Abstract
Background
Tumour infiltrating lymphocytes (TILs) are a prognostic parameter in triple-negative and human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC). However, their role in luminal (oestrogen receptor positive and HER2 negative (ER + /HER2-)) BC remains unclear. In this study, we used artificial intelligence (AI) to assess the prognostic significance of TILs in a large well-characterised cohort of luminal BC.
Methods
Supervised deep learning model analysis of Haematoxylin and Eosin (H&E)-stained whole slide images (WSI) was applied to a cohort of 2231 luminal early-stage BC patients with long-term follow-up. Stromal TILs (sTILs) and intratumoural TILs (tTILs) were quantified and their spatial distribution within tumour tissue, as well as the proportion of stroma involved by sTILs were assessed. The association of TILs with clinicopathological parameters and patient outcome was determined.
Results
A strong positive linear correlation was observed between sTILs and tTILs. High sTILs and tTILs counts, as well as their proximity to stromal and tumour cells (co-occurrence) were associated with poor clinical outcomes and unfavourable clinicopathological parameters including high tumour grade, lymph node metastasis, large tumour size, and young age. AI-based assessment of the proportion of stroma composed of sTILs (as assessed visually in routine practice) was not predictive of patient outcome. tTILs was an independent predictor of worse patient outcome in multivariate Cox Regression analysis.
Conclusion
AI-based detection of TILs counts, and their spatial distribution provides prognostic value in luminal early-stage BC patients. The utilisation of AI algorithms could provide a comprehensive assessment of TILs as a morphological variable in WSIs beyond eyeballing assessment.
Similar content being viewed by others
Introduction
Breast cancer (BC) is a heterogeneous disease with different molecular subtypes and variable clinical behaviours [1]. Despite the good prognosis of early-stage BC expressing oestrogen receptor (ER) and lacking human epidermal growth factor receptor 2 (HER2) overexpression (luminal BC), post-treatment recurrence occurs in approximately 20% of cases [2]. This supports the existence of aggressive subgroups within these luminal tumours, which comprise more than 60% of all BC [3]. Therefore, prognostic stratification of early-stage luminal BC is of paramount importance to inform optimal treatment decision-making for these patients [4].
The role of tumour infiltrating lymphocytes (TILs) in refining BC prognosis and possible targeted immunotherapy has been widely studied [5,6,7,8]. It has been established that TILs play a key prognostic role in triple-negative (TN) (ER-negative, progesterone receptor (PR)-negative and HER2-negative) and HER2-positive BCs [9]. However, their role in luminal BCs remains unclear and conflicting results have been reported. Some studies reported an association between TILs and poor prognostic parameters [10, 11], while others did not find any prognostic significance [12, 13]. This has led to the exclusion of TILs, as currently assessed, as a prognostic stratifier in luminal BCs [13, 14].
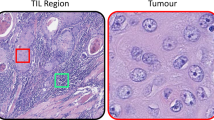
To date, it is recommended that visual assessment of TILs in routine clinical practice depend solely on stromal TILs (sTILs), defined as the percentage of mononuclear cells (lymphocytes and plasma cells) quantifiable in the stromal area [15]. Although this method of assessment would potentially increase reproducibility in routine clinical practice, this approach precludes the assessment of the spatial heterogeneity of TILs distribution which may be clinically informative [15,16,17]. Similarly, TILs in direct contact with or infiltrating tumour cells known as intra-tumoural TILs (tTILs) are not quantified, despite being biologically relevant to interact with tumour cells [15, 16]. Spatial heterogeneity and tTILs are more challenging to quantify and are considered too complex to visually assess in routine practice.
The widespread use of digital pathology and the increasing applications of artificial intelligence (AI) on whole slide images (WSIs) [18,19,20] have opened avenues for re-exploring the prognostic roles of morphological features such as TILs, especially within the molecular classes where TILs role is uncertain. The development of machine learning (ML) algorithms for automated computational TILs assessment to allow precise, rapid and less exhaustive workflow is a current need [9]. This would not only improve diagnostic concordance but will also add more information that cannot be assessed by the human eye [21].
In this study, we hypothesised that using AI-based algorithms would provide improved assessment of TILs in early-stage luminal BC patients and identify additional TILs features that have prognostic implications.
