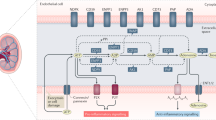
Abstract
Extracellular purines (ATP and adenosine) are ubiquitous intercellular messengers. During tissular damage, they function as damage-associated molecular patterns (DAMPs). In this context, purines announce tissue alterations to initiate a reparative response that involve the formation of the inflammasome complex and the recruitment of specialized cells of the immune system. The present review focuses on the role of the purinergic system in liver damage, mainly during the onset and development of fibrosis. After hepatocellular injury, extracellular ATP promotes a signaling cascade that ameliorates tissue alterations to restore the hepatic function. However, if cellular damage becomes chronic, ATP orchestrates an aberrant reparative process that results in severe liver diseases such as fibrosis and cirrhosis. ATP and adenosine, their receptors, and extracellular ectonucleotidases are mediators of unique processes that will be reviewed in detail.

Similar content being viewed by others
References
Fustin J-M, Doi M, Yamada H et al (2012) Rhythmic nucleotide synthesis in the liver: temporal segregation of metabolites. Cell Rep 1:341–349. https://doi.org/10.1016/j.celrep.2012.03.001
Ananian P, Hardwigsen J, Bernard D, Le Treut YP (2005) Serum acute-phase protein level as indicator for liver failure after liver resection. Hepatogastroenterology 52(63):857–861
Rőszer T (2014) The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res 358:685–695. https://doi.org/10.1007/s00441-014-1985-7
Mataix Verdú J, Martínez de Vitoria E (2009) Chapter 48. Liver and biliary tract. In: Treaty of nutrition and feeding. OCEÁNO/ergon, Spain, pp 1355–1369
Jungermann K, Katz N (1989) Functional specialization of different hepatocyte populations. Physiol Rev 69:708–764. https://doi.org/10.1152/physrev.1989.69.3.708
Ishibashi H, Nakamura M, Komori A et al (2009) Liver architecture, cell function, and disease. Semin Immunopathol 31:399–409. https://doi.org/10.1007/s00281-009-0155-6
Wang G-P, Xu C-S (2010) Reference gene selection for real-time RT-PCR in eight kinds of rat regenerating hepatic cells. Mol Biotechnol 46:49–57. https://doi.org/10.1007/s12033-010-9274-5
Martinez-Hernandez A, Amenta PS (1995) The extracellular matrix in hepatic regeneration. FASEB J 9(14):1401–1410
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426. https://doi.org/10.1016/S1097-2765(02)00599-3
Martinez-Hernandez A, Amenta PS (1993) The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol 423:1–11
Baiocchini A, Montaldo C, Conigliaro A et al (2016) Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS One 11:e0151736. https://doi.org/10.1371/journal.pone.0151736
Elpek GÖ (2014) Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol 20:7260–7276. https://doi.org/10.3748/wjg.v20.i23.7260
McKillop IH, Moran DM, Jin X, Koniaris LG (2006) Molecular pathogenesis of hepatocellular carcinoma. J Surg Res 136:125–135. https://doi.org/10.1016/j.jss.2006.04.013
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Di Virgilio F, Vuerich M (2015) Purinergic signaling in the immune system. Auton Neurosci 191:117–123. https://doi.org/10.1016/j.autneu.2015.04.011
Fredholm BB, IJzerman AP, Jacobson KA et al (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34. https://doi.org/10.1124/pr.110.003285
Webb TE, Simon J, Krishek BJ et al (1993) Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett 324:219–225
Burnstock G, Kennedy C (1985) Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol 16:433–440
Burnstock G (2014) Purinergic signalling: from discovery to current developments. Exp Physiol 99:16–34. https://doi.org/10.1113/expphysiol.2013.071951
von Kügelgen I, Harden TK (2011) Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol 61:373–415. https://doi.org/10.1016/B978-0-12-385526-8.00012-6
Bonora M, Patergnani S, Rimessi A et al (2012) ATP synthesis and storage. Purinergic Signal 8:343–357. https://doi.org/10.1007/s11302-012-9305-8
Ramzan R, Staniek K, Kadenbach B, Vogt S (2010) Mitochondrial respiration and membrane potential are regulated by the allosteric ATP-inhibition of cytochrome c oxidase. Biochim Biophys Acta 1797:1672–1680. https://doi.org/10.1016/j.bbabio.2010.06.005
