Abstract
Screening of 1,000-years old ice layers from the perennial ice block of Scărișoara Ice Cave (NW Romania) revealed the presence of fungal communities. Using culture-dependent methods and molecular techniques based on DGGE fingerprinting of 18S rRNA gene fragments and sequencing, we identified 50 cultured and 14 uncultured fungi in presently-forming, 400 and 900 years old ice layers, corresponding to 28 distinct operational taxonomic units (OTUs). The dominant ice-contained fungal OTUs were related to Ascomycota, Basidiomycota and Cryptomycota phyla. Representatives of Mucoromycota and Chytridiomycota were also isolated from recent and 400 years old ice samples. The cryophilic Mrakia stokesii was the most abundant fungal species found in the cave ice samples of all prospected ages, alongside other cryophilic fungi also identified in various glacial environments. Ice deposits formed during the Little Ice Age (dated between AD 1,250 and 1,850) appeared to have a higher fungal diversity than the ice layer formed during the Medieval Warm Period (prior to AD 1,250). A more complex fungal community adapted to low temperatures was obtained from all analyzed ice layers when cultivated at 4 °C as compared to 15 °C, suggesting the dominance of cold-adapted fungi in this glacial habitat. The fungal distribution in the analyzed cave ice layers revealed the presence of unique OTUs in different aged-formed ice deposits, as a first hint for putative further identification of fungal biomarkers for climate variations in this icy habitat. This is the first report on fungi from a rock-hosted cave ice block.
Similar content being viewed by others
Introduction
Over the last decades, investigation of icy environments became a major scientific direction to unravel the presence, adaptation mechanisms and role of ice-contained microorganisms1,2,3. In spite of low temperatures, life is not absent in frozen environments, and organisms, primarily bacteria, have found various ways to survive and colonize different icy habitats4,5,6,7,8. Such habitats include polar and high mountain ice caps, alpine glaciers, permafrost and rock-hosted cave glaciers. The later – hereafter called ice caves – refer to ice accumulations in caves, formed as seepage water freezes to form underground perennial ice bodies, some of them thousands of years old9. Apart from surface glaciers (polar or alpine) that form as snow slowly metamorphoses into ice, most of cave ice deposits form from water that has percolated through soil and rock and ponded for days through months before freezing. As such, they could host most peculiar microbial assemblages, and are among the poorest investigated cold habitats10. The presence of bacteria in an alpine ice cave was first mentioned by Margesin and collaborators11 who isolated and characterized a psychrophilic Arthrobacter psychrophenolicus strain from the cave non-icy sediments. Bacterial strains belonging to the genera Pseudomonas, Acidovorax and Brevundimonas were isolated from ice deposits of Oregon Cascades lava tube12, while several profiles of prokaryotic communities from Antarctic volcanic ice caves (i.e, caves carved at the interface between rock and glacier surface) were recently described13. Moreover, the occurrence of Archaea in the sediments of an Austrian ice cave was also mentioned14. In Scărișoara Ice Cave (NW Romania), our previous investigations revealed the presence of diverse bacteria in recent ice stalagmites15, including phototrophic microbes in sunlight-exposed ice, and various types of cultured16 and uncultured17 bacteria in ice layers up to 900 years old from the perennial ice block harbored in the cave.
In addition to prokaryotes, icy environments also contain complex fungal communities3,18,19. In the Arctic, various yeast species were detected in sub-glacial water, sea ice and sea water20, permafrost21, glacier surface and ice cores from Northern Greenland, at depths of about 2 km22,23,24. Fungi were also discovered in various Antarctic habitats such as permafrost25,26, decaying wood27, lake28, sub-glacial29 and sea water30, and ice cores as deep as 3.5 km, corresponding to 1 to 2 million years old ice31. While information on psychrophilic fungi from South American, Asian and Himalayan icy environments is rather scarce, several reports mentioned their presence in glacial habitats from Europe, including sediments from alpine glaciers and permafrost18,32,33, sub-glacial sediments and water, as well as glaciers ice cores28,34.
