Abstract
ATP and its ultimate degradation product adenosine are potent extracellular signalling molecules that elicit a variety of pathophysiological functions in the kidney through the activation of P2 and P1 purinergic receptors, respectively. Extracellular purines can modulate immune responses, balancing inflammatory processes and immunosuppression; indeed, alterations in extracellular nucleotide and adenosine signalling determine outcomes of inflammation and healing processes. The functional activities of ectonucleotidases such as CD39 and CD73, which hydrolyse pro-inflammatory ATP to generate immunosuppressive adenosine, are therefore pivotal in acute inflammation. Protracted inflammation may result in aberrant adenosinergic signalling, which serves to sustain inflammasome activation and worsen fibrotic reactions. Alterations in the expression of ectonucleotidases on various immune cells, such as regulatory T cells and macrophages, as well as components of the renal vasculature, control purinergic receptor-mediated effects on target tissues within the kidney. The role of CD39 as a rheostat that can have an impact on purinergic signalling in both acute and chronic inflammation is increasingly supported by the literature, as detailed in this Review. Better understanding of these purinergic processes and development of novel drugs targeting these pathways could lead to effective therapies for the management of acute and chronic kidney disease.
Key points
-
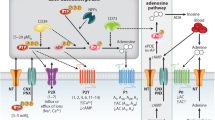
Extracellular ATP and/or ADP acting through P2 receptors exert profound effects on the immune system and also have an impact on kidney pathophysiology.
-
Extracellular adenosine is a signalling molecule in the kidney and in the immune system that acts through P1 or adenosine receptors, often opposing the effects of P2 receptor signalling.
-
The balance between extracellular ATP and adenosine concentrations is largely regulated by the activity of the CD39–CD73 axis and other ATP hydrolysing enzymes, such as the nucleotide pyrophosphatase/phosphodiesterase proteins.
-
CD39 and CD73, an ecto-5′-nucleotidase, constitute a key ‘extracellular master switch’ in the kidney in both health and disease.
-
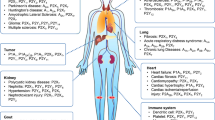
CD39 plays a role in renal inflammation, immunomodulation, acute kidney injury, chronic kidney disease, diabetic nephropathy, polycystic kidney disease, transplantation and renal cell carcinoma.
-
Therapeutic options for kidney disease could include using currently available drugs or developing agents to target purinergic processing.





Similar content being viewed by others
References
Burnstock, G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays 34, 218–225 (2012).
Lohman, A. W., Billaud, M. & Isakson, B. E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 95, 269–280 (2012).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Bailey, M. A., Hillman, K. A. & Unwin, R. J. P2 receptors in the kidney. J. Auton. Nerv. Syst. 81, 264–270 (2000).
Menzies, R. I., Unwin, R. J. & Bailey, M. A. Renal P2 receptors and hypertension. Acta Physiol. 213, 232–241 (2015).
Burnstock, G. Purine and pyrimidine receptors. Cell Mol. Life Sci. 64, 1471–1483 (2007).
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L. & Falzoni, S. The P2X7 receptor in infection and inflammation. Immunity 47, 15–31 (2017).
Kukulski, F., Levesque, S. A. & Sevigny, J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv. Pharmacol. 61, 263–299 (2011).
Blackburn, M. R., Vance, C. O., Morschl, E. & Wilson, C. N. Adenosine receptors and inflammation. Handb. Exp. Pharmacol., 215-269 (2009).
Fredholm, B. B., AP, I. J., Jacobson, K. A., Klotz, K. N. & Linden, J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552 (2001).
Holien, J. K. et al. AMP and adenosine are both ligands for adenosine 2B receptor signaling. Bioorg Med. Chem. Lett. 28, 202–206 (2018).
Rajakumar, S et al. CD73-deficiency protects in kidney ischemia reperfusion injury (IRI) — the role of adenosine A1, A2A and A2B receptors. Nephrology 16, 49 (2011).
Lee, H. T. & Emala, C. W. Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am. J. Physiol. Ren. Physiol 282, F844–F852 (2002).
Zhang, W. et al. Elevated ecto-5’-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ. Res. 112, 1466–1478 (2013).
Vallon, V., Muhlbauer, B. & Osswald, H. Adenosine and kidney function. Physiol. Rev. 86, 901–940 (2006).
Fuxe, K., Guidolin, D., Agnati, L. F. & Borroto-Escuela, D. O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin. Ther. Targets 19, 377–398 (2015).
Moriyama, K. & Sitkovsky, M. V. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J. Biol. Chem. 285, 39271–39288 (2010).
