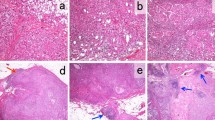
Abstract
The purpose of this study was to assess the prognostic and predictive value of tumor-infiltrating lymphocytes (TILs) in the triple-negative breast cancer (TNBC) cohort of the phase III IBCSG trial 22-00, comparing low-dose oral ‘metronomic’ cyclophosphamide-methotrexate maintenance chemotherapy (CM-maintenance) to no-CM-maintenance in early breast cancer. TILs were evaluated in full-face hematoxylin-and-eosin-stained sections of tumor samples confirmed centrally as TNBC (< 1 % of ER and PgR immunoreactivity and absence of HER2 overexpression or amplification). Mononuclear cells were evaluated in the stromal area within the borders of the invasive tumor. The primary endpoint was breast cancer-free interval (BCFI). Cox proportional hazards regression model assessed the association of BCFI and secondary endpoints with TILs score. In the 647 tumor samples, the median percentage of TILs was 18 % (IQR = 8–40 %), with 18 % having TILs ≥ 50 % (lymphocyte-predominant breast cancer, LPBC). At a median follow-up of 6.9 years, TILs were associated with better prognosis. For every 10 % increase of TILs, BCFI risk reduction was 13 % (HR 0.87, 95 % CI 0.79–0.95,P = 0.003). DFS, DRFI, and OS risk reductions were 11 % (P = 0.005), 16 % (P = 0.003), and 17 % (P < 0.001), respectively. Multivariable analysis confirmed the independent prognostic value of TILs. No significant TILs-by-treatment interaction was observed (P = 0.39) for associations of TILs with BCFI, although patients with LPBC receiving CM-maintenance had a greater breast cancer risk reduction (HR 0.64,95 % CI 0.23–1.78) than those with non-LPBC (TILs < 50 %) (HR 0.96, 95 % CI 0.67–1.40). TILs score is a potent prognostic factor in patients with TNBC. Low-dose chemotherapy confers a greater (not statistically significant) clinical benefit in patients with LPBC.



Similar content being viewed by others
References
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31:860–867
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959–2966
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113
Pruneri G, Vingiani A, Bagnardi V et al (2016) Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 27:249–256
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271
Browder T, Butterfield CE, Kräling BM et al (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60:1878–1886
Orlando L, Cardillo A, Rocca A et al (2006) Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 17:961–967
Colleoni M, Munzone E (2015) Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol 12:631–644
Colleoni M, Gray KP, Gelber SI et al (2016) Low-dose oral cyclophosphamide and methotrexate maintenance for hormone receptor-negative early breast cancer: International Breast Cancer Study Group (IBCSG) Trial 22-00. J Clin Oncol. doi:10.1200/JCO.2015.65.6595
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists: American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Hammond ME, Hayes DF, Wolff AC et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6:195–197
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103:1656–1664
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Ame Statist Assn 53:457–481
Cox DR (1972) Regression models and life-tables. JR Stat Soc B34:187–220
Lazar AA, Cole BF, Bonetti M et al (2010) Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot. J Clin Oncol 28:4539–4544
McShane LM, Altman DG, Sauerbrei W et al (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067–9072
Alizadeh D, Trad M, Hanke NT et al (2014) Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 74:104–118
Ma Y, Adjemian S, Mattarollo SR et al (2013) Anticancer chemotherapy induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38:729–741
Ghiringhelli F, Apetoh L, Tesniere A et al (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1 beta-dependent adaptive immunity against tumors. Nat Med 15:1170–1178
Ba Kamen, Rubin E, Aisner J et al (2000) High-time chemotherapy or high time for low dose. J Clin Oncol 18:2935–2937
Gately S, Kerbel R (2000) Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J 7:427–436
Lutsiak Semnani RT, De Pascalis R et al (2005) Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105:2862–2868
Tongu M, Harashima N, Monma H et al (2013) Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother 62:383–391
Perez EA, Ballman KV, Tenner KS et al (2016) Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol 2:56–64
Salgado R, Denkert C, Campbell C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol 1:448–454
Acknowledgments
We thank the patients, physicians, nurses, and data managers who participated in the International Breast Cancer Study Group trials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the International Breast Cancer Study Group and participating centers. Support for central coordination, data management and statistics were provided by the Swedish Cancer League; The Cancer Council Australia; Australia & New Zealand Breast Cancer Trials Group; The Frontier Science and Technology Research Foundation; The Swiss Group for Clinical Cancer Research; the Swiss Cancer League/Oncosuisse; and the United States National Institutes of Health (CA-75362 to MMR).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Trial Registration
clinicaltrials.gov NCT00022516.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pruneri, G., Gray, K.P., Vingiani, A. et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat 158, 323–331 (2016). https://doi.org/10.1007/s10549-016-3863-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3863-3