Ice cave habitats, mainly screened for prokaryotic organisms, also revealed the presence of uncultured and cultured fungi in ice sediments. Fungal strains were isolated from an Arctic glacier cave located in Svalbard archipelago21, and the diversity of uncultured fungi was reported from an Antarctic volcanic ice cave35. Meanwhile, very few reports refer to fungi from perennial ice deposits in alpine caves. Among these, two psychrophilic Cryptococcus spp. strains and one heterobasidiomycete were isolated from an Austrian ice cave and characterized36. In Scărișoara Ice Cave, the presence of fungi in different layers of the perennial ice block was revealed by 18S rRNA gene amplification17.
While the presence of fungi in caves was reported worldwide37, no studies of the possible use of fungi as biomarker of past climatic changes have been carried out so far. Building on the unique records of past climatic and environmental changes derived from the ice in Scărişoara Ice Cave (Romania), identification of fungal diversity throughout the cave ice block could lead to a novel microbial climatic proxy. In this context, the current data, representing the first report on fungal diversity in an alpine ice cave, led to the identification of cultured and uncultured fungi in perennial ice layers up to 900 years old from Scărişoara Ice Cave, based on DGGE analysis and sequencing of 18S rRNA gene amplicons.
Results and Discussion
Cave ice fungal community based on DGGE analysis
Denaturing gradient gel electrophoresis (DGGE) profiles of 18S rRNA gene fragments retrieved from the 1-S, 1-L, 400-O, 900-O and 900-I cave ice samples (Fig. 1A) and from their enrichment cultures (Fig. 1B) revealed the presence of complex fungal communities throughout the cave ice block, with a relatively different profile of fungal amplicons from ice samples collected from different layers and corresponding cultured microbiota.
DGGE profile of 18S rRNA gene amplicons from Scărișoara ice samples. Fungal 18S rRNA gene fragments were amplified from 1-S, 1-L, 400-O, 900-O and 900-I (A) environmental ice samples, and (B) the corresponding enrichment cultures obtained in T1, T2, LB and LBG media at 4 °C and 15 °C, and analyzed by DGGE as previously described16. The number on each sample amplicon corresponds to the fungal sequence (Table 1).
Amplicon based analysis of the DGGE gels (data not shown) revealed a relatively higher number of fungal OTUs in the recent ice deposits 1-S and 1-L (13.22 ± 3.41) as compared to older ice strata (9.33 ± 3.94). Also, the fungal community profiles of the 400-O ice sample and enrichments appeared to be more complex (average OTUs number 10.44 ± 3.61) than those of the 900-O and 900-I samples (average OTUs number 7.11 ± 2.85). These latter ice deposits were formed during the Medieval Warm Period (MWP; AD 800–1,250), that corresponded to warmer and wetter climate conditions than those formed 400 years ago (sample 400-O) during the cold and dry Little Ice Age (LIA), that lasted between AD 1,250 and 1,8509,38,39. In addition to temperature, the higher fungal diversity identified in the 400-O ice sample could be related to the higher organic content of this ice layer as compared to that of the clear ice sample 900-I16. However, the cultured fungal communities at both 4 °C and 15 °C from 400-O ice sample appeared to be more diverse than those from the 900 years old samples (900-O and 900-I), independent of their organic content. Moreover, no significant differences in fungal amplicon profiles were observed between the 900 years old ice layers of high (900-O) and low (900-I) organic content. In this case, the cultured fungal community at 4 °C contained a larger number of amplicons (12.70 ± 3.76) relative to that grown at 15 °C (8.70 ± 3.73). As expected, the cold-active fungal community from Scărișoara Ice Cave enrichments appeared to be more diverse than that growing at higher temperature, similar to the cultured bacterial community from the same habitat16.