Roberts, V. et al. The differential effect of apyrase treatment and hCD39 overexpression on chronic renal fibrosis after ischemia-reperfusion injury. Transplantation 101, e194–e204 (2017).
Roberts, V., Lu, B., Dwyer, K. M. & Cowan, P. J. Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transpl. Proc. 46, 3257–3261 (2014).
Markovic, D. & Challiss, R. A. Alternative splicing of G protein-coupled receptors: physiology and pathophysiology. Cell Mol. Life Sci. 66, 3337–3352 (2009).
Kreth, S., Ledderose, C., Kaufmann, I., Groeger, G. & Thiel, M. Differential expression of 5’-UTR splice variants of the adenosine A2A receptor gene in human granulocytes: identification, characterization, and functional impact on activation. FASEB J. 22, 3276–3286 (2008).
Nemeth, Z. H. et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 176, 5616–5626 (2006).
Venereau, E., Ceriotti, C. & Bianchi, M. E. DAMPs from cell death to new life. Front. Immunol. 6, 422 (2015).
Robson, S. C., Sevigny, J. & Zimmermann, H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409–430 (2006).
Kishore, B. K. et al. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am. J. Physiol. Ren. Physiol 288, F1032–F1043 (2005).
Vekaria, R. M., Shirley, D. G., Sevigny, J. & Unwin, R. J. Immunolocalization of ectonucleotidases along the rat nephron. Am. J. Physiol. Ren. Physiol 290, F550–F560 (2006).
Le Hir, M. & Kaissling, B. Distribution and regulation of renal ecto-5’-nucleotidase: implications for physiological functions of adenosine. Am. J. Physiol. 264, F377–F387 (1993).
Karczewska, J., Martyniec, L., Dzierzko, G., Stepinski, J. & Angielski, S. The relationship between constitutive ATP release and its extracellular metabolism in isolated rat kidney glomeruli. J. Physiol. Pharmacol. 58, 321–333 (2007).
Lazarowski, E. R., Boucher, R. C. & Harden, T. K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 275, 31061–31068 (2000).
Goldfine, I. D. et al. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr. Rev. 29, 62–75 (2008).
Longhi, M. S., Robson, S. C., Bernstein, S. H., Serra, S. & Deaglio, S. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J. Mol. Med. 91, 165–172 (2013).
Ralto, K. M., Rhee, E. P. & Parikh, S. M. NAD+ homeostasis in renal health and disease. Nat. Rev. Nephrol. 16, 99–111 (2020).
Sortica, D. A., Crispim, D., Zaffari, G. P., Friedman, R. & Canani, L. H. The role of ecto-nucleotide pyrophosphatase/phosphodiesterase 1 in diabetic nephropathy. Arq. Bras. Endocrinol. Metab. 55, 677–685 (2011).
Sortica, D. A. et al. K121Q polymorphism in the ectonucleotide pyrophosphatase/phosphodiesterase 1 gene is associated with acute kidney rejection. PLoS One 14, e0219062 (2019).
Lomashvili, K. A., Cobbs, S., Hennigar, R. A., Hardcastle, K. I. & O’Neill, W. C. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J. Am. Soc. Nephrol. 15, 1392–1401 (2004).
Schlieper, G., Schurgers, L., Brandenburg, V., Reutelingsperger, C. & Floege, J. Vascular calcification in chronic kidney disease: an update. Nephrol. Dial. Transpl. 31, 31–39 (2016).
Fish, R. S. et al. ATP and arterial calcification. Eur. J. Clin. Invest. 43, 405–412 (2013).
Moochhala, S. H., Sayer, J. A., Carr, G. & Simmons, N. L. Renal calcium stones: insights from the control of bone mineralization. Exp. Physiol. 93, 43–49 (2008).
Lomashvili, K. A., Garg, P., Narisawa, S., Millan, J. L. & O’Neill, W. C. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 73, 1024–1030 (2008).
Villa-Bellosta, R., Gonzalez-Parra, E. & Egido, J. Alkalosis and dialytic clearance of phosphate increases phosphatase activity: a hidden consequence of hemodialysis. PLoS One 11, e0159858 (2016).
Lomashvili, K. A., Khawandi, W. & O’Neill, W. C. Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 16, 2495–2500 (2005).
Shirley, D. G., Vekaria, R. M. & Sevigny, J. Ectonucleotidases in the kidney. Purinergic Signal. 5, 501–511 (2009).
Peters, E., Heemskerk, S., Masereeuw, R. & Pickkers, P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am. J. Kidney Dis. 63, 1038–1048 (2014).