Cui JD, Xu ML, Liu EYL et al (2016) Expression of globular form acetylcholinesterase is not altered in P2Y1R knock-out mouse brain. Chem Biol Interact 259:291–294. https://doi.org/10.1016/j.cbi.2016.06.028
Braun M, Wendt A, Karanauskaite J et al (2007) Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. J Gen Physiol 129:221–231. https://doi.org/10.1085/jgp.200609658
Pablo Huidobro-Toro J, Verónica Donoso M (2004) Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur J Pharmacol 500:27–35. https://doi.org/10.1016/j.ejphar.2004.07.008
Choi RCY, Siow NL, Cheng AWM et al (2003) ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J Neurosci 23:4445–4456
Cotrina ML, Lin JH, López-García JC et al (2000) ATP-mediated glia signaling. J Neurosci 20:2835–2844
Abudara V, Retamal MA, Del Rio R, Orellana JA (2018) Synaptic functions of hemichannels and pannexons: a double-edged sword. Front Mol Neurosci 11:435. https://doi.org/10.3389/fnmol.2018.00435
Elliott MR, Chekeni FB, Trampont PC et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286. https://doi.org/10.1038/nature08296
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509:310–317. https://doi.org/10.1038/nature13085
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. https://doi.org/10.1007/s11302-012-9309-4
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32:19–29. https://doi.org/10.1016/j.tins.2008.10.001
Martínez-Ramírez AS, Vázquez-Cuevas FG (2015) Purinergic signaling in the ovary. Mol Reprod Dev 82:839–848. https://doi.org/10.1002/mrd.22537
Burnstock G, Verkhratsky A (2010) Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis 1:e9. https://doi.org/10.1038/cddis.2009.11
Dixon CJ, White PJ, Hall JF et al (2005) Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther 313:1305–1313. https://doi.org/10.1124/jpet.104.082743
Thevananther S, Sun H, Li D et al (2004) Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology 39:393–402. https://doi.org/10.1002/hep.20075
Keppens S, De Wulf H (1986) Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J 240:367–371
Tackett BC, Sun H, Mei Y et al (2014) P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 307:G1073–G1087. https://doi.org/10.1152/ajpgi.00092.2014
Dranoff JA, Kruglov EA, Abreu-Lanfranco O et al (2007) Prevention of liver fibrosis by the purinoceptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS). In Vivo 21:957–965
Ayata CK, Ganal SC, Hockenjos B et al (2012) Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 143:1620–1629.e4. https://doi.org/10.1053/j.gastro.2012.08.049
Emmett DS, Feranchak A, Kilic G et al (2008) Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology 47:698–705. https://doi.org/10.1002/hep.22035
Besnard A, Gautherot J, Julien B et al (2016) The P2X4 purinergic receptor impacts liver regeneration after partial hepatectomy in mice through the regulation of biliary homeostasis. Hepatology 64:941–953. https://doi.org/10.1002/hep.28675
Le Guilcher C, Garcin I, Dellis O et al (2018) The P2X4 purinergic receptor regulates hepatic myofibroblast activation during liver fibrogenesis. J Hepatol 69:644–653. https://doi.org/10.1016/j.jhep.2018.05.020
Peng Z-W, Rothweiler S, Wei G et al (2017) The ectonucleotidase ENTPD1/CD39 limits biliary injury and fibrosis in mouse models of sclerosing cholangitis. Hepatol Commun 1:957–972. https://doi.org/10.1002/hep4.1084
Savio LEB, de Andrade MP, Figliuolo VR et al (2017) CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol 67:716–726. https://doi.org/10.1016/j.jhep.2017.05.021
Feldbrügge L, Jiang ZG, Csizmadia E et al (2018) Distinct roles of ecto-nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) in liver regeneration and fibrosis. Purinergic Signal 14:37–46. https://doi.org/10.1007/s11302-017-9590-3
Chan ESL, Montesinos MC, Fernandez P et al (2006) Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 148:1144–1155. https://doi.org/10.1038/sj.bjp.0706812