Fungal diversity in cave ice
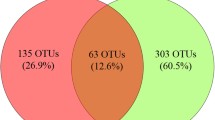
Sequencing of the 64 DGGE-extracted 18S rRNA amplicons, among which 14 from ice samples and 50 from enrichment cultures, led to the identification of 28 different fungal OTUs in the perennial ice from Scărişoara Ice Cave (Table 1). Ten OTUs corresponded to uncultured fungi from cave ice samples, while 23 OTUs resulted from enrichment cultures at 4 °C (16 OTUs) or 15 °C (13 OTUs). The distribution of the identified fungal OTUs in different layers of the cave ice block highlighted 21 specific OTUs for individual ice samples, while 7 fungal OTUs are shared between different ice layers (Fig. 2). Several fungal OTUs from the cave ice block appeared to be homologous to other cold environments-originated strains (Table 1). Among these, the psychrophilic yeast40 Mrakia stokesii was the most represented species (14 OTUs) in the analyzed environmental ice samples and in both 4 and 15 °C enrichment cultures, considering that Mrakia gelida is a synonymous species of M. stokesii41. Another cryophilic yeast, Teberdinia hygrophila, identified in alpine fen soil from Caucasus Mountains42, was present in the clear ice sample 1-L, and recovered from enrichment cultures of both presently-forming and 900 years old ice performed at 4 °C (1-S) and 15 °C (1-S, 900-O and 900-I) (Fig. 2).
Distribution of fungal OTUs in cave ice chronosequence. VENN diagram indicates the number of distinct and shared fungal OTUs in Scărişoara ice samples 1-S, 1-L, 400-O, 900-O and 900-I. The GenBank closest match and distribution (presence/absence: +/−) of 7 fungal strains common to different cave ice layers are indicated in the table.
The ice-originating fungal strain homologous to the Cryptomycota clone KP096139.1 was present in all age ice layers (1-L, 400-O, 900-O, and 900-I samples), while Teberdinia hygrophila, Aureobasidium pullulans, Trametes versicolor and another clone belonging to phylum Cryptomycota (accession no. KP096140.1) were found in recent ice and 900 years old ice layers (Fig. 2). The Aureobasidium pullulans (accession no. KT587333.1) strain was identified in both the 1-S and 400-O samples originating from high organic content ice deposits (Fig. 2). Thelebolus species, also identified in our study (SM.1-L.T1-15), were described from Antarctic lakes43. A close relative of the SM.400-O.LBG-4b enrichment culture from 400 years old ice was Acidomyces acidothermus, previously identified in acidic soils from Czech Republic and Iceland44. Sequences of more ubiquitous fungi (Brachyconidiella monilispora, Valsaria lopadostomoides, Periconia macrospinosa and Trametes versicolor) were also identified in presently-forming and 900 years old ice samples, and recovered in all enrichment cultures at both 4 °C and 15 °C. Thirteen OTUs corresponded to unidentified fungi (Table 1), suggesting a higher diversity of these eukaryotic microorganisms in Scărişoara ice block.
The fungal strains were homologous to known OTUs belonging to five phyla (Table 1). A phylogenetic analysis, using Glomus mosseae [NG017178]45 as outgroup, revealed clearly separated corresponding clades of the fungal tree (Fig. 3). Among these, representatives of the phylum Ascomycota prevailed (25 sequences), followed by those belonging to Basidiomycota (17 sequences), Cryptomycota (13 sequences), Mucoromycota (3 sequences) and Chytridiomycota (1 sequence) phyla. Previous investigations of fungi from glacial environments indicated the dominance of basidiomycetous yeasts. These fungi were identified in Antarctic ice1, Arctic ice21,46, and mountain glaciers in the Apennines32. Fewer studies described the occurrence of ascomycetous fungi in frozen environments, including Arctic ice from Svalbard Archipelago20 and volcanic ice caves on Mt. Erebus in Antarctica35. Our data showed that the ice cave environment selected for particular types of cryophilic fungi. Members of the phylum Cryptomycota were identified in recent ice (1-L) and 400-O samples and recovered in enrichment cultures of ice of all ages. Species belonging to Cryptomycota phylum were identified also in Arctic and Antarctic ice cores from Svalbard47 and from Lake Vostok at 3.5-km depth31, respectively. Viable fungal spores were isolated from Antarctic ice cores more than 1.5 million years old and from Greenland ice cores 140,000 years old48,49. Likewise, the identified fungi in the perennial ice from Scărişoara cave could originate from the surface and trapped within the ice layers that succeeded every year, in the presence of organic debris accumulated on the bottom of supraglacial pond formed during summer time on top of the ice block, while engulfed permanently by the ice during winter50,51.
