Abstract
Sepsis is defined as life-threatening organ dysfunction caused by dysregulated host systemic inflammatory and immune response to infection. Over decades, advanced understanding of host–microorganism interaction has gradually unmasked the genuine nature of sepsis, guiding toward new definition and novel therapeutic approaches. Diverse clinical manifestations and outcomes among infectious patients have suggested the heterogeneity of immunopathology, while systemic inflammatory responses and deteriorating organ function observed in critically ill patients imply the extensively hyperactivated cascades by the host defense system. From focusing on microorganism pathogenicity, research interests have turned toward the molecular basis of host responses. Though progress has been made regarding recognition and management of clinical sepsis, incidence and mortality rate remain high. Furthermore, clinical trials of therapeutics have failed to obtain promising results. As far as we know, there was no systematic review addressing sepsis-related molecular signaling pathways and intervention therapy in literature. Increasing studies have succeeded to confirm novel functions of involved signaling pathways and comment on efficacy of intervention therapies amid sepsis. However, few of these studies attempt to elucidate the underlining mechanism in progression of sepsis, while other failed to integrate preliminary findings and describe in a broader view. This review focuses on the important signaling pathways, potential molecular mechanism, and pathway-associated therapy in sepsis. Host-derived molecules interacting with activated cells possess pivotal role for sepsis pathogenesis by dynamic regulation of signaling pathways. Cross-talk and functions of these molecules are also discussed in detail. Lastly, potential novel therapeutic strategies precisely targeting on signaling pathways and molecules are mentioned.
Similar content being viewed by others
Introduction
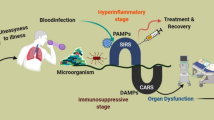
In the past 50 years, sepsis was being defined as the development of host systemic inflammatory response syndrome (SIRS) against microbial infection. The term “SIRS” referred as a clinical syndrome featured with systemic inflammation and extensive tissue injuries. Diagnosis could be confirmed after fulfilling at least two of the following clinical criteria: tachypnea (rapid breathing), tachycardia (rapid heartbeat), pyrexia (fever) or hypothermia, and leukocytosis or leukopenia. With advanced knowledge and initiatives to speed up global awareness of sepsis and to build collaboration between experts groups, Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) jointly initiated the Surviving Sepsis Campaign (SSC) in 2001.1 The global sepsis consensus established in 2004 has speeded up formulation of a standard definition for sepsis (also known as sepsis-2).2 This international task force reviewed and updated the sepsis guideline every 4 years.
Over years, advanced understanding of sepsis pathophysiology has revealed its nature, implicating that sepsis should not be defined simply as syndrome of inflammation. Evidently, the SIRS concept was too broadly applied to critically ill patients and inadequate to meet the clinical heterogeneity and dynamic changes in actual scenario. In the year of 2016, a revised international guideline with modified definition and diagnostic criteria of sepsis and septic shock (sepsis-3) was established, supported with evidenced-based recommendations for improved recognition and appropriate management of sepsis.3 Conceptually, sepsis is now defined as life-threatening organ dysfunction caused by a dysregulated host response against infection, while septic shock is defined as a subset of sepsis patients accompanied with circulatory and cellular/metabolic dysfunction. To better implement this definition, quick assessment tool such as SOFA (sequential organ failure assessment) was introduced to grade sepsis severity and predict in-hospital mortality based on well-defined multi-physiological criteria.
Soon after the pre-existed conceptual definition of adult sepsis, definitions for pediatric sepsis was introduced in 2005.4 In consideration of the distinct physiological features and organ function within each developmental stage, age-adjusted range for main clinical variables were provided in this guideline. In 2017, the American College of Critical Care Medicine proposed a standardized guideline for hemodynamic management of neonatal and pediatric septic shock.5 By reviewing efficacy of various clinical management procedures mentioned in pre-existed studies, guideline recommended implementation of a goal-directed strategy in resource-rich settings and have provided step-wise management tools for practical use. To counteract with the fundamental pathophysiological changes in sepsis, this guideline shed lights on the importance of hemodynamic stabilization and listed supporting procedures for hemodynamics management.
Even though global consensus guideline for sepsis was revised timely, recent version lack discussion regarding management of pediatric sepsis. In response, SCCM and ESICM published an updated statement guideline for management of septic shock and septic organ failure in children.6 Evidence-based recommendations responding to concerns in screening, diagnosis, and treatment of pediatric sepsis were provided to guide “best clinical practice.” Though definition for pediatric sepsis is still pending for renew, concept of sepsis-3 was implemented throughout formulation of these recommendations.
Sepsis remains a burdensome public health problem globally. In a tentative exploration of hospital-treated sepsis data obtained from high-income countries, a global estimate of 31.5 million sepsis cases were reported, with potentially 5.3 million deaths annually.7 Indeed, sepsis were considered to be a huge burden of out-hospital deaths in low-income countries, due to the high prevalence of infectious disease such as malaria, HIV, and dengue fever.8,9 In view of this, a recent study has devoted to provide accurate global sepsis estimates by analyzing both in and out-hospital sepsis-related death among 195 countries. In the year of 2017, an estimated population of 48.9 million were affected by sepsis worldwide. Though mortality rate has decreased by 52.8% from 1990 to 2017, the reported 11.0 million sepsis-related deaths annually still account for 19.7% of the global deaths.10 Moreover, sepsis shows significant higher burden in regions with poorer access to medical care such as sub-Saharan Africa and Southeast Asia, thus explaining discrepancies among epidemiological studies due to study data limited to high- or low-income countries.
In the same report, more than half of sepsis estimates (25.2 million) were aged <20 years, which is substantially higher than previous estimates based on data from high-income countries.11 In the global burden of disease study, infection-related deaths have contributed as the second leading cause for childhood deaths.12 As only types of infection rather than the mutual pathophysiological event “sepsis” were included as causes of death in this study, incidence of sepsis-related deaths might be underestimated. Though incidence of early-onset neonatal sepsis has significantly declined in the past decades following implementation of administrating prophylaxis antibiotics and parental screening in high-income countries,13,14 sepsis remains a leading challenge in pediatric population in less-developed countries.
In a latest global survey of pediatric sepsis, severe sepsis remains a leading cause of death for critically ill patients in pediatric intensive care unit (PICU) within United States, accounting for about one-third of PICU deaths with a mortality rate of 25%.15 In previously healthy pediatric sepsis patients, 75% deaths events occurred within 3 days from sepsis recognition. As one of the most prevalent critical condition in PICU, refractory septic shock was indicated with a mortality rate of 34% and have accounted to one-thirds of early deaths.16 This mortality rate exceeds the prior estimates that relied on retrospective studies. Escalating incidence of sepsis might be attributed to the increased population with chronic critical illness due to improved healthcare services. However, prolonged hospitalization and frequent invasive procedures have increased risk for nosocomial infection, while intensive medications and immunosuppressive state might contribute to multidrug-resistant organism and opportunistic infection, together with the long-term outcomes such as organ failure, immunosuppression, and disabilities, posing an unprecedented great challenge for pediatric management. To cast light on sepsis pathogenesis and novel intervention therapeutic results, we systemically reviewed relevant studies and focused on the molecular signaling pathways and intervention therapies in sepsis.