Imarisio C, Alchera E, Sutti S et al (2012) Adenosine A(2a) receptor stimulation prevents hepatocyte lipotoxicity and non-alcoholic steatohepatitis (NASH) in rats. Clin Sci 123:323–332. https://doi.org/10.1042/CS20110504
Yang P, Chen P, Wang T et al (2013) Loss of A(1) adenosine receptor attenuates alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Toxicol Sci 131:128–138. https://doi.org/10.1093/toxsci/kfs263
Yang P, Han Z, Chen P et al (2010) A contradictory role of A1 adenosine receptor in carbon tetrachloride- and bile duct ligation-induced liver fibrosis in mice. J Pharmacol Exp Ther 332:747–754. https://doi.org/10.1124/jpet.109.162727
Yang P, Wang Z, Zhan Y et al (2013) Endogenous A1 adenosine receptor protects mice from acute ethanol-induced hepatotoxicity. Toxicology 309:100–106. https://doi.org/10.1016/j.tox.2013.05.003
Amaral SS, Oliveira AG, Marques PE, Quintão JLD, Pires DA, Resende RR, Sousa BR, Melgaço JG, Pinto MA, Russo RC, Gomes AKC, Andrade LM, Zanin RF, Pereira RVS, Bonorino C, Soriani FM, Lima CX, Cara DC, Teixeira MM, Leite MF, Menezes GB (2013) Altered responsiveness to extracellular ATP enhances acetaminophen hepatotoxicity. Cell Communication and Signaling 11(1):10
Abdelaziz HA, Shaker ME, Hamed MF, Gameil NM (2017) Repression of acetaminophen-induced hepatotoxicity by a combination of celastrol and brilliant blue G. Toxicol Lett 275:6–18. https://doi.org/10.1016/j.toxlet.2017.04.012
Xie Y, Williams CD, McGill MR et al (2013) Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci 131:325–335. https://doi.org/10.1093/toxsci/kfs283
Hoque R, Sohail MA, Salhanick S et al (2012) P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol 302:G1171–G1179. https://doi.org/10.1152/ajpgi.00352.2011
Shang Y, Li XF, Jin MJ, Li Y, Wu YL, Jin Q, Zhang Y, Li X, Jiang M, Cui BW, Lian LH, Nan JX (2018) Leucodin attenuates inflammatory response in macrophages and lipid accumulation in steatotic hepatocytes via P2x7 receptor pathway: A potential role in alcoholic liver disease. Biomedicine & Pharmacotherapy 107:374–381
Huang C, Yu W, Cui H, Wang Y, Zhang L, Han F, Huang T (2014) P2X7 blockade attenuates mouse liver fibrosis. Molecular Medicine Reports 9(1):57–62
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez J, Shulman GI, Gordon JI, Hoffman HM, Flavell RA (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482(7384):179–185
Vivoli E, Cappon A, Milani S, Piombanti B, Provenzano A, Novo E, Masi A, Navari N, Narducci R, Mannaioni G, Moneti G, Oliveira CP, Parola M, Marra F (2016) NLRP3 inflammasome as a target of berberine in experimental murine liver injury: interference with P2X7 signalling. Clinical Science 130(20):1793–1806
Ni J, Zhang Z, Luo X et al (2016) Plasticizer DBP activates NLRP3 inflammasome through the P2X7 receptor in HepG2 and L02 cells. J Biochem Mol Toxicol 30:178–185. https://doi.org/10.1002/jbt.21776
Gong Z, Zhou J, Zhao S et al (2016) Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget 7:83951–83963. https://doi.org/10.18632/oncotarget.13796
Szuster-Ciesielska A, Sztanke K, Kandefer-Szerszeń M (2012) A novel fused 1,2,4-triazine aryl derivative as antioxidant and nonselective antagonist of adenosine A(2A) receptors in ethanol-activated liver stellate cells. Chem Biol Interact 195:18–24. https://doi.org/10.1016/j.cbi.2011.10.004
Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88:125–172. https://doi.org/10.1152/physrev.00013.2007
Takemura S, Kawada N, Hirohashi K et al (1994) Nucleotide receptors in hepatic stellate cells of the rat. FEBS Lett 354:53–56
Dranoff JA, Ogawa M, Kruglov EA et al (2004) Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287:G417–G424. https://doi.org/10.1152/ajpgi.00294.2003
Benitez-Rajal J, Lorite M-J, Burt AD et al (2006) Phospholipase D and extracellular signal-regulated kinase in hepatic stellate cells: effects of platelet-derived growth factor and extracellular nucleotides. Am J Physiol Gastrointest Liver Physiol 291:G977–G986. https://doi.org/10.1152/ajpgi.00041.2006
Yamaguchi M, Saito S-Y, Nishiyama R et al (2017) Caffeine suppresses the activation of hepatic stellate cells cAMP-independently by antagonizing adenosine receptors. Biol Pharm Bull 40:658–664. https://doi.org/10.1248/bpb.b16-00947