Materials and methods
Study cohorts
This study was conducted on two cohorts including:
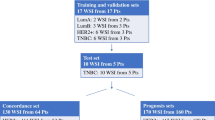
A) Nottingham cohort: a cohort of 2231 endocrine-treated ER + /HER2- BC patients presented to Nottingham City Hospital, Nottingham, United Kingdom. Anonymised clinicopathological data including patient’s age, menopausal status, tumour size, histological subtype, tumour grade, Nottingham prognostic index (NPI), lymph node (LN) status, lymphovascular invasion (LVI), PR and Ki67 expression scores, as well as treatment information were collected. All patients included were treated with adjuvant endocrine therapy only based on local treatment protocol; ER + BC patients were offered chemotherapy only if the NPI score is in the poor prognostic group and the patient tolerates chemotherapy [22, 23]. None of the patients have received neoadjuvant therapy. BC specific survival (BCSS) identified as the time from initial diagnosis to death related to BC was calculated for all patients. The cohort was divided into discovery (n = 1572) and test (n = 659) sets using stratified random sampling to ensure equal distribution of events in each set. Initially, the discovery set underwent an internal 3-fold cross-validation, where the set was stratified into three different splits which were used for training the model. Then the optimised module resulting from this cross-validation process was applied to the test set. To note, the test set remained untouched throughout the AI model upstream steps to ensure a reliable validation. However, for the simplification of the results, the correlation between TILs against the clinicopathological, and outcome data was carried out on the whole discovery set and validated on the test set.
B) External validation cohort: an external validation cohort (n = 318) was also collected from endocrine-treated BC patients presented and managed at the University Hospital Coventry and Warwickshire (UHCW), Coventry, UK from 2011-2014. The clinicopathological data for this cohort was also available.
The patient and tumour characteristics of study cohorts are presented in Supplementary Tables 1 and 2.
Formalin-fixed paraffin-embedded (FFPE) tumour tissue blocks were retrieved for all cases. Fresh sections were prepared and stained with Haematoxylin and Eosin (H&E). Slides were scanned using Philips IntelliSite Ultra-Fast Scanner, Philips Digital Pathology Solutions, Best, the Netherlands and Panoramic 250 Flash III: 3D Histech, Budapest, Hungary on 40x magnification, generating high resolution WSIs. The model was trained on images from both scanners and random brightness/contrast, median blur and colour jitter augmentations were carried out. One representative WSI was used for each case where largest tumour burden with associated TILs was present.
Annotations of the WSIs
Exhaustive region and cell level annotations were performed by six experienced pathologists for supervised ML training models. Regional annotations involved invasive and in situ tumour areas, tumour associated stroma (TAS), and normal breast tissue (terminal duct lobular units) regions. Cell annotations included tumour cells with various morphologies and degrees of pleomorphism, stromal cells either fibroblasts or myofibroblasts, normal epithelial cells and TILs. Areas of necrosis, calcification and tissue/image artefacts were also annotated and excluded from the final image processing. The detailed process of image acquisition and annotation of this cohort was previously described [24].
Deep learning pipeline for TILs quantification and distribution
To quantify sTILs and tTILs, a deep learning (DL) based pipeline is proposed (Fig. 1). Using image thresholding and morphological operations, a tissue mask was generated for a WSI to exclude all irrelevant image background or artefacts from further processing. To exclude regions of carcinoma in situ from the analysis, a convolution neural network (CNN) based model (CNNDCIS) [25] was applied. Different types of nuclei including tumour, stromal and normal epithelium were segmented and classified by CNNNuc. CNNReg was employed to segment stromal and other non-regions of interest (non-ROIs). Further, the stromal regions were restricted to TAS via morphological operations. Finally, the immune, tumour, and stromal nuclei were processed to compute sTILs and tTILs for each WSI.
Ductal carcinoma in situ (DCIS) is filtered out by CNNDCIS prior to CNNNuc performs nuclei segmentation and classification followed by CNNReg performs region segmentation. Both nuclear and regional results are used to generate sTIL and tTIL (T=tumour nuclei, S=stromal nuclei, I=immune nuclei, TR tumour region, TAS tumour associated stroma region).
Region segmentation
Quantification of immune cells within tumour and TAS requires region segmentation. For the purpose of sematic segmentation of the regions, a CNN model known as the U-Net [26] was adapted by adding two additional encoding/decoding blocks. The model was trained on pathologists’ marked regions and the trained model, referred to as CNNReg, was then used for semantic segmentation of stromal and other non-stromal regions in WSIs. Parameters for training CNNReg were set as follows: learning rate 0.01 (initial five epochs), 0.001 (epoch 6-10), 0.0001 (epoch 11-30), momentum 0.9, cross entropy loss function, patch size 512 × 512 with 96 pixels context on all sides and batch size 8. The input was normalised to the [0,1] interval and different augmentation methods (random rotate, random brightness/contrast, median blur) were used during training with values of 0.5. To restrict stromal region only to TAS instead of overall stroma, the following steps were performed: i) stromal regions segmented by CNNReg were combined with tumour regions segmented by CNNDCIS; ii) to capture the TAS, the tumour regions were dilated with a disc of radius 8 pixels. This was followed by filling the holes with a disc of radius 32 pixels; iii) any stroma captured in the final dilated tumours was considered as TAS for further feature calculation. Immune nuclei were then counted separately in the tumour regions as well as in the TAS. Other features included co-occurrences of different nuclei, immune heterogeneity, and contrast.