Signaling pathways in sepsis
Involvement of intricate signaling pathways and dysregulated host response makes sepsis a life-threatening heterogeneous syndrome different from mild infection. The initiating event in sepsis was host recognition of microbial-derived pathogen-associated molecular patterns (PAMPs) or endogenous damage-associated molecular patterns (DAMPs), guaranteed by a series of pattern recognition receptors (PRRs) located at cell membrane or intracellular space. Recognition result in the activation of intracellular signaling pathways. PAMPs and DAMPs range from microbial products, host glycoproteins, lipoproteins, and nucleic acids. In reciprocal, DAMPs and PAMPs bind and interact with Toll-like receptors (TLRs), C-type lectin domain family 7 member A (dectin 1) and C-type lectin domain family 6 member A (dectin 2). Once activated, the subsequent signaling pathways converge toward interferon regulatory factor (IRF) and nuclear factor-κB (NF-κB). IRF is responsible for type I interferon (IFN) production. NF-κB and activator protein 1 (AP-1) signaling are responsible for the early activation of inflammatory genes and those encoding endothelial cell-surface molecules. Immune cells could respond and interact with complexed intracellular signaling system to elicit innate immune responses for elimination of invading pathogens and cell homeostasis, while during sepsis, some of these involved host signaling pathways were abruptly upregulated with robust release of cytokines and alarmins (DAMPs) that serve as potent mediators for excessive systemic inflammation and immune suppression. Modulation and reprogramming of other essential signaling pathways under cellular stress took part to sustain effective innate immune response might also set off detrimental effects.
Cytosolic sensing: STING-IRF3-NF-κB pathway
During sepsis, several types of host plasma cell-free DNA (cfDNA) were significantly elevated. Hepatocyte-derived cfDNA and cardiomyocyte cfDNA were passively released due to tissue damages.17,18 Compared to nuclear DNA (nDNA) derived from neutrophils, elevated levels of mitochondrial DNA (mtDNA) were shown to be associated with septic shock and mortality.19 cfDNA were potent to mediate inflammation and immune responses via interacting with STING (Fig. 1). In mice, the impaired ability to degrade cfDNA due to loss of deoxyribonuclease (DNase) could result in massive inflammation.20 Analogously, administration of DNase could improve survival via cleavage of cfDNA, therefore inhibiting the pro-inflammatory effect mediated by nDNA from neutrophil activation or NETosis.21
Cross-talks of signaling pathway in innate immune cells. At initiation of sepsis, innate immune cells are generally activated at recognition of DAMPs and PAMPs. Membrane-bound and intracellular receptors sense danger signals, which converge with multiple pathways related to activation and regulation of innate immune responses. In general, these converge toward IRF3 and NF-κB signaling pathway that are required to initiate early phase inflammatory responses. Besides, TLR4 agonist (i.e., LPS or HMGB1) provide essential priming signal for the first step of inflammasome activation—upregulation of pro-inflammatory genes. Another substantial group of pathogenic products and endogenous alarmins were required to provide signal 2 for AIM2/NLRP3 inflammasome assembly, which subsequently cleave caspase, GSDMD, and pro-IL-1β/18 to drive canonical inflammasome activation and pyroptosis. Acting as late-phase alarmin, HMGB1 interact with RAGE for delivery of cytosolic LPS, which further trigger pyroptosis via caspase-11-dependent pathway (equivalent to caspase-4 and caspase-5 in human, non-canonical pathway). Intracellularly, cytosolic DNA derived from apoptotic cells or intracellular pathogen could be sensed by AIM2 and cGAS-STING to induce inflammasome assembly and IRF3 phosphorylation, leading to type I interferon responses and inflammasome activation. During sepsis, persisted stress stimuli result in mitochondria dysfunction, ROS production, and metabolism reprogramming that further enhance redox state modification and alarmin production (HMGB1). These cellular changes activate multiple signaling pathways that converge with NLRP3 inflammasome activation. Inflammasome plays a pivotal role in sepsis pathogenesis due to its cross-talk with stress signaling, immune cell activation, and cell homeostasis. Interaction between inflammatory and coagulation cascades serve as underlined mechanism for DIC pathogenesis. As a cytosolic LPS receptor, caspase-11 participated in initiation of coagulation by enhancing tissue factor (TF) activity, performed by GSDMD pore-mediated Ca2+ efflux that trigger PS externalization, while macrophage pyroptosis facilitate release of cellular contents including procoagulant products (TF) and alarmins to sustain systemic coagulation. ROS reactive oxygen species, DAMP damage-associated molecular pattern, PAMP pathogen-associated molecular pattern, cfDNA cell-free DNA, EC endothelial cells, ER endoplasmic reticulum, ASC apoptosis-associated speck-like protein, TRX thioredoxin, TXNIP thioredoxin-interacting protein, HMGB1 high mobility group box protein 1, RAGE receptor for advanced glycation end products, GPX4 glutathione peroxidase 4, PLCG phospholipase C gamma 1, PKM2 pyruvate kinase M2, PKR RNA-activated protein kinase, HDAC1 histone deacetylase 1, GLUT1 glucose transporter 1, HIF1a hypoxia-inducible factor-1, PS phosphatidylserine, GSDMD gasdermin D
It was previously manifested that inappropriate digested apoptotic DNA accumulated in lysosomal compartments were potent to stimulate production of type I IFNs in a STING-dependent manner. This phenomenon could be observed in engulfed apoptotic or necrotic cells and serve as vehicles for delivery of cfDNA to cytosol.22 Other studies propose that extracellular vesicles such as apoptosis-derived membrane vesicles and exosomes participated in delivery of cfDNA to immune cells, which enhances type I IFN through cGAS-STING pathway.23
With established role as an adapter protein during IFN induction in response to cytosolic DNA sensing, the endoplasmic reticulum (ER)-bounded STING also serve as innate immune sensor for bacterial cyclic dinucleotides (CDNs), a group of conserved signaling molecules specifically released by bacteria. CDNs (c-di-GMP or c-di-AMP) gain access into immune cells and directly bind with STING.24 As a consequence, STING forms activated homodimer and was facilitated to form oligomeric state that lead to phosphorylation of downstream TANK-binding kinase 1 (TBK1) dimers, which consequently activate IRF3 and NF-κB signaling pathway.25,26 In lipopolysaccharide (LPS)-induced cardiac dysfunction model, LPS stimulation mediated by TLR4 is observed to induce STING perinuclear translocation, downstream IRF3 phosphorylation, and NLRP3 expression, while knockdown of STING in mice could alleviate sepsis-induced cardiac dysfunction with better survival rate via suppressing myocardial and serum inflammatory cytokines, apoptosis, and pyroptosis.27 STING-mediated cytokines also showed diverse immunoregulatory role via activating Janus-activated kinase (JAK)-signal transducer and activator of transcription factor (STAT) pathway.