Wang H, Guan W, Yang W et al (2014) Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS One 9:e92482. https://doi.org/10.1371/journal.pone.0092482
Che J, Chan ESL, Cronstein BN (2007) Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol 72:1626–1636. https://doi.org/10.1124/mol.107.038760
Pinzani M (2002) PDGF and signal transduction in hepatic stellate cells. Front Biosci 7:d1720–d1726
Hashmi AZ, Hakim W, Kruglov EA et al (2007) Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292:G395–G401. https://doi.org/10.1152/ajpgi.00208.2006
Yang Y, Wang H, Lv X et al (2015) Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie 115:59–70. https://doi.org/10.1016/j.biochi.2015.04.019
Velasco-Loyden G, Pérez-Carreón JI, Agüero JFC et al (2010) Prevention of in vitro hepatic stellate cells activation by the adenosine derivative compound IFC305. Biochem Pharmacol 80:1690–1699. https://doi.org/10.1016/j.bcp.2010.08.017
Pérez-Carreón JI, Martínez-Pérez L, Loredo ML et al (2010) An adenosine derivative compound, IFC305, reverses fibrosis and alters gene expression in a pre-established CCl(4)-induced rat cirrhosis. Int J Biochem Cell Biol 42:287–296. https://doi.org/10.1016/j.biocel.2009.11.005
Ikeda N, Murata S, Maruyama T et al (2011) Platelet-derived adenosine 5′-triphosphate suppresses activation of human hepatic stellate cell: in vitro study. Hepatol Res 42:91–102. https://doi.org/10.1111/j.1872-034X.2011.00893.x
Toki Y, Takenouchi T, Harada H et al (2015) Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1β, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem Biophys Res Commun 458:771–776. https://doi.org/10.1016/j.bbrc.2015.02.011
Kojima S, Negishi Y, Tsukimoto M et al (2014) Purinergic signaling via P2X7 receptor mediates IL-1β production in Kupffer cells exposed to silica nanoparticle. Toxicology 321:13–20. https://doi.org/10.1016/j.tox.2014.03.008
Mihm S (2018) Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci 19:E3104. https://doi.org/10.3390/ijms19103104
Englezou PC, Rothwell SW, Ainscough JS et al (2015) P2X7R activation drives distinct IL-1 responses in dendritic cells compared to macrophages. Cytokine 74:293–304. https://doi.org/10.1016/j.cyto.2015.05.013
Ainscough JS, Frank Gerberick G, Zahedi-Nejad M et al (2014) Dendritic cell IL-1α and IL-1β are polyubiquitinated and degraded by the proteasome. J Biol Chem 289:35582–35592. https://doi.org/10.1074/jbc.M114.595686
Ting JP-Y, Lovering RC, Alnemri ES et al (2008) The NLR gene family: a standard nomenclature. Immunity 28:285–287. https://doi.org/10.1016/j.immuni.2008.02.005
Davis BK, Wen H, Ting JP-Y (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. https://doi.org/10.1146/annurev-immunol-031210-101405
Di Virgilio F (2013) The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev 65:872–905. https://doi.org/10.1124/pr.112.006171
Coddou C, Yan Z, Obsil T et al (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63:641–683. https://doi.org/10.1124/pr.110.003129
Muñoz-Planillo R, Kuffa P, Martínez-Colón G et al (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. https://doi.org/10.1016/j.immuni.2013.05.016
Pétrilli V, Papin S, Dostert C et al (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589. https://doi.org/10.1038/sj.cdd.4402195
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203
Shi H, Wang Y, Li X et al (2016) NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 17:250–258. https://doi.org/10.1038/ni.3333
He Y, Zeng MY, Yang D et al (2016) NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530:354–357. https://doi.org/10.1038/nature16959
Franceschini A, Capece M, Chiozzi P et al (2015) The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J 29:2450–2461. https://doi.org/10.1096/fj.14-268714
de Rivero Vaccari JP, Bastien D, Yurcisin G et al (2012) P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci 32:3058–3066. https://doi.org/10.1523/JNEUROSCI.4930-11.2012
Chen K, Zhang J, Zhang W et al (2013) ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45:932–943. https://doi.org/10.1016/j.biocel.2013.02.009