Nuclei segmentation and classification
To calculate different features related to immune, tumour and stroma nuclei, an in-house developed state-of-the-art nuclei segmentation and classification model CNNNuc based on HoVer-Net [27] was fine-tuned to classify and segment different types of nuclei. Weights from a pretrained version of the model on BC only subset of PanNuke dataset [28] were used in the finetuning process. Training parameters were set as follows: learning rate 0.0001 (initial two epochs), 0.00001 (3rd epoch onwards), patch size 256 × 256 pixels and batch size 8. During inference on a WSI, a nuclei mask was generated by mapping the type/class of each detected nucleus at its centroid in a five-times down sampled WSI. Combined with segmented tumour and TAS regions, the counts of immune nuclei were used to get the tTILs and sTILs counts (Fig. 1). Furthermore, a co-occurrence matrix (CM) was created for tumour, stromal and immune nuclei (Fig. 2 and Supplementary Fig. 1). Based on the CM, different features were calculated including immune heterogeneity, stroma and immune co-occurrences, tumour and immune co-occurrences.
Deep learning-based scoring of sTILs and tTILs
sTILs count refers to the number of sTILs scattered in stroma, while tTILs count describes number of tTILs in direct contact with tumour cells with no intervening stroma [29]. The percentage of tumour stroma occupied by TILs, mimicking the visual score performed by pathologists [15] was added to the features and is referred to as the “AI-based sTILs percentage”. The co-occurrence of sTILs and stromal cells indicates the number of times sTILs and stromal cells are found within certain distance of each other. Similarly, for tTILs, co-occurrence of tTILs with tumour cells within certain distance to each other was also assessed. Supplementary Table 3 illustrates a simplified description of the assessed features.
Eyeballing assessment of TILs
A subset of cases (n = 300) was scored by a well-trained pathologist as a continuous parameter following the proposed scoring method by TILs Working Group [15], where TILs within the tumour borders were only considered, excluding areas of necrosis or tissue artefacts. TILs around normal breast lobules or in situ carcinoma were omitted. Scoring was carried out blindly to the generated features and was then compared with the AI-based sTILs percentage for measuring the concordance as a validation method.
Statistical analysis
Analysis was performed using Statistical Package SPSS v28 for Windows (Chicago, IL, USA). Assessed features were used as continuous variables and association with clinicopathological parameters was evaluated using Mann-Whitney and Kruskal-Wallis tests. Each feature was dichotomised, into low and high for outcome analysis based on X-tile software version 3.6.1 (Yale University, New Haven, CT) [30] using BCSS as an end point. The cut-off used for analysis was generated on the discovery set and applied to the test set and external validation cohort. Kaplan Meier curves and log-rank test were used to assess the significance between low and high groups and outcome. Cox-regression analysis was performed, hazard ratios (HR) and confidence intervals (CI) were calculated. The power of survival analysis was calculated using Stata Statistical Software, Release 18 (College Station, TX: StataCorp LLC). The level of agreement between visual and AI-based sTILs percentage was assessed using intraclass correlation coefficient (ICC). A p-value of less than 0.05 (two-tailed) was considered significant in all the statistical tests.
Results
ML modules for TILs quantification and distribution
Analytical performance of different ML modules used in TILs quantification and distribution are shown in Supplementary Table 4. F1-scores for tumour and DCIS segmentation were 0.71 and 0.90, respectively, whereas dice scores of 0.76 and 0.69 were achieved for stromal versus other region segmentation. Similarly, average F1-scores of 0.82, 0.92 and 0.81 were achieved for classifying immune, tumour and stromal cells, respectively. Supplementary Table 5 shows the confusion matrix for cell classification where immune, tumour and stroma cells were classified with accuracies of 80%, 96% and 84.7%, respectively.
TILs distribution and characteristics
AI-based sTILs percentage had a range from 1% to 76%, which was close to results obtained by visual assessment (ranged from 0 to 80%). The concordance between the visual and AI-based sTILs percentage, showed good agreement (ICC = 0.7).