Complement system
Activation of complement system marked the prelude of host recognition of hazardous signals and cascades of defensive signaling pathways related to inflammation, coagulation, and bacterial cell lysis. These distinct properties of complements regulate early innate immune responses and are essential for protecting host from uncontrolled dissemination of invasive pathogens. The complement cascade was known to be activated via three separate pathways: classical pathway in response to antigen–antibody complex, alternative pathway by factor B/D, and lectin pathway via recognition of pathogen-derived mannose (by host mannose-binding lectin (MBL)). C3 act as the central component that all three pathways converge. Activating stimuli were classified into two main types: (1) pathogen-derived products (PAMP) such as LPS, mannose, and antigen–antibody complex; (2) endogenous cellular debris (DAMP) resulted from disturbances of cell homeostasis. These pattern signals were detected during initiating phase and assembly to propagate cleavage of complements. Cascade of complement cleavage and assembly result in formation of catalytic C3 convertase (C4b2b). C3 was then cleaved to obtain excess C3b that amplifies complement response. Accumulation of C3 cleavage products covalently bound to pathogen surface and facilitate phagocytosis by neutrophils, an antimicrobial process specifically known as opsonization. Adequate surface density of C3b subsequently lead to functional shift from C3 to C5 convertase. C5 convertase marked the formation of terminal C5b-9 complement complex—also known as membrane attack complex (MAC). Incorporation of MAC into pathogen surfaces create pores in the bacterial cell wall that induce cell lysis and guarantee clearance of specific type of intracellular bacterial (i.e., Neisseria species), while anaphylatoxins C3a and C5a are potent in coordinating with various inflammatory responses via directly binding to reciprocal receptors so to allow recruitment and activation of innate immune cells (neutrophils, monocytes, and macrophages).
Complement-mediated neutrophil activation is not only responsible for various prominent effector events in sepsis but also plays an ambivalent role in innate defense. Complement activation products C3a, C5a, and C5b interact with cell membrane receptors to induce antimicrobial response and pro-inflammatory effect via cross-talk with multiple signaling pathways. Extracellular signal-regulated kinase (ERK1/2) and p38-mitogen-activated protein kinase (MAPK) might be involved in the generation of interleukin (IL)-6 in neutrophils,28 while phosphatidylinositide 3-kinase (PI3K) control C5a-mediated response by regulating oxidative burst of neutrophils and macrophages as well as phagocytosis activity in neutrophils.29 C5a also facilitate phosphorylation and translocation of dormant intracellular enzyme (p47phox) so to induce activation of NADPH oxidase complex and generate oxidative burst for pathogen killing.30 However, sustained neutrophil activation might exhibit excessive response that are pathogenic, resulting in increased risks of disseminated infection and tissue damages.
As complement activation proceed, sustained generation of C5a might lead to paralysis of innate immune, resulting in dampened inflammatory and bacterial-killing effect.30 Such suppressed responses were mainly observed in neutrophils. Apparently, reduced tumor necrosis factor (TNF) secretion by neutrophils could be resulted from inhibition of NF-κB transcription due to increased levels of C5a-mediated IκBα.31 Besides, high levels of C5a might result in less targeted migration property of neutrophils.32 Disruption of C3a–C3aR axis by C5a promote egress of premature granulocytes and hematopoietic stem cells from bone marrow, presented with less targeted but more progressive inflammatory responses.33 C5a signal could simultaneously downregulate CXCR4 on granulocytes and facilitate release of protease that causes matrix protein degradation and inhibition of homing effect of stroma cell-derived factor 1, resulting in altered neutrophil phenotype.34
The direct activation of complement components by certain coagulation protease were known as “extrinsic protease pathway.”35 Thrombin potentially generate active C5a in the absence of C3 and might substitute as an independent novel complement pathway,36 while plasmin, kallikrein, and Hageman factor fragment were potent C3 and C5 convertase in in vitro studies and these biological fragments were related with increased chemotactic activity and cell proliferation.37,38,39 Aside from protease, pentraxins (CRP, SAP, PTX3) released in response to infection were potent in initiating classical pathway via interaction with C1q. Such mechanism was implicated to remove cellular debris during infection or tissue injuries.40
Stress signaling mediated via reactive oxygen species (ROS)/NLRP3 inflammasome activation
Stress signaling is a highly conserved mechanism essential for exhibiting host defense response. Capable in sensing harmful signals (whole pathogen, PAMPs, environmental irritants) and damage-associated alarmins (extracellular ATP), stress signaling engage with pathways associated with appropriate cellular repairing and usually followed by a general scheme—stress stimuli detected by sensor proteins, oligomerization of sensor that allowed subsequent recruitment of effector protein, and finally activation of effector proteins for repairing responses.41 NLRP3 inflammasome assembly is a well-known sensing model that function to modulate such protective innate immune response during sepsis.42 In reviewing underlying signaling pathways and mechanisms in massive candidates of NLRP3 activators, ROS is noted as a group of crucial intermediators for engaging stress sensors and effectors due to its ability in integrating multiple signaling pathways associated with NLRP3 inflammasomes and innate immune responses.43
Injury-released extracellular ATP trigger generation of short-lived ROS via a pore-forming channel mechanism coupled with P2X7 receptor activation (Fig. 1), while large particulates (silica, alum) produce ROS depending on lysosome rupture process amid phagocytosis (Fig. 1).44,45 When confronting cellular stress, TLR-mediated mitochondria ROS and NADPH oxidase generation upon phagocytosis are recognized as essential source of cellular ROS for inflammasome activation.44,46 During ROS-dependent inflammasome activation, elevated intracellular ROS allow dissociation of the NLRP-ligand (thioredoxin-interacting protein (TXNIP)) from the ROS-sensitive TXNIP–thioredoxin complex, which specifically bind with the leucine-rich repeat domain of NLRP3 to trigger inflammasome activation. In the channel model of NLRP3 inflammasome activation, the rapid K+ efflux facilitated by activation of P2X7 ATP-gated ion channel not only serve as requirement crucial for inflammasome activation but also produce low levels of intracellular ROS,47 suggesting an inflammasome–dependent positive feedback for sustained ROS production.45 These findings proposed potential mechanisms engaging cellular stress recognition and ROS production for subsequent NLRP3 inflammasome activation.48
Inflammasome and pyroptosis
Pyroptosis, a form of programmed necrosis associated with release of cellular contents and pro-inflammatory cytokines, have essential roles in mediating protective innate immune response to combat invading pathogens and microbial infections.49 Hallmark events include (1) inflammasome priming, (2) NLRP3 inflammasome assembly and activation, (3) cleavage of gasdermin D (GSDMD) and pore formation as well as (4) pro-inflammatory molecules secretion (Fig. 1). Ample evidences detected pyroptosis activities and elevated IL-18/IL-1β levels in neutrophils observed in LPS- and cecal ligation and puncture (CLP)-induced sepsis models.50 Serum PCR array performed on clinical sepsis patients also proved the presence of an altered inflammasome-related gene profile, featured with a greater magnitude of altered genes and higher intensity of gene expression disturbance compared to normal controls. Expression levels for genes such as NLRP3, NLRC4, TLR5, NOD, IL-1β, and IL-18 showed intricate interconnection that could eventually lead to a robust inflammasome gene profile, while in sepsis non-survivors, a higher magnitude of the same gene modulation pattern were observed, revealing its clinical relevance with sepsis severity.51