Burnstock G, Vaughn B, Robson SC (2014) Purinergic signalling in the liver in health and disease. Purinergic Signal 10:51–70. https://doi.org/10.1007/s11302-013-9398-8
Cover C, Liu J, Farhood A et al (2006) Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 216:98–107. https://doi.org/10.1016/j.taap.2006.04.010
Lang CH, Silvis C, Deshpande N et al (2003) Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock 19:538–546. https://doi.org/10.1097/01.shk.0000055237.25446.80
Ganz M, Csak T, Nath B, Szabo G (2011) Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol 17:4772–4778. https://doi.org/10.3748/wjg.v17.i43.4772
Imaeda AB, Watanabe A, Sohail MA et al (2009) Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest 119:305–314. https://doi.org/10.1172/JCI35958
Masumoto J, Taniguchi S, Nakayama J et al (2001) Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J Histochem Cytochem 49:1269–1275. https://doi.org/10.1177/002215540104901009
Boaru SG, Borkham-Kamphorst E, Tihaa L et al (2012) Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J Inflamm (Lond) 9:49. https://doi.org/10.1186/1476-9255-9-49
Xiao J, Zhu Y, Liu Y et al (2014) Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol 69:73–78. https://doi.org/10.1016/j.ijbiomac.2014.05.034
Csak T, Ganz M, Pespisa J et al (2011) Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 54:133–144. https://doi.org/10.1002/hep.24341
Watanabe A, Sohail MA, Gomes DA et al (2009) Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 296:G1248–G1257. https://doi.org/10.1152/ajpgi.90223.2008
Inzaugarat ME, Johnson CD, Holtmann TM et al (2019) NLR family pyrin domain-containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice. Hepatology 69:845–859. https://doi.org/10.1002/hep.30252
Zhang W-J, Fang Z-M, Liu W-Q (2019) NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB. Parasit Vectors 12:29. https://doi.org/10.1186/s13071-018-3223-8
Wang F, Guan M, Wei L, Yan H (2019) IL-18 promotes the secretion of matrix metalloproteinases in human periodontal ligament fibroblasts by activating NF-κB signaling. Molecular Medicine Reports; Athens 19:703. https://doi.org/10.3892/mmr.2018.9697
Imamura M, Tsutsui H, Yasuda K et al (2009) Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol 51:333–341. https://doi.org/10.1016/j.jhep.2009.03.027
Jiang S, Zhang Y, Zheng J-H et al (2017) Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol Res 117:82–93. https://doi.org/10.1016/j.phrs.2016.11.040
Vivoli E, Cappon A, Milani S et al (2016) NLRP3 inflammasome as a target of berberine in experimental murine liver injury: interference with P2X7 signalling. Clin Sci 130:1793–1806. https://doi.org/10.1042/CS20160400
Gao H, Lv Y, Liu Y et al (2019) Wolfberry‐derived zeaxanthin dipalmitate attenuates ethanol‐induced hepatic damage. Mol Nutr Food Res 63:e1801339. https://doi.org/10.1002/mnfr.201801339
Kanneganti TD, Lamkanfi M, Kim YG et al (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. - PubMed - NCBI. Immunity 26(4):433–443
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082. https://doi.org/10.1038/sj.emboj.7601378
Gicquel T, Victoni T, Fautrel A et al (2014) Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages. Clin Exp Pharmacol Physiol 41:279–286. https://doi.org/10.1111/1440-1681.12214
Pelegrin P, Barroso-Gutierrez C, Surprenant A (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol 180:7147–7157
Acknowledgments
We are grateful to Jessica González Norris for proofreading.
Funding
This work was funded by PAPIIT-UNAM, number IN201017 to FGV-C and IN201618 to MD-M, and CONACyT-México, number 284-557 to MD-M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Velázquez-Miranda declares that he has no conflict of interest.
M. Díaz-Muñoz declares that he has no conflict of interest.
Francisco Gabriel Vázquez-Cuevas declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Velázquez-Miranda, E., Díaz-Muñoz, M. & Vázquez-Cuevas, F.G. Purinergic signaling in hepatic disease. Purinergic Signalling 15, 477–489 (2019). https://doi.org/10.1007/s11302-019-09680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-019-09680-3