Lam, K. W. et al. Improved immunohistochemical detection of prostatic acid phosphatase by a monoclonal antibody. Prostate 15, 13–21 (1989).
Boison, D. & Yegutkin, G. G. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36, 582–596 (2019).
Vallon, V., Unwin, R., Inscho, E. W., Leipziger, J. & Kishore, B. K. Extracellular nucleotides and P2 receptors in renal function. Physiol. Rev. 100, 211–269 (2020).
Schnermann, J. & Levine, D. Z. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu. Rev. Physiol. 65, 501–529 (2003).
Castrop, H. Mediators of tubuloglomerular feedback regulation of glomerular filtration: ATP and adenosine. Acta Physiol. 189, 3–14 (2007).
Kishore, B. K., Nelson, R. D., Miller, R. L., Carlson, N. G. & Kohan, D. E. P2Y2 receptors and water transport in the kidney. Purinergic Signal. 5, 491–499 (2009).
Zhang, Y. et al. Genetic deletion of P2Y2 receptor offers long-term (5 months) protection against lithium-induced polyuria, natriuresis, kaliuresis, and collecting duct remodeling and cell proliferation. Front. Physiol. 9, 1765 (2018).
Kishore, B. K. et al. Targeting renal purinergic signalling for the treatment of lithium-induced nephrogenic diabetes insipidus. Acta Physiol. 214, 176–188 (2015).
Zhang, Y., Pop, I. L., Carlson, N. G. & Kishore, B. K. Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am. J. Physiol. Ren. Physiol. 302, F70–F77 (2012).
Zhang, Y. et al. P2Y12 receptor localizes in the renal collecting duct and its blockade augments arginine vasopressin action and alleviates nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 26, 2978–2987 (2015).
Zhang, Y. et al. Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice. Purinergic Signal. 13, 239–248 (2017).
Zhang, Y. et al. Genetic deletion of ADP-activated P2Y12 receptor ameliorates lithium-induced nephrogenic diabetes insipidus in mice. Acta Physiol. 225, e13191 (2019).
Dwyer, K. M. et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J. Clin. Invest. 113, 1440–1446 (2004).
Zhang, Y. et al. Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am. J. Physiol. Ren. Physiol. 303, F420–F430 (2012).
Zhang, Y. et al. Impaired natriuretic response to high-NaCl diet plus aldosterone infusion in mice overexpressing human CD39, an ectonucleotidase (NTPDase1). Am. J. Physiol. Ren. Physiol. 308, F1398–F1408 (2015).
Leipziger, J. Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr. Opin. Nephrol. Hypertens. 20, 518–522 (2011).
Leipziger, J. Control of epithelial transport via luminal P2 receptors. Am. J. Physiol. Ren. Physiol. 284, F419–F432 (2003).
Praetorius, H. A. & Leipziger, J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin. Cell Dev. Biol. 24, 3–10 (2013).
Pandit, M. M. et al. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am. J. Physiol. Ren. Physiol. 308, F541–F552 (2015).
Burnstock, G., Evans, L. C. & Bailey, M. A. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 10, 71–101 (2014).
Solini, A., Usuelli, V. & Fiorina, P. The dark side of extracellular ATP in kidney diseases. J. Am. Soc. Nephrol. 26, 1007–1016 (2015).
Menzies, R. I., Tam, F. W., Unwin, R. J. & Bailey, M. A. Purinergic signaling in kidney disease. Kidney Int. 91, 315–323 (2017).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Hutton, H. L., Ooi, J. D., Holdsworth, S. R. & Kitching, A. R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 21, 736–744 (2016).
Baron, L. et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 6, e1629 (2015).
Dosch, M. et al. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. eLife 8, e42670 (2019).
Sakaki, H., Tsukimoto, M., Harada, H., Moriyama, Y. & Kojima, S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS One 8, e59778 (2013).
Riteau, N. et al. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 3, e403 (2012).
Brandao-Burch, A., Key, M. L., Patel, J. J., Arnett, T. R. & Orriss, I. R. The P2X7 receptor is an important regulator of extracellular ATP levels. Front. Endocrinol. 3, 41 (2012).
Fan, J., Xie, K., Wang, L., Zheng, N. & Yu, X. Roles of inflammasomes in inflammatory kidney diseases. Mediators Inflamm. 2019, 2923072 (2019).
Kim, Y. G., Kim, S. M., Kim, K. P., Lee, S. H. & Moon, J. Y. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells 8, 1389 (2019).
Vilaysane, A. et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 21, 1732–1744 (2010).