The NLRP3 inflammasome activation event required at least two activating signals for triggering distinct processes (Fig. 1).52 Initially, pathogenic-derived molecules recognized by pattern-recognition receptors (PRRs) triggered nuclear translocation of NF-κB and transcription of pro-IL-1β and pro-IL-18. Studies using synthetic LPS and TLR4 activators were potent to induce apoptosis-associated speck-like protein (ASC) pyroptosome formation, illustrating their indispensable role for NLRP3 priming.53 Under LPS stimulation, a strong NF-κB-dependent NLRP3 mRNA expression was observed in macrophages in wild-type mouse, while a dose-dependent reduction of NLRP3 protein was observed in cells lacking TLR4 or double deficient in myeloid differentiation factor 88 (MyD88) and TRIF. Together with the generally low expression of NLRP3 levels observed in inactive immune cell lines,54 NF-κB signaling is suggested as the crucial priming event that guarantee inflammasome to respond accurately to stimuli and prevent inappropriate NLRP3 activation.53 However, recent study showed that mitochondrial ROS induce priming by directly leading to deubiquitination of NLRP3 inflammasome but independent of protein synthesis, suggesting a non-transcription priming mechanism for NLRP3 deubiquitination.55
Yet, priming signals provided by NF-κB activators were insufficient for inflammasome activation. In the absence of established NLRP3 activators, enhanced NLRP3 expression were inadequate for caspase-1 activation, revealing the requirement of a crucial inducing agent for NLRP3 inflammasome assembly.53 Experimentally, NLRP3 inflammasome activation was mediated by a diverse group of endogenous or exogenous agonists including DAMPS, extracellular ATP, pore-forming toxins (nigericin), or biochemical crystals (silica and alum).56,57,58 For instance, apoptosis inhibitor of macrophage 2 (AIM2) specifically recognizes cytosolic dsDNA and initiates inflammasome activation (Fig. 2).59 Extracellular ATP found in transfected models activates P2X7 receptor and NLRP3 signaling in an autocrine manner (Fig. 1),58 while significant switch of metabolism status (i.e., elevated saturated fatty acids, absence of ketogenesis) during sepsis converge with inflammasome signaling (Fig. 1) and was associated with increased lethality.59 Mechanically, these secondary signal activators function to increase membrane permeability for potassium efflux and subsequent downstream binding of NEK7 with NLRP3, therefore regulating NLRP3 oligomerization, inflammasome assembly, and subsequent catalytic cleavage of pro-caspases (Fig. 1).60,61
Stress signaling and cell homeostasis. Intracellularly, oxidative stress-mediated redox state is pivotal to maintain host cell survival and homeostasis via cross-talks with inflammasome, cell death pathways, and stress-responsive proteins. Mitochondria-derived ROS and mtDNA provide stimuli for upregulation of JAK-STAT pathway and inhibition of HDAC1, which is required for hyperacetylation and cytosolic translocation of HMBG1. In the presence of pathogenic stimuli, substantial release of all thiol-reduced HMGB1 by exosomes serve as inflammatory mediators that marked the prelude of sepsis. This redox form interacts with AIM2 and dsDNA to initiate inflammasome activation and caspase-1-mediated responses, which serve as pre-requisite for autophagy/mitophagy induction via beclin1-mediated pathways. Besides, prolonged oxidative stress oxidize HMGB1 into the disulfide form, which is potent to displace Bcl2 from its association with beclin1, allowing autophagy initiation and removal of hazardous oxidative stress stimulus. Interaction of beclin-1 with PINK/Parkin further facilitate mitochondria priming and autophagosome formation, which subsequently interact with lysosome to induce mitophagy. Autophagy and mitophagy are essential mechanisms to regulate innate immune response and prevent stress-induced injuries. During systemic inflammation context (sepsis), failure to initiate protective autophagy/mitophagy might result in profound cell stress signaling and deleterious pro-inflammatory cell deaths. HDAC1 histone deacetylase 1, Bcl-2 B cell lymphoma-2, GSDMD gasdermin D, SESN2 sestrin 2, PINK PTEN-induced putative kinase 1, LC3 microtubule-associated protein 1A/1B-light chain 3
Some bacterial-specific toxin also interacts with host cells to exhibit NLRP3 activating properties. During Staphylococcus aureus infection, massive potassium efflux induced by potassium channel-forming toxins such as α-toxin was potent in activating inflammasome.62 Besides, mitochondria dysfunction during apoptosis or programmed cell death might result in significant reduction in negative potential within mitochondria, thus triggering release of oxidized mtDNA and cardiolipin to cytosol. Both molecules bound and induce NLRP3 inflammasome activation licensed with IL-1β production (Fig. 1).63,64
Recently, a TLR4-independent mechanism for inflammasome activation was found in intracellular Gram-negative bacterial infection. Intracellular LPS transfected by Escherichia coli,65 Salmonella typhimurium,66 and Legionella pneumophila67 were sensed and respond via a caspase-11 non-canonical inflammasome mechanism. Intracellular LPS trigger pyroptosis by directly binding to the recruitment domains (CARD) of pro-caspase-11, which subsequently underwent recruitment, oligomerization, and activation of pro-caspase-11 (Fig. 1).68 Caspase-11-dependent pyroptosis is a critical event for endotoxin shock in mice, representing an innate sensing mechanism. Analogously, similar pyroptotic cell death mediated by homologous caspase-4/5 were observed in human monocytic cell lines.69 To promote cytokine production, activated caspase-11 require NLRP3/ASC complex formation in a cell-intrinsic manner, independent of danger signals or pro-inflammatory cytokines released by adjacent cells. In this process, caspase-11 is rather upstream of NLRP3 activation and the caspase-11-mediated potassium efflux was a necessary intrinsic event for triggering non-canonical NLRP3 inflammasome activation.60,70 Noteworthy, the LPS-activated caspase-11 catalytically cleave GSDMD to release the intramolecular inhibition on the gasdermin-N domain but have no effect on pro-IL-1β and pro-IL-18 activation.71
Cleavage of GSDMD by active caspase-1/4/5/11 release functional active gasdermin-N domains that contain liposome-leakage-inducing and intrinsic pore-forming properties, subsequently forming membrane pores that facilitate active release of inflammatory cytokines (IL-18 and IL-1β) and intracellular components.72,73 Pore-forming properties of the gasdermin family contributed to the distinctive molecular and structural mechanism in pyroptosis. Unlike the explosive-like membrane blebbing and cell swelling that resulted from MLKL selective diffusion of ions during necroptosis, GSDMD-N pore facilitate non-selective ion diffusion without increasing osmolality.74
Mitochondria dysfunction, inflammasome activation, and cell death