Phylogenetic tree of fungi from ice samples collected from Scărişoara Ice Cave and from the corresponding enrichment cultures, based on 18S rDNA. The cave ice 18S rRNA gene sequences and closest fungal match (Table 1) were used for the phylogenetic tree construction, using Glomus mosseae [NG017178]45 as outgroup for tree rooting.
In Scărişoara Ice Cave, new layers of ice form on at the surface every year, trapping the microbial communities within the ice, with no further contact with the outer surface of the ice block50. In these settings, the microbial communities may form spores that allow them to endure for centuries22,26. Alternatively, these microorganisms may survive deep in the ice in micro-spaces surrounding various types of debris insoluble in frozen water. A thin film of unfrozen water may form around mineral particles allowing microorganisms to perform metabolic reactions in icy habitats4. To decipher their metabolic status, in the case of culturable fungi found in the cave ice block, further investigations are needed using high-throughput RNA sequencing, a study that is presently underway. Diversity of cultured and uncultured fungi present in perennial ice collected from layers of different ages in Scărişoara Ice Cave can provide evidence for climate change effects. Melting of the cave perennial ice due to global warming could release viable microorganisms trapped for the last hundreds of years in the ice block51. These microbes could be of significant value both for basic science and applications in bionanotechnologies and cryopreservation52.
Ice melting could lead to revival of microorganisms considered extinct, and isolation and characterized of entirely new taxa entrapped in ice. In our study, 19 OTUs shared less than 97% identity with the closest known relatives in GenBank database, constituting putative novel strains. Such new fungi may have major influences of the existing environment by interfering with strains of same species that have evolved and adapted to different environmental conditions, while the new arrivals have rested for centuries of even millennia. The emergence of the new microbes could have a major impact on other organisms, including humans, possibly generating disease outbreaks. Among these, Aureobasidium pullulans, also detected in Scărişoara ice samples, is known to produce various skin infections53.
Our data not only provided a first glimpse on the fungal diversity in different aged ice layers of an ice cave, a pioneering study in this type of habitat, but led to isolation of a series of cultured cold-active fungal strains of putative applicative potential. The presence of some dissimilar fungal OTUs in different aged ice layers of Scărişoara ice block could constitute promising leads for future identification of fungal climate indicators. A deeper sequencing of an extended ice block chronosequence using NGS, currently underway, should provide more insight into the ice cave fungal diversity and the possible use of these microorganisms as a proxy for climate variation.
Material and Methods
Ice sampling
Scărişoara Ice Cave harbors one of the largest (100,000 m3) and the oldest (>10,000 years old) perennial cave ice blocks in the world9. The cave is located in Bihor Mountains, NW Romania, (46°29′23″N, 22°48′35″E), at an altitude of 1,165 m51. The ice block is formed of ice layers deposited annually by freezing of percolating water, containing debris with microorganisms carried to the cave from the surface54,55,56. As a result of this depositional mechanism, each annual stratum is formed by an upper layer of clear ice and a bottom layer of debris-rich ice. Further, two mechanisms are responsible for the formation of extremely rich layers of debris: during periods of enhanced melting, several annual layers of ice are melted and the debris they contain is concentrated in one single, thicker layer, while inflow of water from the surface during wetter periods lead to the formation of similarly thick layers of debris. The two can be distinguished by their content, with the latter being richer in macrofossils washed-in from the surface37. Five ice samples of variable age and sediment content were collected aseptically from newly formed ice (1-S and 1-L) corresponding to direct (S) and indirect (L) sunlight exposed areas, 400 (400-O) and 900 (900-O and 900-I) years old ice deposits, from organic-rich (O) and clear ice (I) layers of the ice block16.
