Abstract
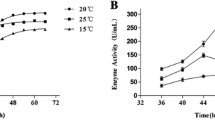
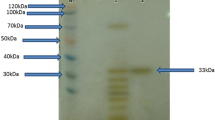
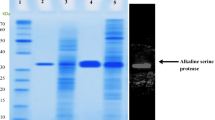
An extracellular serine proteinase, lap2, from the psychrophilic antarctic yeast Leucosporidium antarcticum 171 was purified to homogeneity and characterized. The enzyme is a glycoprotein with a molecular mass of 34.4 kDa and an isoelectric point of pH 5.62. The proteinase is halotolerant, and its activity and stability are dependent neither on Ca2+ nor on other metal ions. Lap2 is a true psychrophilic enzyme because of low optimal temperature (25°C), poor thermal stability, relatively small values of free energy, enthalpy and entropy of activation, and high catalytic efficiency at 0–25°C. The 35 N-terminal amino acid residues of lap2 have homology with subtilases of the proteinase K subfamily (clan SB, family S8, subfamily C). The proteinase lap2 is the first psychrophilic subtilase in this family.






Similar content being viewed by others
References
Anson ML (1938) Estimation of pepsin, trypsin, papain and cathepsin with haemoglobin. J Gen Physiol 22:79–82
Ásgeirsson BK, Bjarnason JB (1993) Properties of elastase from Atlantic cod, a cold-adapted proteinase. Biochim Biophys Acta 1164:91–100
Bae IH, Kang KH (1985) Studies on extracellular protease of Saccharomycopsis lipolytica (Candida lipolytica): purification and properties of enzyme. Korean J Appl Microbiol Bioeng 15:286–292
Bergero R, Girlanda M, Varese GC, Intili D, Luppi AM (1999) Psychrooligotrophic fungi from Arctic soils of Franz Joseph Land. Polar Biol 21:361–368
Bowman JP, McCammon SA, Nichols DS, Skerratt JS, Rea SM, Nichols PD, McMeekin TA (1997a) Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov.—novel species with the ability to produce eicosapentaenoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int J Syst Bacteriol 47:1040–1047
Bowman JP, Nichols DS, McMeekin TA (1997b) Psychrobacter glacincola sp. nov., a halotolerant, psychrophilic bacterium isolated from Antarctic sea ice. Syst Appl Microbiol 20:209–215
Bowman JP, Gosink JJ, McCammon SA, Lewis TE, Nichols DS, Nicols PD, Skerratt JH, Staley JT, McMeekin TA (1998a) Colwellia demingae sp. nov., Colwellia horneae sp. nov., Colwellia rossensis sp. nov. and Colwellia psychrotropica sp. nov.: psychrophilic Antarctic species with the ability to synthesize docosahexaenoic acid (22:6 ω-3). Int J Syst Bacteriol 48:1171–1180
Bowman JP, McCammon SA, Brown JL, McMeekin TA (1998b) Glaciecola punicea gen. nov., sp. nov. and Glaciecola pallidula gen. nov., sp. nov.: psychrophilic bacteria from Antarctic sea-ice. Int J Syst Bacteriol 48:1205–1212
Chattopadhyay MK (2000) Cold-adaptation of Antarctic microorganisms—possible involvement of viable but nonculturable state. Polar Biol 13:223–224
Davail S, Feller G, Narinx E, Gerday C (1994) Cold-adaptation of proteins. Purification, characterization and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA. J Biol Chem 269:17448–17453
Davidow LS, O'Donnell MM, Kaczmarek FS, Pereira DA, DeZeeuw JR, Franke AE (1987) Cloning and sequencing of the alkaline extracellular protease gene of Yarrowia lipolytica. J Bacteriol 169:4621–4629
DelMar EG, Largmann C, Brodrick JW, Geokas MC (1979) A sensitive new substrate for chymotrypsin. Anal Biochem 99:316–320
Denner BM, Mark B, Busse HJ, Turkiewicz M, Lubitz W (2001) Psychrobacter proteolyticus sp. nov., a psychrotrophic, halotolerant bacterium isolated from Antarctic krill (Euphausia superba Dana) excreting a cold-adapted metalloprotease. Syst Appl Microbiol 24:44–53
Donachie SP (1995) Ecophysiological description of Marine Bacteria from Admiralty Bay (Antarctica), and the digestive tracts of selected Euphausiidae. PhD thesis, Department of Antarctic Biology, Polish Academy of Sciences, Warsaw
Durham DR (1993) The elastynolytic properties of subtilisin GX from alkalophilic Bacillus sp. strain 6644 provides a means of differentiation from other subtilisins. Biochem Biophys Res Commun 194:1365–1370
Eklund MW, Spinelli J, Miyauchi D, Groninger H (1965) Characteristic of yeast isolated from Pacific crab meat. Appl Microbiol 13:985–990
Fell JW, Statzell AC, Hunter IL, Phaff HJ (1969) Leucosporidium gen. nov., the heterobasidiomycetous stage of several yeast of the genus Candida. Antonie Van Leeuwenhoek 35:433–462