A significant positive correlation between the numbers of sTILs and tTILs was found (r = 0.6, p < 0.001) and this correlation was observed in the discovery and test sets. Similarly, the sTILs count and AI-based sTILs percentage showed significantly positive correlation (r = 0.6, p < 0.001) in both sets. In the external validation cohort, the correlation between sTILs and tTILs count and between sTILs count and AI-based sTILs percentage was positive (r = 0.8 and r = 0.6, respectively, p < 0.001). The median count of TILs and TILs co-occurrence scores in WSIs, was calculated in the study cohorts (Supplementary Table 3).
Association of TILs with clinicopathological parameters
High sTILs and tTILs counts as well as the combined total TILs counts were associated with unfavourable clinicopathological prognostic parameters including younger age at diagnosis, larger tumour size, higher grade, LN metastasis, poorer NPI and high Ki67 index (Table 1). Similarly high AI-based sTILs percentage was significantly associated with higher grade, poorer NPI and a high ki67 index (Table 2).
The presence of high sTILs-stromal cells co-occurrences, representing the close distribution of TILs to their neighboured stromal cells and similarly tTILs-tumour cells co-occurrences was significantly correlated with unfavourable tumour characteristics in both the discovery and test sets (Table 3). Similar findings were revealed when tested on the external validation cohort (Tables 1–3).
Outcome analysis
Patients with BC showing high sTILs had significantly shorter survival (HR = 1.6, 95% CI = 1.01-2.5, p = 0.04, in the discovery set and HR = 2.5, 95% CI = 1.3-4.5, p = 0.004 in the test set) than tumours with low sTILs (Fig. 3a, b). The presence of high tTILs was also associated with shorter survival (HR = 1.7, 95% CI = 1.08-2.6, p = 0.01 and HR = 2, 95% CI = 1.06-3.7, p = 0.03 in discovery and test sets, respectively) (Fig. 3c, d). The same findings were observed using total TILs count. However, sTILs percentage (the current recommended method for TILs assessment) did not show any significant association with patient survival, neither using the AI-based sTILs percentage nor eyeballing scoring (p > 0.05).
Worse breast cancer specific survival was associated with tumours with high stromal tumour infiltrating lymphocytes count in discovery (a) and test (b) sets, high intratumoural tumour infiltrating lymphocytes count in discovery (c) and test (d) sets, high stromal tumour infiltrating lymphocytes- stromal cells co-occurrence in discovery (e) and test (f) sets, and high intratumoural tumour infiltrating lymphocytes- tumour cells co-occurrence in discovery (g) and test (h) sets.
In terms of the spatial distribution, high sTILs-stromal cells co-occurrence was significantly correlated with worse outcome compared to tumours with low co-occurrence (HR = 1.6, 95% CI = 1.08-2.4, p = 0.03 and HR = 2.2, 95% CI = 1.2-4.2, p = 0.01 in discovery and test sets, respectively) (Fig. 3e, f). Similar results were observed with high tTILs-tumour cells cooccurrence (HR = 1.7, 95% CI = 1.1-2.7, p = 0.01 and HR = 1.9, 95% CI = 1.08-3.7, p = 0.04 in discovery and test sets, respectively) (Fig. 3g, h). The power of survival analyses ranged from 60% to 80%.
Multivariate Cox Regression analysis of all significant features, adjusted for tumour grade, LN metastasis, Ki67 index and tumour size, revealed that tTILs count is an independent predictor of outcome. Every 1000 unit increase in tTILs count is associated with 20% increase in BC- related death risk (HR = 1.2, 95% CI = 1.1-1.5 and p = 0.007).
However, a statistically meaningful outcome analysis on the external validation cohort could not be carried out due to the limited number of events and the short-term follow-up.
Discussion
TILs have been extensively studied for their prognostic significance [31,32,33,34]. A good prognostic value of TILs in TN and HER2-positive BCs was reported [35,36,37,38]. However, TILs findings in luminal ER + /HER2- BCs were inconsistent [10, 12, 39, 40]. Luminal BCs are biologically heterogeneous tumours with divergent clinicopathological characteristics and variable outcomes [12]. A considerable proportion of ER-expressing BCs are resistant to endocrine therapy which highlights the need for further prognostic indicators to stratify high from low-risk early stage luminal BC patients [41].
In the era of immunotherapy, the role of the tumour microenvironment (TME) in predicting patient outcomes, and the possible influence of chemotherapy and hormonal treatment on TME has been demonstrated [42]. Luminal BCs are characterised by low chromosomal instability compared to TNBC, explaining low antigenicity and immune response [43]. Additionally, ER has the capability of T regulators (T-reg) activation and inhibition of antigen-presenting cell (APC) function, promoting immunogenic tolerance [44, 45]. The presence of relatively low TILs levels and studies addressing the absence of prognostic or predictive significance of TILs in ER + /HER2- BCs has shifted attention away from the study of TILs in this patient subgroup [46].
