M. Beneficial effect of N-acetylcysteine on cisplatin nephrotoxicity in rats. J. Appl. Toxicol. 13(3), 189–192 (1993).
Zhao, C. & Shichi, H. Prevention of acetaminophen-induced cataract by a combination of diallyl disulfide and N-acetylcysteine. J. Ocul. Pharmacol. Ther. 14(4), 345–355 (1998).
Author information
Authors and Affiliations
Contributions
A.E., A.E., S.F., A.S., and M.A. measured biochemistry. R.E. and W.A. worked pathology. G.Y. and E.Y.A. induced toxicity by using cisplatin. M.A., S.F., E.Y.A., and W.A. revised final article. M.A. made interpretation of the results and write original article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, A., Elkomy, A., Elkammar, R. et al. Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats. Sci Rep 11, 13979 (2021). https://doi.org/10.1038/s41598-021-93196-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93196-7
- Springer Nature Limited
This article is cited by
-
An integrated view of cisplatin-induced nephrotoxicity, hepatotoxicity, and cardiotoxicity: characteristics, common molecular mechanisms, and current clinical management
Clinical and Experimental Nephrology (2024)
-
Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats
Environmental Science and Pollution Research (2023)
-
Protective Effect of N-Acetylcysteine Against Aluminum-Induced Kidney Tissue Damage in Rats
Biological Trace Element Research (2023)
-
Safety assessment of fish oil green extraction and in vivo acute toxicity evaluation
Environmental Science and Pollution Research (2022)
-
Therapeutic effect of lycopene in lipopolysaccharide nephrotoxicity through alleviation of mitochondrial dysfunction, inflammation, and oxidative stress
Molecular Biology Reports (2022)