It was proven that engagement of specific NLRP3 inflammasome activators could amplify mitochondria destabilization, leading to pyroptotic cell death with enhanced loss of plasma membrane integrity and release of intracellular proteins (DAMP) via a caspase-1-dependent mechanism,75 while excessive ROS resulting from impaired electron transport chain, Ca2+ overload, or attenuation of endogenous anti-oxidants also triggered cell death modalities, such as apoptosis and autophagy.76,77 During mitochondria damage, the highly expressed mtROS promote apoptosis via triggering opening of mitochondrial permeability transition pores (mPTPs),78 while in an animal model of acute peritonitis, inhibition of mPTPs was sufficient to reduce sepsis-induced myocardial dysfunction.79
Induction of these ROS-mediated events partially relied on inflammasome activities, then interplay with cellular processes that would exacerbate to cell death. Physiological events leading to calcium influx not only provide calcium signaling required for regulation of NLRP3 inflammasome assembly but also initiate robust inflammasome activation by inducing the intermediate step—mitochondria destabilization.80 Therefore, calcium mobilization that lead to Ca2+ overload in mitochondria damage play a vital role in NLRP3 inflammasome activation.81 In the process of apoptosis, free oxidized mtDNA and cardiolipin released to cytosol also serves as a potent ligand for NLRP3 inflammasome activation (Fig. 1).63 During sepsis, activated caspase-1 interact with molecular events in precipitating mitochondria dysfunction, such as mitochondria ROS production, perturbation of membrane permeability, and fragmentation of mitochondria network, thus exacerbating apoptosis featured with pro-inflammatory responses.75 While demonstrating the role of a serum pro-inflammatory factor (S100A12) on NLRP3 inflammasome activation, the resulting apoptotic events orchestrated by elevated intracellular oxidative stress was suggested for pathogenesis of sepsis-induced acute respiratory distress syndrome.82 Likewise, anti-apoptotic proteins were mentioned to be effective in attenuating NLRP3 inflammasome responses.42
The types of mitochondria dysfunction might have consequences on the inflammatory nature of the ongoing cell death, regulated by formulation of apoptosome or inflammasome in a context-dependent manner.83 Cleaved caspase-1 by NLRP3 inflammasome could engage with multiple pathways in parallel to trigger mitochondrial damage that result in either pyroptosis or apoptosis. Mechanically, caspase-1 inactivates mitophagy process via catalytic cleavage of the key mitophagy regulator Parkin, thus allowing accumulation of dysfunctional mitochondria that augment pyroptosis.75 This additional caspase-dependent event corresponds to the inactivation of pro-survival and homeostatic pathways previously observed during apoptosis.
Necroptosis
Different from the caspase-dependent type apoptosis, molecularly controlled forms of necrosis play predominant role in pathogenesis of cell death in sepsis. With defined signaling transduction pathways, necroptosis showed additional physiological significance and perform distinctly from typical necrosis. Via interacting with death receptors and various cytoplasmic protein kinases, necroptosis also drives inflammation and immunogenic events for various diseases.84,85 Unlike necrosis that often lead to irreversible pathological damages, necroptosis has been utilized by organism to obtain appropriate levels of cellular activity and embryonic development.86
Necroptosis was first notified as a TNF-induced type of necrosis and later confirmed as kinase-dependent process after the successful inhibition by RIPK1-inhibitor (necrostatin).87 Unlike caspase-8-regulated apoptosis, necroptosis was specifically regulated by signal transduction proteins known as RIPK1 and RIPK3, both of which function as complex of membrane-associated proteins.88,89 Ligation of stimuli induce interaction between coupled proteins and kinases that trigger formation of necroptosis-initiating cytosolic complexes, so as to respond with relevant cell death/survival outcome via inducing signal transduction. Aside from the established TNF/TNFR1-induced necroptosis, stimuli such as immune death signals (FASL/TRAIL),90 bacterial and cellular stress signals (LPS/TLR4, poly(I:C)/TLR3)91,92 as well as type-I/II IFNs (viral RNA/PKR or autocrine loop for sustained RIPK3 activation)93,94 were capable of inducing necrosome formation via stimulation of death receptors. TLRs (TLR3, TLR4) that signals through a RHIM-domain-containing protein (TRIF, DAI) could allow interaction of RIPK1 and RIPK3 and lead to necroptosis.95,96 Besides, recruitment of RIPK1 to Fas in the absence of inhibitor of apoptosis (IAP) trigger transformation of cytosolic complex I to complex II, which lead to assembly of necrosome and necroptosis.97 Conversely, activation of cytosolic complex I was known to trigger pro-inflammatory signaling and promote cell survival by NF-κB and MAPK activation.98
Though mechanisms determining apoptosis, necroptosis, or cell survival remains unclear, genetic studies revealed that FADD or caspase-8 deficiency could sensitize cells to undergo RIPK3-mediated necroptosis, thus triggering embryonic lethality and inflammation.99,100 Similarly, sufficient levels of catalytically active RIPK3 is required to induce necroptosis and suppress apoptosis.101 In clinical sepsis, RIPK3 levels were significantly elevated at various time points with positive correlation to SOFA score. Regarding its relationship with clinical outcome, lower levels of TRAIL, a potent inducer of apoptosis, is associated with increased levels of RIPK3, while RIPK3 levels is positively associated with higher incidence of organ dysfunction and septic shock, suggesting the pathological impacts of RIPK3-mediated pathways.102 In analog, in vivo sepsis studies have also proven the participation of RIPK3 necroptosis during organ deterioration.103
Unlike the irrelevant physiological roles between apoptosis and necroptosis, necroptosis and pyroptosis collaborate to amplify inflammatory signals and aggravate tissue injuries, both playing pivotal roles in the progress of sepsis. Though both processes were activated by a similar pool of stimuli signals, intracellular signals were transduced via distinct signaling pathways and target to their respective death regulator proteins—RIPK3 or GSDMD. In the effective phase, release of intracellular component varied between the type of programmed cell death—DAMPs such as high mobility group box protein 1 (HMGB1) released during necroptosis while pro-inflammatory cytokines IL-1β and IL-18 during pyroptosis. In view of the potential synergistic impact on sepsis-induced injuries, one study aimed to investigate the protective effect on double deletion of RIPK3 and GSDMD. Double blockage of necroptosis and pyroptosis by deletion of RIPK3/GSDMD or MLKL/GSDMD showed accumulated protective effects against septic shock, systemic blood clotting, and multi-organ injuries. Both RIPK3 and GSDMD perform lytic cell death that collaborate to amplify necro-inflammation and tissue factor (TF) release in macrophages and endothelial cells, resulting in massive tissue injuries.104
In neonatal polymicrobial sepsis mice, systemic and pulmonary inflammation were ameliorated with improved survival after injection of RIPK1 inhibitor (necrostatin-1). Decreased expression of local IL-6, IL-1β, and IL-18 as well as neutrophil chemoattractant mRNA were also observed,105 while deletion of RIPK3 confer complete protection against SIRS, with reduced amount of circulating DAMPs and cytokines.106 Similar protective effects were confirmed in CLP-induced sepsis models without negative effect on apoptosis or NK-kB activation, thus indicating the therapeutic potential by targeting necroptosis process.107 Having noted that evidence of complex II formation for necroptosis in tissue samples is hard to demonstrate, the above results had attempted to prove the involvement of necroptosis in sepsis-induced lung injury and presented the protective effect of RIPK inhibition on sepsis.