PCR-DGGE
The enrichment cultures of melted ice samples in T1, T2, LB and LBG media57, and the DNA extractions from both the environmental ice samples and enrichments were performed as previously described16. The 18S rRNA gene fragments were amplified using the fungal-specific primers FF390 (CGA TAA CGA ACG AGA CCT) and FR1-GC (the GC clamp is underlined, CCC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GCC G AIC CAT TCA ATC GGT AIT)58. PCR amplification was performed in a 50-μl reaction mixture containing 110 ng cave-ice DNA, 100 pmol of both forward and reverse primers, 0.2 mM dNTP, and 1.25 U MyTaq HS DNA Polymerase (Bioline, London, UK), in the presence of 1× MyTaq Reaction Buffer. Amplification was performed in a Thermal Cycler C1000™ (Bio-Rad Laboratories, Hercules, CA, USA), using an initial denaturation of 95 °C for 8 min, followed by 30 cycles of 95 °C for 30 s, 50 °C for 45 s and 72 °C for 2 min, and a final elongation step at 72 °C for 10 min.
DGGE was carried out using a DGGE-4801-220 system (C.B.S. Scientific Company Inc., Del Mar, CA, USA). PCR products were loaded onto 8% (wt/vol) polyacrylamide (ratio of acrylamide to bisacrylamide 37.5:1) gels. A 20 to 35% linear denaturing gradient was used, where 100% denaturant was defined as 7 M urea and 40% (vol/vol) formamide. Electrophoresis was performed in 1 × TAE buffer (40 mM Tris–acetate, 1 mM Na-EDTA; pH 8.0) at 200 V and 60 °C for 4 h. The gels were stained in 1 × TAE buffer containing 1 μg ml−1 ethidium bromide, and the amplicon profile of the DGGE gels was used to compare the fungal diversity in different environmental samples and enrichment cultures, using statistical tests.
Sequencing and data analysis
18S rDNA amplicons were excised from the DGGE gels, incubated 48 h at 4 °C in 20 µl sterile water, and re-amplified by PCR as described above. Sanger sequencing was performed (Macrogen, Amsterdam, Netherlands) using the FF390 primer58. The DNA sequences were edited using CodonCode Aligner and BioEdit for eliminating the sequencing errors. The closest match of each OTU was determined using the BLAST-NCBI Megablast algorithm and the nucleotide collection database59. Sequence alignment was performed using ClustalW and the phylogenetic tree was generated using the maximum likelihood analysis, using the www.phylogeny.fr platform60. The 18S rDNA sequences were deposited in the GenBank DNA database under accession numbers KY614706-KY614719 (uncultured fungi) and KY614552-KY614602 (cultured fungi).
References
Abyzov, S. S. Microorganisms in the Antarctic ice in Antarctic Microbiology (ed. Friedmann, E. I.) 265-295 (Wiley-Liss, Inc., 1993).
Abyzov, S. S. et al. Microflora in the basal strata at Antarctic ice core above the Vostok lake. Adv. Space Res. 28, 701–706 (2001).
Anesio, A. M., Lutz, S., Chrismas, N. A. M. & Benning, L. G. The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes. 3, 10, https://doi.org/10.1038/s41522-017-0019-0 (2017).
Price, P. B. Microbial life in glacial ice and implications for a cold origin of life. FEMS Microbiol. Ecol. 59, 217–231 (2007).
Priscu, J. C., Christner, B. C., Foreman, C. M. & Royston-Bishop, G. Biological material in ice cores in Encyclopedia of Quaternary Science. (ed. Elias S. A.) 1156–1167 (Elsevier, 2007).
Margesin, R. & Miteva, V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 162, 346–361 (2010).