It has been proposed that TILs abundance is sufficient to be assessed in TNBC to guide a prognostic benefit, while in luminal tumours, biological stratification of TILs should be more informative [46, 47]. However, TILs visual evaluation performed by pathologists is limited to a single parameter, stromal TILs [15], which failed to have a prognostic significance in several studies [48, 49]. The TILs working group has recommended assessing only sTILs, taking into account the correspondence with tTILs and the better reproducibility of sTILs between pathologists [13]. Visual assessment cannot capture all complex morphological features and the geometric distribution of TILs in relation to tumour cells which may play a role in BC behaviour. Additionally, low reproducibility and challenges in visual assessment of TILs, highlight the need for reliable automated methods [50, 51]. The agreement in TILs scoring between pathologists has been investigated in several studies with an ICC range from 0.5 to 0.8 [9, 52,53,54], which emphasises the necessity for a validated standardised method of assessment.
The use of the readily available H&E WSIs in such automated analyses will enable more cost effective use of tissue and clinical resources [17]. Automated TILs detection is expected to enable better identification of TILs features, which are challenging to score by a pathologist owing to their relatively low numbers [47] and difficult recognition among tumour nests [15] and the complexity of assessment of distribution of TILs in relation to stromal and tumour cells. Moreover, computational TILs assessment allows the detection of underestimated parameters in an easy quantitative method, allowing spatial distribution estimation [14]. In the current study, a good level of concordance between visual and automated TILs scores was achieved, which was encouraging for further testing and validation.
Although most previous studies did not focus on tTILs due to their lower density compared to sTILs [40], interestingly, our study revealed that tTILs are an independent prognostic indicator of BCSS, which highlights the under-recognised role of tTILs. In the present study, both sTILs and tTILs were strongly positively correlated, which is consistent with previous studies [55,56,57], but contrary to Heindl et.al., who proposed that TILs tend to either infiltrate the tumour nests or the stroma [58].
Automated TILs assessment could provide data about the distribution of TILs in relation to stromal and tumour cells. Here the histological ecology of TILs, or how TILs interact with their neighbouring tumour and stromal cells, was investigated in terms of their spatial distribution [59]. It has recently been found that focusing on the spatial relationship may be more predictive than the routinely used density scores [58, 60]. In our study, TILs found in close proximity to tumour and stroma cells were significantly associated with worse clinicopathological features and poorer BCSS, which was consistent with a previous study [58]. However, the co-localisation of tumours and immune cells was reported to have a positive predictive association in luminal A subtype [61]. Their results were regardless of type of therapy received and lacked validation. This controversy encourages further research aiming to study the spatial distribution of TILs, adding to our understanding of tumour progression, managed by the biological interactions between tumour and immune cells [61, 62].
One of the features that can be generated by AI algorithms is the TILs count, which may be a more powerful prognostic indicator than the usual routine assessment method. In relation to patient outcome, TILs count was significantly associated with poorer survival, unlike the sTILs percentage scored both manually and AI-assisted, which lacked prognostic significance. This was consistent with previous studies that reported high TILs were negatively associated with recurrence-free survival and distant metastasis-free survival [2, 63]. It has been reported that high expression of TILs related genes in ER+ BCs was associated with poor overall survival [64]. On the other hand, no prognostic significance of TILs was detected in several studies and meta-analyses [12, 55, 65]. In another AI-based study, none of the TILs abundance scores showed significant association with outcomes, though the discordance may owe to the normalisation to the count of cancer cells [58].
TILs counts were significantly associated with unfavourable clinicopathological parameters in this study in line with the findings of former studies [12, 63]. High TILs in ER + /HER2- BCs have been associated with highly proliferating tumours shown with Ki67 testing which was consistent with several studies [11, 12, 66, 67], that can be explained by increased antigenicity caused by high proliferation. Previous publications reported no additional significance of high TILs in relation to any of the clinicopathological parameters [67, 68], explaining the limited information gained from the visual score.
We propose that one of the contributors to disagreement between studies was the use of different methodologies, whether total TILs or subclasses quantification through IHC detected subpopulations or using different cut-off values for defining low versus high TILs. In our analysis, the TILs count was used as continuous variables to avoid a biased cut-off point.
Our cohort was endocrine treated with poor prognostic outcomes of tumours enriched with TILs. Dunbier and colleagues reported poor response to aromatase inhibitors in ER+ tumours rich TILs suggesting that TILs are involved in resistance to hormonal therapy [69]. Moreover, high TILs was associated with unfavourable outcome in patients who received neoadjuvant letrozole [67]. This suggests that endocrine monotherapy in the subgroup of luminal BC patients with high TILs infiltration is insufficient for optimal patient management and outcomes. Although a clinical trial on immunotherapy for Programmed cell death 1 (PD1)-rich ER + BC patients revealed poor response, neoadjuvant trials had a promising pathological complete response advantage suggesting that ER+ patients encompass a group with possible benefit from immunotherapy [70,71,72].