Ayna, G. et al. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS One 7, e40069 (2012).
Petrovski, G. et al. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1beta release in human macrophages. Autophagy 7, 321–330 (2011).
Yadav, V. et al. Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1beta-driven venous thrombosis. J. Clin. Invest. 129, 2872–2877 (2019).
Ouyang, X. et al. Adenosine is required for sustained inflammasome activation via the A2A receptor and the HIF-1alpha pathway. Nat. Commun. 4, 2909 (2013).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Tecklenborg, J., Clayton, D., Siebert, S. & Coley, S. M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 192, 142–150 (2018).
Maliszewski, C. R. et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 153, 3574–3583 (1994).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Dwyer, K. M. et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transpl. 10, 2410–2420 (2010).
Allard, B., Longhi, M. S., Robson, S. C. & Stagg, J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 (2017).
Mizumoto, N. et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 8, 358–365 (2002).
Yoon, J. et al. Plasma cell alloantigen ENPP1 is expressed by a subset of human B cells with potential regulatory functions. Immunol. Cell Biol. 94, 719–728 (2016).
Pan, W. et al. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 301, E901–E911 (2011).
Nowak-Machen, M. et al. Lysophosphatidic acid generation by pulmonary NKT cell ENPP-2/autotaxin exacerbates hyperoxic lung injury. Purinergic Signal. 11, 455–461 (2015).
Barbayianni, E., Kaffe, E., Aidinis, V. & Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 58, 76–96 (2015).
Pettengill, M. et al. Soluble ecto-5’-nucleotidase (5’-NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J. Biol. Chem. 288, 27315–27326 (2013).
Gibson, D. J. et al. Heightened expression of CD39 by regulatory T lymphocytes is associated with therapeutic remission in inflammatory bowel disease. Inflamm. Bowel Dis. 21, 2806–2814 (2015).
Borsellino, G. et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110, 1225–1232 (2007).
Liao, H., Hyman, M. C., Baek, A. E., Fukase, K. & Pinsky, D. J. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J. Biol. Chem. 285, 14791–14805 (2010).
Aswad, F., Kawamura, H. & Dennert, G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J. Immunol. 175, 3075–3083 (2005).
Mascanfroni, I. D. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 21, 638–646 (2015).
Roncarolo, M. G., Gregori, S., Bacchetta, R. & Battaglia, M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr. Top. Microbiol. Immunol. 380, 39–68 (2014).
Dwyer, K. M. et al. CD39 and control of cellular immune responses. Purinergic Signal. 3, 171–180 (2007).
Schenk, U. et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 4, ra12 (2011).
Longhi, M. S. et al. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One 9, e87956 (2014).
Longhi, M. S., Moss, A., Jiang, Z. G. & Robson, S. C. Purinergic signaling during intestinal inflammation. J. Mol. Med. 95, 915–925 (2017).
Mascanfroni, I. D. et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14, 1054–1063 (2013).
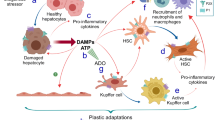
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80, 915–925 (2011).
Cao, Q., Wang, Y. & Harris, D. C. Pathogenic and protective role of macrophages in kidney disease. Am. J. Physiol. Ren. Physiol 305, F3–F11 (2013).
Tian, S. & Chen, S. Y. Macrophage polarization in kidney diseases. Macrophage 2, e679 (2015).
Lopez-Castejon, G., Baroja-Mazo, A. & Pelegrin, P. Novel macrophage polarization model: from gene expression to identification of new anti-inflammatory molecules. Cell Mol. Life Sci. 68, 3095–3107 (2011).
Pelegrin, P. & Surprenant, A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 28, 2114–2127 (2009).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Levesque, S. A., Kukulski, F., Enjyoji, K., Robson, S. C. & Sevigny, J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur. J. Immunol. 40, 1473–1485 (2010).
Ferrante, C. J. et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 36, 921–931 (2013).
Roberts, V. S., Cowan, P. J., Alexander, S. I., Robson, S. C. & Dwyer, K. M. The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 86, 685–692 (2014).
Kanthi, Y. M., Sutton, N. R. & Pinsky, D. J. CD39: interface between vascular thrombosis and inflammation. Curr. Atheroscler. Rep. 16, 425 (2014).
Kanthi, Y. et al. Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J. Clin. Invest. 125, 3027–3036 (2015).
Jiang, Z. G. et al. Characterization of circulating microparticle-associated CD39 family ecto-nucleotidases in human plasma. Purinergic Signal. 10, 611–618 (2014).