Boetius, A., Anesio, A. M., Deming, J. W., Mikucki, J. A. & Rapp, J. Z. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat. Rev. Microbiol. 13, 677–90 (2015).
Garcia-Lopez, E. & Cid, C. Glaciers and ice sheets as analog environments of potentially habitable icy worlds. Front. Microbiol. 8, 01407, https://doi.org/10.3389/fmicb.2017.01407 (2017).
Perşoiu, A. et al. Holocene winter climate variability in Central and Eastern Europe. Sci. Rep. 7, 1196–1203 (2017).
Purcarea, C. Microbial life in ice caves in Ice caves (eds Perșoiu, A. & Lauritzen, S.-E.) 173-187 (Elsevier, 2018).
Margesin, R., Schumann, P., Sproer, C. & Gounot, A. M. Arthrobacter psychrophenolicus sp. nov., isolated from an alpine ice cave. Int. J. Syst. Evol. Microbiol. 54, 2067–2072 (2004).
Popa, R., Smith, A. R., Popa, R., Boone, J. & Fisk, M. Olivine-respiring bacteria isolated from the rock-ice interface in a lava-tube cave, a Mars analog environment. Astrobiology 12, 9–18 (2012).
Tebo, B. M. et al. Microbial communities in dark oligotrophic volcanic ice cave ecosystems of Mt. Erebus, Antarctica. Front. Microbiol. 6, 179–192 (2015).
Reitschuler, C., Lins, P., Wagner, A. P. & Illmer, P. Cultivation of moonmilk-born non-extremophilic Thaum and Euryarchaeota in mixed culture. Anaerobe 29, 73–79 (2014).
Hillebrand-Voiculescu, A. et al. Bacterial 16S rRNA gene clone library from recent ice stalagmites of Scărişoara cave. Romanian Journal of Biochemistry 50, 109–118 (2013).
Iţcuş, C., Pascu, M.-D., Brad, T., Perşoiu, A. & Purcarea, C. Diversity of cultured bacteria from the perennial ice block of Scărişoara Ice Cave, Romania. Int. J. Speleol. 45, 89–100 (2016).
Hillebrand-Voiculescu, A. et al. Searching for cold-adapted microorganisms in the underground glacier of Scărişoara ice cave, Romania. Acta Carsologica 43, 319–329 (2014).
Buzzini, P., Branda, E., Goretti, M. & Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 82, 217–241 (2012).
Hassan, M., Rafiq, M., Hayat, M., Shah, A. A. & Hasan, F. Psychrophilic and psychrotrophic fungi: a comprehensive review. Rev. Environ. Sci. Biotechnol. 15, 147–172 (2016).
Butinar, L., Strmole, T. & Gunde-Cimerman, N. Relative Incidence of Ascomycetous Yeasts in Arctic Coastal Environments. Microb. Ecol. 61, 832–843 (2011).
Butinar, L., Spencer-Martins, I. & Gunde-Cimerman, N. Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie Van Leeuwenhoek 91, 277–289 (2007).
Ma, L. J., Catranis, C. M., Starmer, W. T. & Rogers, S. O. Revival and characterization of fungi from ancient polar ice. Mycologist 13, 70–73 (1999).
Miteva, V., Rinehold, K., Sowers, T., Sebastian, A. & Brenchley, J. Abundance, viability and diversity of the indigenous microbial populations at different depths of the NEEM Greenland ice core. Polar Research 34, 25057, https://doi.org/10.3402/polar.v34.25057 (2015).
Starmer, W. et al. Yeasts in the genus Rhodotorula recovered from the Greenland ice sheet in Life in ancient ice (eds Castello J. D. & Rogers S. O.) 181–196 (Princeton University Press, 2005).
Connell, L. B. et al. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb. Ecol. 56, 448–459 (2008).
Kochkina, G. et al. Ancient fungi in Antarctic permafrost environments. FEMS Microbiol. Ecol. 82, 501–509 (2012).