Recently demonstrated in in vivo study using LPS-challenged human kidney tubular epithelial cells, enhanced RIPK3 expression subsequently promote oxidative stress and mitochondrial dysfunction via upregulation of NADPH oxidase-4 and downregulation of mitochondrial complex I and III. These activated components are important evidences associated with sepsis-induced acute kidney injury (AKI). In this study, RIPK3-mediated responses work independently with RIPK1 and MLKL and were untypical to the necroptosis process. Together with observation of mitochondrial depolarization in in vitro study, these findings have shed insights into RIPK3’s unique role in regulating mitochondrial function during sepsis-induced AKI.108
Autophagy and mitophagy
Autophagy and mitophagy are conserved processes essential for cell homeostasis and initiated under various stress conditions. Both function to facilitate intracellular degradation of dysfunctional mitochondria, damaged cytosolic organelles, and invading microorganisms, therefore mitigating the extent and severity of cellular-induced inflammatory responses.109 Autophagy might have alleviated inflammasome activity via multiple aspects, such as elimination of damaged mitochondria, removal of cytoplasmic HMGB1, and activated inflammasomes.110 Involvement of caspase-1-dependent activities in mitochondria damages have already been discussed in the previous section. Notably, ROS production by mitochondria subsequently oxidized HMGB1 released from apoptotic cells, resulting in neutralization of immunogenic activity and fail to activate innate immune cells (Fig. 2). Though reduction of oxidized HMGB1 by thioredoxin is sufficient to maintain the reduced form, such reaction is low in efficiency during sepsis.111
In contrary to the pathological role of serum HMGB1 in amplifying systemic inflammation, intracellular HMGB1 shows cross-talk with cell homeostasis and exert protective effect under specific circumstances. As for hepatocytes and macrophages, cytosolic HMGB1 is capable to prevent deleterious cell death from endotoxemia by mediating autophagy and mitophagy (Fig. 2).112 Stress stimuli that enhance ROS could promote nucleocytoplasmic shuttling of HMGB1, where it directly interacts with the autophagy protein beclin-1 by displacing Bcl-2, thus resulting in formation of autophagy initiation complexes and removal of hazardous oxidative stress stimulus (Fig. 2).113 The various redox states of intracellular HMGB1 have contributed to its important regulatory role for AIM inflammasome activation. All thiol reduced form of intracellular HMGB1 showed highest affinity when binding with AIM2, which subsequently initiate inflammasome signaling during redox stress (Fig. 2). Initiation of inflammasome pathway serves as an important prerequisite for stimulating protective autophagy and mitophagy for cell survival.114
The disulfide bridge formed between HMGB1 cysteines and beclin-1 is an essential conformation structure required for sustained autophagy.115 Furthermore, HMGB1 controls the checkpoint process that proceed to autophagy, via preventing the calpain-mediated cleavage of autophagic regulator beclin-1 and ATG5 during inflammation.116,117 Though autophagy level was downregulated proportionally according to severity of sepsis condition, injection of cell-permeable TAT-beclin-1 successfully restore mitochondrial biogenesis and preserve sepsis cardiac function via PINK1/Parkin and AMPK/ULK1 signaling. Initially, PINK1 protein on outer mitochondria membrane recruit and activate Parkin that builds ubiquitin chains on damaged mitochondria, facilitating its binding to LC3 on the autophagosome to induce mitophagy (Fig. 2). By interacting with Parkin, Beclin-1 is potent to support PINK1/Parkin-mediated mitophagy via localization of mitochondria-associated membrane for directing ER–mitochondria tethering and inducing the formation of autophagosome precursors for mitophagy.118,119
As an immunomodulatory molecule, endogenous nitric oxide (NO) have been identified as a negative regulator for NLRP3 inflammasome activation via stabilization of mitochondria in macrophages.120 In response to stress-mediated cellular dysfunction, NO is potent to balance inflammasome responses and promote survival via inducing autophagy. Through elimination of mtROS and mtDNA, the ROS-mediated autophagy helps suppress NLRP inflammasome hyperactivation and maintain stability of mitochondrial function.110,121 In recent study, a novel negative regulator for macrophage pyroptosis during sepsis was identified. As an anti-oxidant enzyme responsible for repairing oxidative lipid damage, glutathione peroxidase 4 (GPX4) catalyzes reduction process of phospholipid hydroperoxide to inhibit lipid peroxidation. Besides, GPX4 exhibit coordinated role for oxidative stress, inflammasome activation, and pyroptotic cell death. During sepsis, GPX4 was proposed to inhibit phospholipase C gamma 1-mediated GSDMD activity and caspase-dependent events, therefore reducing excessive macrophage pyroptosis.122
However, excessive NO production due to TXNIP deficiency increase sensitivity to lethal endotoxic shock. After LPS treatment, TXNIP expression was observed to decrease dramatically and then gradually restored, which coincidentally accompany with a significant increase of inducible NO synthase (iNOS) expression. With multiple biological functions on oxidative stress, cell proliferation, and inflammation, these might have contributed to the distinct susceptibility phenotype observed in TXNIP-deficient status. Previously noted, TXNIP exhibit a protective negative regulatory role on NO production, induction of iNOS expression as well as on NF-κB activation, while in TXNIP-deficient mice, increased sepsis susceptibility proceed despite reduced IL-1β processing by S-nitrosylation of NLRP3. These results emphasize the involvement of other crucial roles of TXNIP on inflammatory response. Finally, it was suggested that TXNIP participated with the regulation of NO production via the NF-κB/iNOS pathway.123