Yegutkin, G. G., Wieringa, B., Robson, S. C. & Jalkanen, S. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. FASEB J. 26, 3875–3883 (2012).
Thompson, L. F. et al. Crucial role for ecto-5’-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395–1405 (2004).
Eckle, T. et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111, 2024–2035 (2008).
Hechler, B. & Gachet, C. Purinergic receptors in thrombosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 35, 2307–2315 (2015).
Gremmel, T. et al. Synergistic inhibition of both P2Y1 and P2Y12 adenosine diphosphate receptors as novel approach to rapidly attenuate platelet-mediated thrombosis. Arterioscler. Thromb. Vasc. Biol. 36, 501–509 (2016).
Baroni, M. et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 21, 1926–1933 (2007).
Johnston-Cox, H. A., Koupenova, M. & Ravid, K. A2 adenosine receptors and vascular pathologies. Arterioscler. Thromb. Vasc. Biol. 32, 870–878 (2012).
Kishore, B. K., Robson, S. C. & Dwyer, K. M. CD39-adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signal. 14, 109–120 (2018).
Roberts, V., Lu, B., Rajakumar, S., Cowan, P. J. & Dwyer, K. M. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 9, 135–143 (2013).
Grenz, A. et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21, 2863–2873 (2007).
Lu, B. et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation 86, 1707–1712 (2008).
Crikis, S. et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am. J. Transpl. 10, 2586–2595 (2010).
Eltzschig, H. K. et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113, 224–232 (2009).
Grenz, A. et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 5, e137 (2008).
Grenz, A. et al. Protective role of ecto-5’-nucleotidase (CD73) in renal ischemia. J. Am. Soc. Nephrol. 18, 833–845 (2007).
Jian, R. et al. CD73 protects kidney from ischemia-reperfusion injury through reduction of free radicals. APMIS 120, 130–138 (2012).
Sharma, R. & Kinsey, G. R. Regulatory T cells in acute and chronic kidney diseases. Am. J. Physiol. Ren. Physiol 314, F679–F698 (2018).
Hu, M. et al. Regulatory T cells in kidney disease and transplantation. Kidney Int. 90, 502–514 (2016).
Kinsey, G. R. et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 20, 1744–1753 (2009).
Kinsey, G. R., Huang, L., Vergis, A. L., Li, L. & Okusa, M. D. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 77, 771–780 (2010).
Grenz, A. et al. Use of a hanging-weight system for isolated renal artery occlusion during ischemic preconditioning in mice. Am. J. Physiol. Ren. Physiol 292, F475–F485 (2007).
Kinsey, G. R. et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 23, 1528–1537 (2012).
Rissiek, A. et al. The expression of CD39 on regulatory T cells is genetically driven and further upregulated at sites of inflammation. J. Autoimmun. 58, 12–20 (2015).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Hill, N. R. et al. Global prevalence of chronic kidney disease — a systematic review and meta-analysis. PLoS One 11, e0158765 (2016).
Venkatachalam, M. A. et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am. J. Physiol. Ren. Physiol 298, F1078–F1094 (2010).
Dai, Y. et al. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J. Am. Soc. Nephrol. 22, 890–901 (2011).
Wilkinson, P. F., Farrell, F. X., Morel, D., Law, W. & Murphy, S. Adenosine signaling increases proinflammatory and profibrotic mediators through activation of a functional adenosine 2B receptor in renal fibroblasts. Ann. Clin. Lab. Sci. 46, 339–345 (2016).
Basile, D. P., Donohoe, D. L., Roethe, K. & Mattson, D. L. Chronic renal hypoxia after acute ischemic injury: effects of L-arginine on hypoxia and secondary damage. Am. J. Physiol. Ren. Physiol 284, F338–F348 (2003).
Nangaku, M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25 (2006).
Fine, L. G. & Norman, J. T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 74, 867–872 (2008).
Zhang, L. et al. Adenosine 2A receptor is protective against renal injury in MRL/lpr mice. Lupus 20, 667–677 (2011).
Ozuyaman, B. et al. Adenosine produced via the CD73/ecto-5’-nucleotidase pathway has no impact on erythropoietin production but is associated with reduced kidney weight. Pflugers Arch. 452, 324–331 (2006).
Blume, C. et al. Autoimmunity in CD73/Ecto-5’-nucleotidase deficient mice induces renal injury. PLoS One 7, e37100 (2012).
Picher, M., Burch, L. H., Hirsh, A. J., Spychala, J. & Boucher, R. C. Ecto 5’-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 278, 13468–13479 (2003).
Yoshida, O. et al. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl. Immunol. 32, 76–83 (2015).