Arenz, B. E., Held, B. W., Jurgens, J. A., Farrell, R. L. & Blanchette, R. A. Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil Biol. Biochem. 38, 3057–3064 (2006).
Turchetti, B. et al. Psychrophilic yeasts from Antarctica and European glaciers: description of Glaciozyma gen. nov., Glaciozyma martinii sp. nov. and Glaciozyma watsonii sp. nov. Extremophiles 15, 573–586 (2011).
Turkiewicz, M., Pazgier, M., Donachie, S. P. & Kalinowska, H. Invertase and a-glucosidase production by the endemic Antarctic marine yeast Leucosporidium antarcticum. Pol. Polar. Res. 26, 125–136 (2005).
Turkiewicz, M., Pazgier, M., Kalinowska, H. & Bielecki, S. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 7, 435–442 (2003).
D’Elia, T., Veerapaneni, R., Theraisnathan, V. & Rogers, S. O. Isolation of fungi from Lake Vostok accretion ice. Mycologia 101, 751–763 (2009).
Branda, E. et al. Yeast and yeast-like diversity in the southernmost Glacier of Europe (Calderone glacier, Apennines, Italy). FEMS Microbiol. Ecol. 72, 354–369 (2010).
Frey, B. et al. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 92; https://doi.org/10.1093/femsec/fiw018 (2016).
Turchetti, B. et al. Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol. Ecol. 63, 73–83 (2008).
Connell, L. & Staudigel, H. Fungal Diversity in a Dark Oligotrophic Volcanic Ecosystem (DOVE) on Mount Erebus, Antarctica. Biology 2, 798–809 (2013).
Margesin, R., Gander, S., Zacke, G., Gounot, A. M. & Schinner, F. Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7, 451–458 (2003).
Vanderholf, K. J., Malloch, D., McAlpine, D. F. & Forbes, G. J. A world review of fungi, yeasts, and slime molds in caves. Int. J. Speleol. 42, 77–96 (2013).
Feurdean, A., Perşoiu, A., Pazdur, A. & Onac, B. P. Evaluating the palaeoecological potential of pollen recovered from ice in caves: a case study from Scărişoara Ice Cave, Romania. Rev. Palaeobot. Palynol. 165, 1–10 (2011).
Perşoiu, A. Climate evolution during the Late Glacial and the Holocene. In Landform dynamics and evolution in Romania (eds Rădone, M. & Vespremeanu-Stroe, A.) 57–66 (Springer, 2017).
Fell, J. W., Statzell, A. C., Hunter, I. L. & Phaff, H. J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie van Leeuwenhoek 35, 433–462 (1969).
Messner, R. et al. Molecular Characterization and Application of Random Amplified Polymorphic DNA Analysis of Mrakia and Sterigmatomyces Species. Int. J. System. Bacteriol. 44, 694–703 (1994).
Sogonov, M. V., Schroers, H.-J., Gams, W., Dijksterhuis, J. & Summerbell, R. C. The hyphomycete Teberdinia hygrophila gen. nov., sp. nov. and related anamorphs of Pseudeurotium species. Mycologia 97, 695–709 (2005).
De Hoog, G. S. et al. Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud. Mycol. 51, 33–76 (2005).
Hujslová, M., Kubátová, A., Kostovčík, M. & Kolařík, M. Acidiella bohemica gen. et sp. nov. and Acidomyces spp. (Teratosphaeriaceae), the indigenous inhabitants of extremely acidic soils in Europe. Fungal Diversity 58, 33–45 (2013).
James, T. Y. et al. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98, 860–871 (2006).
Ma, L. J., Rogers, S. O., Catranis, C. M. & Starmer, W. T. Detection and characterization of ancient fungi entrapped in glacial ice. Mycologia 92, 286–295 (2000).
Singh, P., Tsuji, M., Singh, S. M., Roy, U. & Hoshino, T. Taxonomic characterization, adaptation strategies and biotechnological potential of cryophilic yeasts from ice cores of Midre Lovénbreen glacier, Svalbard, Arctic. Cryobiology 66, 167–175 (2013).