We recognise the study has some limitations. Firstly, this study was conducted on H&E-stained sections only which did not allow for the identification of different immune subpopulations and their distinct roles. In routine practice, H&E sections are the standards for TILs evaluation and its association with tumour behaviour and patient outcome, however, the identification of the makeup of the various subpopulations would help in understanding the underlying biology. Various immune cells, including CD8 + , CD4 + , FOXP3 T lymphocytes, B lymphocytes, plasma cells and macrophages (including M1 and M2 subtypes) would have different roles in tumour microenvironment and behaviour. The predominate immune cell type may exert significant control over the tumour behaviour. Secondly, although our model showed high accuracy in identifying and distinguishing TILs from other cell types, we must acknowledge the potential presence of false TILs identification. As with any automated or AI-based system, there is an inherent risk of misclassifications. Thirdly, although using large well characterised cohort, the small number of events in our study warrants careful interpretation of the results and further validation utilising external independent cohorts with long term follow up is recommended.
In conclusion, automated assessment of both TILs counts, and spatial distribution provides independent prognostic value in early-stage luminal BCs. The utilisation of AI algorithms would add to the limited information pathologist can retrieve from visual assessment.
Data availability
The data presented in the current study are available upon reasonable request.
References
Ohtani H, Mori‐Shiraishi K, Nakajima M, Ueki H. Defining lymphocyte‐predominant breast cancer by the proportion of lymphocyte‐rich stroma and its significance in routine histopathological diagnosis. Pathol Int. 2015;65:644–51.
Sobral-Leite M, Salomon I, Opdam M, Kruger DT, Beelen KJ, van der Noort V, et al. Cancer-immune interactions in ER-positive breast cancers: PI3K pathway alterations and tumor-infiltrating lymphocytes. Breast Cancer Res. 2019;21:1–12.
Ignatiadis M, Sotiriou C. Luminal breast cancer: from biology to treatment. Nat Rev Clin Oncol. 2013;10:494–506.
Locy H, Verhulst S, Cools W, Waelput W, Brock S, Cras L, et al. Assessing tumor-infiltrating lymphocytes in breast cancer: a proposal for combining immunohistochemistry and gene expression analysis to refine scoring. Front Immunol. 2022;13:794175.
Blackley EF, Loi S. Targeting immune pathways in breast cancer: review of the prognostic utility of TILs in early stage triple negative breast cancer (TNBC). Breast. 2019;48:S44–8.
Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology. 2017;49:141–55.
Nelson MA, Ngamcherdtrakul W, Luoh S-W, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021;40:519–36.
Rizzo A, Ricci AD. Biomarkers for breast cancer immunotherapy: PD-L1, TILs, and beyond. Expert Opin Investig Drugs. 2022;31:549–55.
Sun P, He J, Chao X, Chen K, Xu Y, Huang Q, et al. A computational tumor-infiltrating lymphocyte assessment method comparable with visual reporting guidelines for triple-negative breast cancer. EBioMedicine. 2021;70:103492.
Montagna E, Vingiani A, Maisonneuve P, Cancello G, Contaldo F, Pruneri G, et al. Unfavorable prognostic role of tumor-infiltrating lymphocytes in hormone-receptor positive, HER2 negative metastatic breast cancer treated with metronomic chemotherapy. Breast. 2017;34:83–8.
Tsang J, Hui S-W, Ni Y-B, Chan S-K, Yamaguchi R, Kwong A, et al. Lymphocytic infiltrate is associated with favorable biomarkers profile in HER2-overexpressing breast cancers and adverse biomarker profile in ER-positive breast cancers. Breast Cancer Res Treat. 2014;143:1–9.
Criscitiello C, Vingiani A, Maisonneuve P, Viale G, Curigliano G. Tumor-infiltrating lymphocytes (TILs) in ER+/HER2− breast cancer. Breast Cancer Res Treat. 2020;183:347–54.
El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. npj Breast Cancer. 2021;7:1–17.
Amgad M, Stovgaard ES, Balslev E, Thagaard J, Chen W, Dudgeon S, et al. Report on computational assessment of tumor infiltrating lymphocytes from the International Immuno-Oncology Biomarker Working Group. NPJ breast cancer. 2020;6:1–13.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71.
Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol. 2018;26:523–32.