Roberts, V., Lu, B., Chia, J., Cowan, P. J. & Dwyer, K. M. CD39 overexpression does not attenuate renal fibrosis in the unilateral ureteric obstructive model of chronic kidney disease. Purinergic Signal. 12, 653–660 (2016).
Xiao, H. et al. The effects of adenosine A2A receptor knockout on renal interstitial fibrosis in a mouse model of unilateral ureteral obstruction. Acta Histochem. 115, 315–319 (2013).
Xiao, H. et al. Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One 8, e60173 (2013).
Goncalves, R. G. et al. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 70, 1599–1606 (2006).
Kim, M. J. et al. Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol. Dial. Transpl. 29, 1350–1361 (2014).
Kawano, A. et al. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem. Biophys. Res. Commun. 420, 102–107 (2012).
Rennert, L. et al. P2Y2R signaling is involved in the onset of glomerulonephritis. Front. Immunol. 9, 1589 (2018).
Zhao, J. et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 65, 3176–3185 (2013).
Garcia, G. E. et al. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 22, 445–454 (2008).
Scheuher, C. A review of organ transplantation: heart, lung, kidney, liver, and simultaneous liver-kidney. Crit. Care Nurs. Q. 39, 199–206 (2016).
Nankivell, B. J. & Kuypers, D. R. Diagnosis and prevention of chronic kidney allograft loss. Lancet 378, 1428–1437 (2011).
McRae, J. L., JSJ, C., S, P. & KM, D. Evaluation of CD4+CD25+/−CD39+ T cell populations in peripheral blood of patients following renal transplantation and during acute allograft rejection. Nephrology 22, 505–512 (2016).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Braza, F. et al. Central role of CD45RA− Foxp3hi memory regulatory T cells in clinical kidney transplantation tolerance. J. Am. Soc. Nephrol. 26, 1795–1805 (2015).
Durand, M. et al. Increased degradation of ATP is driven by memory regulatory T cells in kidney transplantation tolerance. Kidney Int. 93, 1154–1164 (2018).
Mahajan, D. et al. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J. Am. Soc. Nephrol. 17, 2731–2741 (2006).
Wang, Y. M. et al. Regulatory T cells participate in CD39-mediated protection from renal injury. Eur. J. Immunol. 42, 2441–2451 (2012).
Sitkovsky, M. V. et al. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol. Res. 2, 598–605 (2014).
Ohta, A. et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA 103, 13132–13137 (2006).
Fong, L. et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. 10, 40–53 (2020).
Finke, J. H. et al. Modification of the tumor microenvironment as a novel target of renal cell carcinoma therapeutics. Cancer J. 19, 353–364 (2013).
Siddiqui, S. A. et al. Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 13, 2075–2081 (2007).
Stagg, J. et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 72, 2190–2196 (2012).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
Li, X. Y. et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 9, 1754–1773 (2019).
Perrot, I. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27, 2411–2425.e9 (2019).
Lau, W. M. et al. Enpp1: a potential facilitator of breast cancer bone metastasis. PLoS One 8, e66752 (2013).
Takahashi, R. U. et al. Loss of microRNA-27b contributes to breast cancer stem cell generation by activating ENPP1. Nat. Commun. 6, 7318 (2015).
Hu, M. et al. Dysregulated ENPP1 increases the malignancy of human lung cancer by inducing epithelial-mesenchymal transition phenotypes and stem cell features. Am. J. Cancer Res. 9, 134–144 (2019).
Su, S. C. et al. Autotaxin-lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin. Cancer Res. 19, 6461–6472 (2013).
Narres, M. et al. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: a systematic review. PLoS One 11, e0147329 (2016).
Tomino, Y. & Gohda, T. The prevalence and management of diabetic nephropathy in Asia. Kidney Dis. 1, 52–60 (2015).
Teng, J. et al. Spectrum of renal disease in diabetes. Nephrology 19, 528–536 (2014).
Persson, P., Hansell, P. & Palm, F. Reduced adenosine A2a receptor-mediated efferent arteriolar vasodilation contributes to diabetes-induced glomerular hyperfiltration. Kidney Int. 87, 109–115 (2015).
Moritz, C. E. et al. Physical training normalizes nucleotide hydrolysis and biochemical parameters in blood serum from streptozotocin-diabetic rats. Arch. Physiol. Biochem. 118, 253–259 (2012).
Rucker, B. et al. E-NTPDases and ecto-5’-nucleotidase expression profile in rat heart left ventricle and the extracellular nucleotide hydrolysis by their nerve terminal endings. Life Sci. 82, 477–486 (2008).