Taylor, K. C. et al. The Holocene-Younger dryas transition recorded at Summit, Greenland. Science 278, 825–827 (1997).
Christner, B. C., Mosley-Thompson, E., Thompson, L. G. & Reeve, J. N. Bacterial recovery from ancient glacial ice. Environ. Microbiol. 5, 433–436 (2003).
Perşoiu, A. Ice dynamics in caves in Ice caves (eds Perșoiu, A. & Lauritzen, S.-E.) 97–108 (Elsevier, 2018).
Kern, Z. & Perşoiu, A. Cave ice - the imminent loss of untapped mid-latitude cryospheric palaeoenvironmental archives. Quat. Sci. Rev. 67, 1–7 (2013).
Margesin, R. & Feller, G. Biotechnological applications of psychrophiles. Environ. Technol. 31, 835–44 (2010).
Chan, G. F., Puad, M. S. A., Chin, C. F. & Rashid, N. A. A. Emergence of Aureobasidium pullulans as human fungal pathogen and molecular assay for future medical diagnosis. Folia Microbiol. 56, 459–467 (2011).
Perşoiu, A. & Pazdur, A. Ice genesis and its long-term mass balance and dynamics in Scărişoara Ice Cave, Romania. The Cryosphere 5, 45–53 (2011).
Perşoiu, A., Onac, B. P. & Perşoiu, I. The interplay between air temperature and ice dynamics in Scărişoara Ice Cave, Romania. Acta Carsologica 40, 445–456 (2011).
Perşoiu, A., Onac, B. P., Wynn, J. G., Bojar, A. V. & Holmgren, K. Stable isotope behavior during cave ice formation by water freezing in Scărişoara Ice Cave, Romania. J. Geophys. Res. Atmos. 116, D02111, https://doi.org/10.1029/2010JD014477 (2011).
Bidle, K. D., Lee, S., Marchant, D. R. & Falkowski, P. G. Fossil genes and microbes in the oldest ice on Earth. Proc. Natl. Acad. Sci. USA 104, 13455–13460 (2007).
Vainio, E. J. & Hantula, J. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 104, 927–936 (2000).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Dereeper, A. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–9, https://doi.org/10.1093/nar/gkn180 (2008).
Acknowledgements
We thank Christian A. Ciubotărescu (“Sfinx” Speleological Association Garda) for speleological support during field trips, and the Apuseni Natural Park Administration and the Commission of Speleological Patrimony, Romania, for granting permissions for sampling in Scărişoara Ice Cave. This study was supported by the H2020 ELAC2014_DCC-0178 Joint Program and the UEFISCDI grants PN-II-ID-PCE-2011-3-074, PN-III-P1-1.1-TE-2016-2210 and PN-II-RU-TE-2014-4-1993.
Author information
Authors and Affiliations
Contributions
C.P. conceived and coordinated the project; T.B., C.I., M.-D.P., A.P., A.H.-V., C.P. participated to sampling field trips; C.I., M.-D.P. and A.H.-V. performed the culture experiments; T.B., C.I., M.-D.P. performed the DNA analysis.; T.B., C.I., L.I., C.P. analyzed the fungal DNA sequences; T.B. wrote the manuscript with substantial contribution from C.P. and A.P.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brad, T., Itcus, C., Pascu, MD. et al. Fungi in perennial ice from Scărișoara Ice Cave (Romania). Sci Rep 8, 10096 (2018). https://doi.org/10.1038/s41598-018-28401-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28401-1
- Springer Nature Limited
This article is cited by
-
Unravelling the hidden diversity of cave mycobiota in Thailand’s Satun Geopark
Scientific Reports (2023)
-
First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core
Scientific Reports (2021)
-
Microbiota entrapped in recently-formed ice: Paradana Ice Cave, Slovenia
Scientific Reports (2021)
-
Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species
Fungal Diversity (2021)
-
Bacterial and archaeal community structures in perennial cave ice
Scientific Reports (2018)