Le H, Gupta R, Hou L, Abousamra S, Fassler D, Torre-Healy L, et al. Utilizing automated breast cancer detection to identify spatial distributions of tumor-infiltrating lymphocytes in invasive breast cancer. Am J Pathol. 2020;190:1491–504.
Amrane M, Oukid, S, Gagaoua, I & Ensari, T in 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting (EBBT). 1-4 (IEEE).
Fatima N, Liu L, Hong S, Ahmed H. Prediction of breast cancer, comparative review of machine learning techniques, and their analysis. IEEE Access. 2020;8:150360–76.
Osareh A & Shadgar, B in 2010 5th international symposium on health informatics and bioinformatics. 114-20 (IEEE).
Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288:62–81.
Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast cancer Res Treat. 1992;22:207–19.
Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–8.
Wahab N, Miligy IM, Dodd K, Sahota H, Toss M, Lu W, et al. Semantic annotation for computational pathology: Multidisciplinary experience and best practice recommendations. J Pathol: Clin Res. 2022;8:116–28.
Jahanifar M, Tajeddin, NZ, Koohbanani, NA & Rajpoot, NM in Proceedings of the IEEE/CVF International Conference on Computer Vision. 674-83.
Ronneberger O, Fischer, P & Brox, T in International Conference on Medical image computing and computer-assisted intervention. 234-41 (Springer).
Graham S, Vu QD, Raza SEA, Azam A, Tsang YW, Kwak JT, et al. Hover-net: Simultaneous segmentation and classification of nuclei in multi-tissue histology images. Med Image Anal. 2019;58:101563.
Gamper J, Alemi Koohbanani, N, Benet, K, Khuram, A & Rajpoot, N in European congress on digital pathology. 11-19 (Springer).
Kos Z, Roblin E, Kim RS, Michiels S, Gallas BD, Chen W, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6:17.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin cancer Res. 2004;10:7252–9.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J cancer. 2011;105:93–103.
Oble DA, Loewe, R, Yu, P & Mihm, MC Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer immunity 9 (2009).
Orhan A, Vogelsang RP, Andersen MB, Madsen MT, Hölmich ER, Raskov H, et al. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis. Eur J cancer. 2020;132:71–84.
Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour‐infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol: A J Pathological Soc Gt Br Irel. 1997;182:318–24.
Denkert C, Loibl S, Noske A, Roller M, Muller B, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50.
Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64.
Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast cancer Res Treat. 2016;158:323–31.
Al‑Saleh K, El‑Aziz A, Ali A, Abozeed W, El‑Warith A, Ibraheem A, et al. Predictive and prognostic significance of CD8+ tumor‑infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncol Lett. 2017;14:337–44.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. lancet Oncol. 2018;19:40–50.
Lindström LS, Yau C, Czene K, Thompson CK, Hoadley KA, Van’t Veer LJ, et al. Intratumor heterogeneity of the estrogen receptor and the long-term risk of fatal breast cancer. JNCI: J Natl Cancer Inst. 2018;110:726–33.
Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–41.
Zeppellini A, Galimberti S, Leone BE, Pacifico C, Riva F, Cicchiello F, et al. Comparison of tumor microenvironment in primary and paired metastatic ER+/HER2-breast cancers: results of a pilot study. BMC cancer. 2021;21:1–10.
Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines. 2016;4:28.
Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen‐mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD‐1 costimulatory pathway. J Neurosci Res. 2006;84:370–8.
Dieci MV, Griguolo G, Miglietta F, Guarneri V. The immune system and hormone-receptor positive breast cancer: Is it really a dead end? Cancer Treat Rev. 2016;46:9–19.
Caparica R, Bruzzone M, Agostinetto E, Franzoi MA, Ceppi M, Radosevic-Robin N, et al. Tumour-infiltrating lymphocytes in non-invasive breast cancer: A systematic review and meta-analysis. Breast. 2021;59:183–92.
Althobiti M, Aleskandarany MA, Joseph C, Toss M, Mongan N, Diez‐Rodriguez M, et al. Heterogeneity of tumour‐infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology. 2018;73:887–96.
He L, Wang Y, Wu Q, Song Y, Ma X, Zhang B, et al. Association between levels of tumor-infiltrating lymphocytes in different subtypes of primary breast tumors and prognostic outcomes: A meta-analysis. BMC women’s health. 2020;20:1–11.
Klauschen F, Müller KR, Binder A, Bockmayr M, Hägele M, Seegerer P, et al. Scoring of tumor-infiltrating lymphocytes: From visual estimation to machine learning. Semin Cancer Biol. 2018;52:151–7.
Kos Z, Roblin E, Kim RS, Michiels S, Gallas BD, Chen W, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ breast cancer. 2020;6:1–16.
Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G, et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017;30:1204–12.
Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–64.