Xia, J. F. et al. Correlations of six related purine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin. Biochem. 42, 215–220 (2009).
Tam, F. W. Monocyte chemoattractant protein-1 (MCP-1) is a prognostic biomarker and a therapeutic target in diabetic nephropathy. Meta Gene 17, S12–S13 (2018).
Friedman, D. J., Rennke, H. G., Csizmadia, E., Enjyoji, K. & Robson, S. C. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56, 2371–2379 (2007).
Menzies, R. I. et al. Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related injury. EBioMedicine 19, 73–83 (2017).
Friedman, D. J. et al. Functional ENTPD1 polymorphisms in African Americans with diabetes and end-stage renal disease. Diabetes 58, 999–1006 (2009).
Sommerer, C. & Zeier, M. Clinical manifestation and management of ADPKD in western countries. Kidney Dis. 2, 120–127 (2016).
Wilson, P. D., Hovater, J. S., Casey, C. C., Fortenberry, J. A. & Schwiebert, E. M. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J. Am. Soc. Nephrol. 10, 218–229 (1999).
Turner, C. M., Ramesh, B., Srai, S. K., Burnstock, G. & Unwin, R. J. Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cell Tissues Organs 178, 168–179 (2004).
Xu, C. et al. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am. J. Physiol. Ren. Physiol. 296, F1464–F1476 (2009).
Hovater, M. B., Olteanu, D., Welty, E. A. & Schwiebert, E. M. Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic Signal. 4, 109–124 (2008).
Chang, M. Y. et al. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J. Am. Soc. Nephrol. 22, 1696–1706 (2011).
Hillman, K. A. et al. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem. Biophys. Res. Commun. 322, 434–439 (2004).
Palygin, O. et al. Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal. 14, 485–497 (2018).
Ilatovskaya, D. V., Palygin, O. & Staruschenko, A. Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am. J. Physiol. Ren. Physiol 311, F1135–F1139 (2016).
Ghimire, G., Hage, F. G., Heo, J. & Iskandrian, A. E. Regadenoson: a focused update. J. Nucl. Cardiol. 20, 284–288 (2013).
Ananthasubramaniam, K. et al. A randomized, double-blind, placebo-controlled study of the safety and tolerance of regadenoson in subjects with stage 3 or 4 chronic kidney disease. J. Nucl. Cardiol. 19, 319–329 (2012).
Vij, A., Golzar, Y. & Doukky, R. Regadenoson use in chronic kidney disease and end-stage renal disease: a focused review. J. Nucl. Cardiol. 25, 137–149 (2018).
Hinz, S., Lacher, S. K., Seibt, B. F. & Muller, C. E. BAY60-6583 acts as a partial agonist at adenosine A2B receptors. J. Pharmacol. Exp. Ther. 349, 427–436 (2014).
Sitkovsky, M. V. Lessons from the A2A adenosine receptor antagonist-enabled tumor regression and survival in patients with treatment-refractory renal cell cancer. Cancer Discov. 10, 16–19 (2020).
Zhang, Y. et al. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am. J. Physiol. Ren. Physiol. 295, F1715–F1724 (2008).
Kishi, Y. et al. Perindopril augments ecto-ATP diphosphohydrolase activity and enhances endothelial anti-platelet function in human umbilical vein endothelial cells. J. Hypertens. 21, 1347–1353 (2003).
Kaneider, N. C. et al. Reversal of thrombin-induced deactivation of CD39/ATPDase in endothelial cells by HMG-CoA reductase inhibition: effects on Rho-GTPase and adenosine nucleotide metabolism. Arterioscler. Thromb. Vasc. Biol. 22, 894–900 (2002).
Kaneider, N. C., Mosheimer, B., Reinisch, N., Patsch, J. R. & Wiedermann, C. J. Inhibition of thrombin-induced signaling by resveratrol and quercetin: effects on adenosine nucleotide metabolism in endothelial cells and platelet-neutrophil interactions. Thromb. Res. 114, 185–194 (2004).
Abu-Zaid, M. H., Ghany, S. E. A. & Gaber, R. A. Effect of statins as modulators of CD39+ tregs in patients with rheumatoid arthritis who were unsuccessfully treated with methotrexate. Egypt. Rheumatol. Rehabil. 45, 1–8 (2018).
Cao, J. et al. Protective properties of sesamin against fluoride-induced oxidative stress and apoptosis in kidney of carp (Cyprinus carpio) via JNK signaling pathway. Aquat. Toxicol. 167, 180–190 (2015).