Feller G, Gerday C (1997) Psychrophilic enzymes: molecular basis of cold adaptation. Cell Mol Life Sci 53:830–841
Gerday C, Aittaleb M, Bentahir M, Chessa JP et al. (2000) Cold-adapted enzymes: from fundamental to biotechnology. Trends Biotechnol 18:103–107
Gomes J, Gomes I, Steiner W (2000) Thermolabile xylanase of the Antarctic yeast Cryptococcus adeliae: production and properties. Extremophiles 4:227–235
Gosink JJ, Herwig RP, Staley JT (1997) Octadecobacter arcticus gen. nov., sp. nov., and O. Antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst Appl Microbiol 20:356–365
Gosink JJ, Woese CR, Staley JT (1998) Polaribacter gen. nov., with three new species, P. irgensii sp. nov., P. franzmannii sp. nov., and P. filamentus sp. nov., gas vacuolate polar marine bacteria of the Cytophaga-Flavobacterium-Bacteroides group and reclassification of Flectobacillus glomeratus as Polaribacter glomeratus comb. nov. Int J Syst Bacteriol 48:223–235
Hummel BCW (1959) A modified spectrophotometric method of determination of chymotrypsin, trypsin and thrombin. Can J Biochem Physiol 37:1393–1400
Jany KD, Lederer G, Mayer B (1986) Amino acid sequence of proteinase K from the mold Tritirachium album Limber. FEBS Lett 199:139–144
Kulakova L, Galkin A, Kurihara T, Yoshimura T, Esaki N (1999) Cold-active serine protease from psychrophilic Shewanella strain Ac10: gene cloning and enzyme purification and characterization. Appl Environ Microbiol 65:611–617
Kwon ST, Terada I, Matsuzawa H, Ohta T (1988) Nucleotide sequence of the gene for aqualysin I (a thermophilic alkaline serine protease) of Thermus aquaticus YT-1 and characteristics of the deduced primary structure of the enzyme. Eur J Biochem 173:491–497
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:681–685
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:165–275
Nicaud JM, Fabre E, Beckerich JM, Fournier P, Gaillardin C (1989) Cloning, sequencing, and amplification of the alkaline extracellular protease (XPR2) gene of the yeast Yarrowia lipolytica. J Biotechnol 12:285–297
Nichols D, Bowman J, Sanderson K, Nichols CM, Lewis T, McMeekin T, Nichols PD (1999) Developments with Antarctic microorganisms: culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr Opin Microbiol 10:240–246
Ogrydziak DM (1993) Yeast extracellular proteases. Crit Rev Biotechnol 13:1–55
Ogrydziak DM, Scharf SJ (1982) Alkaline extracellular protease produced by Saccharomycopsis lipolytica CX161-1B. J Gen Microbiol 128:1225–1234
Petrescu I,·Brasseur-Lamotte J, Chessa JP, Ntarima P, Claeyssens M, Devreese B, Marino G, Gerday C (2000) Xylanase from the psychrophilic yeast Cryptococcus adeliae. Extremophiles 4:137–144
Rawlings ND, Barret AJ (1994) Families of serine peptidases. Methods Enzymol 244:19–61
Ray MK, Uma Devi K, Seshu Kumar G, Shivaji S (1992) Extracellular protease from the Antarctic yeast Candida humicola. Appl Environ Microbiol 58:1992–1923
Sheridan PP, Panasik N, Coombs JM, Brenchley JE (2000) approaches for deciphering the structural basis of low temperature enzyme activity. Biochim Biophys Acta 1543:417–433
Sietzen RJ, Leunissen JAM (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci 6:501–523
Tobe S, Takami T, Ikeda S, Mitsugi K (1976) Production and some enzymatic properties of alkaline proteinase Candida lipolytica. Agric Biol Chem 40:1087–1092
Wagner L, Geisen H, Zahn H (1983) Histochemical localization of high sulphur keratins with silver nitrate. Colloid Polym Sci 261:365–369
Zacharius RM, Zell TE, Norrison JH, Woodlock JJ (1969) Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem 30:148–152
Zucconi L, Pagano S, Fenice M, Selbmann L, Tosi S, Onfri S (1996) Growth temperature preference of fungal strains from Victoria Land, Antarctica. Polar Biol 1653–61
Acknowledgment
This work was supported by funding from the Polish Committee of Scientific Researches (grant number 3P04B 026 22 ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Turkiewicz, M., Pazgier, M., Kalinowska, H. et al. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum . Extremophiles 7, 435–442 (2003). https://doi.org/10.1007/s00792-003-0340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0340-9