However, inhibition or upregulation of autophagy process depends to the context-specific activated pathways and confronted stress. In models of severe sepsis, autophagy is downregulated and insufficient to counteract the NLRP3-induced negative outcomes due to exhaustion of autophagic proteins (Fig. 2).118 In endotoxemia models with severe abdominal infection, inefficient fusion of autophagosomes with lysosomes result in impaired autophagy (Fig. 2). Inefficient clearance of autophagic vacuoles and bacterial products remain source of stress stimuli, leading to hyper-inflammatory response via induction of cGAS-STING pathway.124 Besides, the stress-inducible proteins SESNs (sestrins) suppress prolonged NLRP3 inflammasome activation via inducing mitophagy by a two-phase cooperative mechanism (Fig. 2). First, SESNs facilitate priming of damaged mitochondria by inducing aggregation of SQSTM1 to the Lys 63-ubiquitinated (U) mitochondria. Coupled with maintained levels of ULK1, a specific autophagic machinery was finally triggered for degradation of primed mitochondria.125 This SESN-mediated mitophagy provides a previously unknown mechanism aside from regulating antioxidant expression and lowering ROS levels.126,127
Warburg effect and metabolic reprogramming
Pyruvate kinase M2 (PKM2), a kinase that interact with hypoxia-inducible factor 1α (HIF-1α), was capable to mediate HMGB1 release via exerting inhibitory signal for histone deacetylases (HDACs), providing a novel mechanism for metabolic control on inflammation.128 Previously, LPS was shown potent in elevating the transcriptional regulator HIF-1α, via a TLR4-depedent fashion. With evidences of massive inflammatory cytokines (TNF-α, IL-1, IL-4, IL-6, and IL-12) associated with HIF-1α expression, HIF-1α was proven as a critical determinator for sepsis phenotype.129 Similar signs of altered metabolism were later observed in activated innate immune cells (dendrite cells and macrophages).130,131 During sepsis, presence of hypoxia, inflammatory, or infectious signals prevented HIF-1α from degradation. Accumulated levels of HIF-1α specifically interact with PKM2 to promote targeted gene expression (aerobic glycolysis-related genes) in a positive feedback so as to mimic Warburg effect (Fig. 1).132,133 Warburg effect was first observed in cancer cells,134 characterized with upregulated levels of glycolysis and lactate products even under normoxic conditions. Such effect is essential to provide biosynthetic requirements conducive for cell proliferation rather than efficient ATP production.132,135 During aerobic glycolysis, excessive production of PKM2-mediated lactates inhibit HDAC activity and result in elevated acetylated levels of HMGB1 comparable to that stimulated by LPS and HDAC inhibitors.128 Acetylated HMGB1 was then translocated to the cytosol and subsequently released into extracellular space. This reprogrammed mechanism parallel with previous studies that have also demonstrated the pivotal role of HDAC in regulating mobilization of acetylated HMGB1 during liver ischemia and reperfusion (I/R) injury.136
More importantly, PKM2-dependent glycolysis promote NLRP and AIM inflammasome activation via PKR autophosphorylation (Fig. 1).137 PKR (also termed as EIF2AK2), a double-stranded RNA-dependent protein kinase, was previously known as an intracellular viral RNA sensor and recently proven to be primed by endogenous cellular and metabolic stress signals. Lactate-induced PKR phosphorylation and activation promote release of IL-1β, IL-18, and HMGB1 in macrophages, indicating the regulatory role of PKM2-PKR complex in inflammasome activation (Fig. 2). Via reducing PKR phosphorylation and caspase-1 activity, both PKM2 inhibitors (shikonin) and PKR inhibitors (C16) were capable to protect mice from lethal sepsis. Autophosphorylated PKR could physically interact with specific inflammasome components (NLRP3, NLRP1, NLRC4, AIM2) and subsequently trigger corresponding events of inflammasome activation. Therefore, PKR was indicated as an integral component between innate immune responses and stress stimuli, while inhibitors of PKR were suggested as novel therapeutic targets to counteract inflammation.138 In contrast, other studies suggested that PKR is not required for inflammasome activation and might even inhibit inflammasome activity to avoid initial priming during innate immune response.139,140
Pink1-Park2 pathway: mitophagy and immunomodulatory role
Participation of Pink1-Park2 pathway in maintenance of mitochondrial quality control via mitophagy has been widely studied,141 while a novel Pink1-Park2 protective neuro-immune pathway during sepsis was recently mentioned. In genetic depletion of genes encoding for Pink and Park2, a subsequent decrease in neurotransmitter dopamine was accompanied with increase of late sepsis mediator—HMGB1, via mechanism of HIF-1α-dependent anaerobic glycolysis and NLRP3 inflammasome activation. This finding has proposed the involvement of Pink1 and Park2 neuro-immune pathway in regulating dopamine release and HMGB1 secretion, which exacerbate sepsis severity via activating NLRP3 inflammasome.142
In consistent with previous observation of PKM2-HIF-1α metabolic pathway that promote AIM and NLRP3 inflammasome activation, the HIF-1α-mediated anaerobic glycolysis upregulated after depletion of neurotransmitter-mediated immune responses showed association with sepsis lethality. Together with the pro-inflammatory role of PKM2-HIF-1α on LPS-activated macrophages,133 HIF-1α-mediated immune-metabolic dysfunction was emphasized as a mechanism for lethal sepsis. Indeed, recent studies shed lights on the protective role of Pink1-parkin pathway by mitophagy in AKI.143,144 Featured with accelerated elimination of damaged mitochondria, Pink1- and Park2-mediated mitophagy prevented cell apoptosis and tissue damages through reducing mitochondrial ROS and subsequent NLRP3 inflammasome activation. These findings further support the regulatory role of a novel Pink1-Park2 pathway on immune-metabolism during sepsis, providing rationale basis for cross-interaction of host defense system with intrinsic cellular responses.