Kong, X. et al. Sesamin ameliorates advanced glycation end products-induced pancreatic beta-cell dysfunction and apoptosis. Nutrients 7, 4689–4704 (2015).
Monteiro, E. M. et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 6, 1931–1944 (2014).
Nakano, D. et al. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol. Pharm. Bull. 26, 1701–1705 (2003).
Wu, X. Q. et al. Sesamin exerts renoprotective effects by enhancing NO bioactivity in renovascular hypertensive rats fed with high-fat-sucrose diet. Eur. J. Pharmacol. 683, 231–237 (2012).
Robson, S. C. et al. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 31, 217–233 (2005).
Li, K. et al. Sesamin protects against renal ischemia reperfusion injury by promoting CD39-adenosine-A2AR signal pathway in mice. Am. J. Transl. Res. 8, 2245–2254 (2016).
Correa-Costa, M. et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl Acad. Sci. USA 115, E2302–E2310 (2018).
Eckle, T. et al. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115, 1581–1590 (2007).
Eckle, T. et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 18, 774–782 (2012).
Zhou, Q. et al. Ferulic acid protected from kidney ischemia reperfusion injury in mice: possible mechanism through increasing adenosine generation via HIF-1alpha. Inflammation 41, 2068–2078 (2018).
Dorneles, G. P., da Silva, I. M., Peres, A. & Romao, P. R. T. Physical fitness modulates the expression of CD39 and CD73 on CD4+ CD25− and CD4+ CD25+ T cells following high intensity interval exercise. J. Cell Biochem. 120, 10726–10736 (2019).
Didsbury, M. et al. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation 95, 679–687 (2013).
Sashindranath, M. et al. Development of a novel strategy to target CD39 antithrombotic activity to the endothelial-platelet microenvironment in kidney ischemia-reperfusion injury. Purinergic Signal. 13, 259–265 (2017).
Acknowledgements
S.C.R. is funded by the Department of Defence grant W81XWH-16-1-0464 and also previously the National Institutes of Health grants R21-CA221702, R01-DK108894, R01-DK10372, 1R01-AI132389 and 5R01DK104714. B.K.K. has been supported by the US Department of Veterans Affairs Merit Review Program and also previously by the National Institutes of Health grants R01-DK061183 and R21-DK081041, and a pre-clinical research grant from AstraZeneca.
Author information
Authors and Affiliations
Contributions
K.M.D., B.K.K. and S.C.R. all contributed to researching data for the article, writing the article and reviewing and editing the manuscript before submission. All authors substantially contributed to the discussion of the review contents.
Corresponding author
Ethics declarations
Competing interests
K.M.D. has no conflicts to declare. S.C.R. has had grant support from Tizona for development and characterization of antibodies to CD39. B.K.K. received research grant support and collaborated with AstraZeneca, and has United States patents issued for the use of P2Y2 and P2Y12 antagonists for the treatment of nephrogenic diabetes insipidus and other diseases.
Additional information
Peer review information
Nature Reviews Nephrology thanks Gennady Yegutkin, Matthew Bailey and the other, anonymous, reviewer(s) for their contributions to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- G proteins
-
A family of GTPase proteins involved in transmitting signals from extracellular stimuli to the cell interior.
- Damage-associated molecular pattern
-
(DAMP). Molecules released from damaged or dying cells that trigger immune responses by interacting with pattern recognition receptors.
- Purinome
-
All the proteins in a cell that utilize purine cofactors.
- Treg-specific demethylated region
-
An evolutionarily conserved non-coding element within the FOXP3 gene locus that controls FOXP3 expression.
Rights and permissions
About this article
Cite this article
Dwyer, K.M., Kishore, B.K. & Robson, S.C. Conversion of extracellular ATP into adenosine: a master switch in renal health and disease. Nat Rev Nephrol 16, 509–524 (2020). https://doi.org/10.1038/s41581-020-0304-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-0304-7
- Springer Nature Limited
This article is cited by
-
Abcc6 deficiency prevents rhabdomyolysis-induced acute kidney injury
Scientific Reports (2023)
-
Physical exercise as a modulator of the purinergic system in the control of sarcopenia in individuals with chronic kidney disease on hemodialysis
Purinergic Signalling (2023)
-
Overcoming Debye length limitations: Three-dimensional wrinkled graphene field-effect transistor for ultra-sensitive adenosine triphosphate detection
Frontiers of Physics (2023)
-
The role of dorsomedial striatum adenosine 2A receptors in the loss of goal-directed behaviour
Psychopharmacology (2023)
-
Contribution of common and rare variants to Asian neovascular age-related macular degeneration subtypes
Nature Communications (2023)