Abstract
Purpose
The prognostic role of tumor-infiltrating lymphocytes (TILs) in ER+/HER2− breast cancer (BC) is debated. We evaluated the association of TILs and clinico-pathological features with distant disease-free survival (DDFS) in patients with ER+/HER2− BC treated at a single institution.
Patients and methods
A mono-institutional case-cohort series of 987 patients with early ER+/HER2− BC was retrospectively analyzed. TILs were considered both as continuous variable, and dichotomized in low (< 5%) vs high (≥ 5%). The main outcome was DDFS. Median follow-up was 7.5 years (0.1–10). Univariate and multivariable Cox proportional hazards regression with inverse sub-cohort sampling probability weighting were used to evaluate the risk across groups.
Results
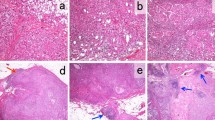
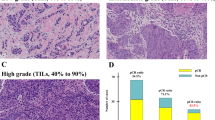
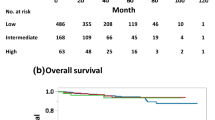
Median TIL count was 2% (Q1–Q3 1–4%). Higher TILs were positively associated with number of lymph nodes involved (p = 0.003), tumor grade (p < 0.0001), peritumoral vascular invasion (p = 0.003), higher Ki-67 (p = 0.0001), luminal B subtype (p < 0.0001), and chemotherapy use (p < 0.00019). In multivariable regression analysis, only higher Ki-67 expression retained significant association with TILs. At univariate Cox regression analysis, TIL expression (≥ 5% vs. < 5%) was not associated with DDFS (HR 1.08, 95% CI 0.80–1.46, p = 0.62). In patients treated with adjuvant chemotherapy, high TILs were associated with better DDFS (HR 0.52, 95%CI 0.33–0.83, p = 0.006), particularly in the group with Ki-67 ≥ 20% (HR 0.50, 95%CI 0.29–0.86, p = 0.01).
Conclusion
High TILs in ER+/HER2− BC are significantly associated with clinico-pathological features of dismal outcome. TIL prognostic value seems different in patients treated with or without chemotherapy. Our findings suggest that the high-risk subgroup might be more immunogenic, thus deserving the exploration of immunotherapy approaches.





Similar content being viewed by others
References
Prat A, Fan C, Fernandez A et al (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13:303
Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444
Savas P, Salgado R, Denkert C et al (2016) Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 13(4):228–241
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31(7):860–867
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25(8):1544–1550
Ali HR, Provenzano E, Dawson SJ et al (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 25(8):1536–1543
Salgado R, Denkert C, Campbell C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol 1(4):448–454
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966
Park JH, Jonas SF, Bataillon G et al (2019) Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 30:1941
Dieci MV, Criscitiello C, Goubar A et al (2015) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 26(7):1518
Dieci MV, Griguolo G, Miglietta F, Guarneri V (2016) The immune system and hormone-receptor positive breast cancer: is it really a dead end? Cancer Treat Rev 46:9–19
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50
Maisonneuve P, Disalvatore D, Rotmensz N et al (2014) Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res 16(3):R65
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Denkert C, Wienert S, Poterie A et al (2016) Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol 29(10):1155–1164
Stanton SE, Disis ML (2016) Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 4:59
Mostafa AA, Codner D, Hirasawa K et al (2014) Activation of ERalpha signaling differentially modulates IFN-gamma induced HLA-class II expression in breast cancer cells. PLoS ONE 9(1):e87377
Fujimoto Y, Watanabe T, Hida AI et al (2019) Prognostic significance of tumor-infiltrating lymphocytes may differ depending on Ki67 expression levels in estrogen receptor-positive/HER2-negative operated breast cancers. Breast Cancer (Tokyo, Japan) 26:738
Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24(34):5373–5380
Sobral-Leite M, Salomon I, Opdam M et al (2019) Cancer-immune interactions in ER-positive breast cancers: PI3K pathway alterations and tumor-infiltrating lymphocytes. Breast Cancer Res 21(1):90
Dunbier AK, Ghazoui Z, Anderson H et al (2013) Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin Cancer Res 19(10):2775–2786
Gao Q, Patani N, Dunbier AK et al (2014) Effect of aromatase inhibition on functional gene modules in estrogen receptor-positive breast cancer and their relationship with antiproliferative response. Clin Cancer Res 20(9):2485–2494
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CC: honoraria for speakers' bureau or consultancy from Roche, Pfizer, Novartis, Lilly. AV, PM, CV: no competing interests to disclose. GV: personal honoraria from MSD Oncology, Pfizer; Consulting or Advisory Role from Dako, Roche/Genentech, Astellas Pharma, Novartis, Bayer, Daiichi Sankyo; Speakers' Bureau from Roche/Genentech; Institutional research Funding from Roche/Genentech, Ventana Medical Systems, Dako/Agilent Technologies. GC: honoraria for speaker, consultancy, or advisory role from Roche, Pfizer, Novartis, Seattle Genetics, Lilly, Ellipsis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Criscitiello, C., Vingiani, A., Maisonneuve, P. et al. Tumor-infiltrating lymphocytes (TILs) in ER+/HER2− breast cancer. Breast Cancer Res Treat 183, 347–354 (2020). https://doi.org/10.1007/s10549-020-05771-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05771-7