Swisher SK, Wu Y, Castaneda CA, Lyons GR, Yang F, Tapia C, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Ann surgical Oncol. 2016;23:2242–8.
Dieci M, Mathieu M, Guarneri V, Conte P, Delaloge S, Andre F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–704.
Goldberg J, Pastorello RG, Vallius T, Davis J, Cui YX, Agudo J, et al. The immunology of hormone receptor positive breast cancer. Front Immunol. 2021;12:674192.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7.
Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y. Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. JNCI: J Natl Cancer Inst. 2018;110:166–75.
Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl immunohistochemistry Mol morphology: AIMM. 2018;26:523.
Saltz J, Gupta R, Hou L, Kurc T, Singh P, Nguyen V, et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 2018;23:181–93.e187.
Maley CC, Koelble K, Natrajan R, Aktipis A, Yuan Y. An ecological measure of immune-cancer colocalization as a prognostic factor for breast cancer. Breast Cancer Res. 2015;17:1–13.
Anderson AR, Hassanein M, Branch KM, Lu J, Lobdell NA, Maier J, et al. Microenvironmental independence associated with tumor progression. Cancer Res. 2009;69:8797–806.
Takada K, Kashiwagi S, Asano Y, Goto W, Kouhashi R, Yabumoto A, et al. Prediction of distant metastatic recurrence by tumor-infiltrating lymphocytes in hormone receptor-positive breast cancer. BMC women’s health. 2021;21:1–11.
Calabrò A, Beissbarth T, Kuner R, Stojanov M, Benner A, Asslaber M, et al. Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast cancer Res Treat. 2009;116:69–77.
Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PloS one. 2016;11:e0152500.
Honda C, Kurozumi S, Katayama A, Hanna‑Khalil B, Masuda K, Nakazawa Y, et al. Prognostic value of tumor‑infiltrating lymphocytes in estrogen receptor‑positive and human epidermal growth factor receptor 2‑negative breast cancer. Mol Clin Oncol. 2021;15:1–6.
Skriver SK, Jensen M-B, Knoop AS, Ejlertsen B, Laenkholm A-V. Tumour-infiltrating lymphocytes and response to neoadjuvant letrozole in patients with early oestrogen receptor-positive breast cancer: analysis from a nationwide phase II DBCG trial. Breast Cancer Res. 2020;22:1–8.
Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res. 2018;38:2311–21.
Dunbier AK, Ghazoui Z, Anderson H, Salter J, Nerurkar A, Osin P, et al. Molecular Profiling of Aromatase Inhibitor–Treated Postmenopausal Breast Tumors Identifies Immune-Related Correlates of ResistanceGenomic Profiling Predicts Response to Aromatase Inhibitor Treatment. Clin Cancer Res. 2013;19:2775–86.
de Boo L, Cimino-Mathews A, Lubeck Y, Daletzakis A, Opdam M, Sanders J, et al. Tumour-infiltrating lymphocytes (TILs) and BRCA-like status in stage III breast cancer patients randomised to adjuvant intensified platinum-based chemotherapy versus conventional chemotherapy. Eur J Cancer. 2020;127:240–50.
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H-T, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167:671–86.
Nanda R, Liu, MC, Yau, C, Asare, S, Hylton, N, Veer, LV t. et al. (American Society of Clinical Oncology, 2017).
Acknowledgements
The authors are part of the Path LAKE digital pathology consortium. These new Centres are supported by a £50 m investment from the Data to Early Diagnosis and Precision Medicine strand of the government’s Industrial Strategy Challenge Fund, managed and delivered by UK Research and Innovation (UKRI).
Funding
S.M. is supported and funded by the Egyptian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Contributions
Concept and Design: S.M., E.R., Collection of Data: S.M., M.T., A.I., A.L., Annotations: M.T., A.L. and A.I., Analysis of Data: S.M., N.W., M.T., E.R., Cell and tumour Segmentation: M.J., S.G., N.W., N.R., Interpretation of Results: All Authors, Writing and Reviewing Manuscript: All Authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declares no competing interests.
Ethical approval
This work was approved by South Central - Oxford C Research Ethics Committee under the title; PathLAKE, reference number19/SC/0363. All procedures performed in studies were in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makhlouf, S., Wahab, N., Toss, M. et al. Evaluation of tumour infiltrating lymphocytes in luminal breast cancer using artificial intelligence. Br J Cancer 129, 1747–1758 (2023). https://doi.org/10.1038/s41416-023-02451-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02451-3
- Springer Nature Limited
This article is cited by
-
Current status and prospects of artificial intelligence in breast cancer pathology: convolutional neural networks to prospective Vision Transformers
International Journal of Clinical Oncology (2024)