Coagulation cascades
Disseminated intravascular coagulation (DIC) is a life-threatening syndrome with excessive activation of intravenous coagulation cascades, exhaustion of anti-coagulants, and suppressed fibrinolysis. In sepsis, approximately 35% of the severe cases showed complication of DIC.145 Incidence of DIC was significantly increased in late phase sepsis correlated with irreversible septic shock and organ dysfunction.146 Mortality rate in septic DIC patients is almost twofolds higher compared to those without DIC.147 Systemic activation of the coagulation and inflammation cascades have central role in the pathogenesis of DIC. During initial phase, extensive expression of inflammatory cytokine-mediated TF was known as the principal mechanism of DIC (Fig. 3). In both endotoxemia and sepsis models, coagulation largely depends on the levels of TF expressed on macrophages and monocytes,148,149 while damaged endothelial cells and neutrophils might provide alternative sources of TF.150
Endothelial barrier and coagulation cascades. Lined by membrane-binding proteoglycans and glycosaminoglycan side chains, dearrangement of endothelial glycocalyx result in loss of anti-thrombogenicity and exposure of adhesion molecules, which allow leukocyte adhesion, platelet recruitment, and thrombus formation. Increased vascular permeability trigger leukocyte extravasation, plasma protein leakage, and tissue edema. Under immunogenic stimuli, procoagulant tissue factors were substantially expressed in the form of microvesicles by macrophages and neutrophils, which triggered extrinsic coagulation cascade and cleavage of prothrombin. Neutrophils and platelets work reciprocally to regulate innate immune response and bacterial clearance via NETosis and platelet-mediated responses. During sepsis, NET-derived histones HMGB1 and cfDNA abundantly found serve as procoagulants that accelerate thrombus formation, while activated platelets and RBC recruitment result in thrombocytopenia and platelet-rich thrombus. In order to monitor coagulation dynamics, thrombin interacts with endothelial-bound TM to exhibit profound anti-coagulant and anti-inflammatory effects. In the presence of APC-EPCR, TM/thrombin interact with coagulation–fibrolysis system to act as negative feedback loop. APC–EPCR interaction also allow switching of PAR-1 signaling to anti-inflammatory pattern and strengthened endothelial integrity via activities mediated by S1P and Ang/Tie axis. Besides, TM/thrombin directly inactivate procoagulant alarmins to further restrict immunocoagulation. During severe infection, dysregulation of Ang/Tie axis were associated with weakened vascular stability, which include inhibition of junctional protein, destabilization of cortical actin, and increased vascular adhesion and permeability. Finally, extensive formation of inflammatory thrombus and activation of coagulation cascades result in microcirculatory dysfunction and organ injury. TM thrombomodulin, PAR-1 protease activated receptor 1, EPCR endothelial protein C receptor, S1P sphingosine-1 phosphate, TFPI tissue factor pathway inhibitor, AT antithrombin, TAFI thrombin-activatable fibrinolysis inhibitor, Rac RAS-related C3 botulinum toxin substrate, RhoA ras homolog family member A, Ang1 angiopoietin 1, Ang2 angiopoietin 2, VEGF vascular endothelial growth factor, VE-PTP vascular endothelial protein tyrosine phosphatase, WPB Weibel–Palade body, MLCK myosin light chain kinase, ROCK Rho-associated kinase, ADAM disintegrin and metalloproteinase domain-containing protein
Insufficient balance of TF-dependent coagulation events by tissue factor pathway inhibitor (TFPI) in early phase supported the impaired physiological function of anti-coagulants recognized during sepsis.151 Systemic inflammation amplified coagulation cascade via inflammasome mechanism and synergistic effect with other innate immune components. Besides, bacterial-derived products and DAMPs (NETs, HMGB1, cfDNAs, and histones) participated and accelerated development of DIC via multiple aspects (Fig. 3).152,153,154 Minimal levels of TF derived from perivascular cells due to increased vascular permeability have also contributed to the coagulopathy.155,156 Exposure of TF attract interaction with the FVII and FX that activate both coagulation cascades,157 which gradually generate prothrombinase complex that covert prothrombin to thrombin and induce fibrin formation for platelet activation. Conversely, thrombin and other coagulation factors exhibit arrays of pro-inflammatory activities via cleavage of protease-activated receptors (PARs), forming a positive feedback loop that augments inflammation and coagulation (Fig. 3).
Endothelial barrier dysfunction
Endothelial dysfunction is one of the hallmark features of sepsis. Stability in endothelial cell contacts allows systemic blood flow, supply of essential biological molecules, and effective immune defense to take place. Integrity of endothelial barrier maintained by intracellular junction molecules regulates anti-coagulant and anti-inflammatory properties of endothelial lining. In response to pathogen-derived molecules and cell stress signals, secretion of cytokines and chemokines recruit neutrophils to localized infection sites for phagocytosis and oxidative killing to take place, while upregulation of endothelial adhesion molecules and loosening of vascular tight junction favor cell migration to targeted tissues. However, increased endothelial permeability further enhance microvascular leakage that trigger vascular hypotension, tissue edema, organ failure, and shock (Fig. 3).
Endothelial cell barrier destabilization was activated via alteration in intracellular junction molecule expression and dynamics of cytoskeleton contraction. Such functional changes were exclusively regulated by endothelial-specific receptors known as Tie receptors (including Tie1 and Tie2). Signaling was diversely mediated by two secreted Angiopoietin (Ang) family proteins—Ang1 as agonist while Ang2 as context-dependent agonist/antagonist.158 Investigation of circulating biomarkers reveal a correlation between plasma Ang2 levels and severity of acute respiratory distress syndrome (ARDS), while in sepsis studies, Ang2 serves as a biomarker for sepsis severity and is related to sepsis progression.159,160
Under stable vasculature, Angs (Ang1 and Ang2) interact with Tie receptors and enhance formation of Tie1/Tie2 heterodimer, which result in Tie2 trafficking to cell–cell junctions (Fig. 3).161 Tie2 then trigger cortical actin formation and upregulate pathways related to anti-adhesion and anti-inflammatory properties of endothelial cells, therefore maintaining vascular stability. The Ang/Tie2 pathway poses broad impacts on vascular remodeling, inflammation, and cell survival of endothelial cells. Ang/Tie2 activation induce the expression of KLF2 via PI3K/Akt pathway and counteract with the vascular endothelial growth factor (VEGF)-mediated vascular permeability, while elevation of intracellular NO by endothelial NOS (eNOS) expression is potent to inhibit exocytosis of Ang2 from Weibel–Palade bodies (WPB) found in endothelial cell cytoplasm (Fig. 3).162,163
Essential cells and signaling molecules
High mobility group box protein 1
As an evolutionarily conserved DNA-binding protein presenting with high electrophoretic mobility, HMGB1 was abundantly found in the nucleus and initially known to play essential roles in maintaining genome homeostasis and cell survival.164,165 During sterile injuries and infections, extracellular HMGB1 is able to alert innate immune response and regulate inflammation by acting as an endogenous prototypical DAMP.166 Later, HMGB1 is proven as a late-phase inflammatory mediator that drives endotoxin lethality in sepsis. Elevation of HMGB1 was detected considerably later than secretion of acute-phase cytokines (such as TNF and IL-1) and reach a persistent plateau at 16–32 h in LPS-induced endotoxemia models and sepsis patients.165,167 Besides, remarkable elevation of HMGB1 is observed in severe sepsis patients and correlate to disease progression.168,169
In resting cells, HMGB1 was found anchored to nucleus and stabilized by chromatins.165 HMGB1 could be released extracellularly either by active post-translational modification that facilitate nucleocytoplasmic translocation or by passive diffusion from damaged lytic cells. HMGB1 functions were determined by the types of post-translational modification, sites of activation, sources of cell types, and redox states.111,170 Various studies have demonstrated HMGB1’s role in regulating inflammasome activation, autophagy, cell survival, coagulation, and innate immunity by using sepsis model,171 suggesting the potentially multifaceted roles of HMGB1 in sepsis pathogenesis. In response to exogenous and endogenous danger signals, activated immune (macrophages, monocytes, and neutrophils) and non-immune (hepatocytes) cells release HMGB1 through multiple signaling pathways. HMGB1 released extracellularly act as alarmins to promote arrays of inflammatory cytokines with a delayed and biphasic pattern.172 Moreover, HMGB1 release was shown as the downstream mediator of inflammasome activation.173 Recent finding revealed the essential role of HMGB1 in delivering extracellular LPS required for non-canonical caspase-11 inflammasome activation and pyroptotic cell death (Fig. 1).174 Functioning as a DAMP, researchers also argued that HMGB1 secretion induced by type I IFN and TNF signal in necrotic cells might be linked with necroptotic pathway and required for necroptosis-induced inflammation.175,176,177
Biological effects of HMGB1 largely depend on the levels of nucleocytoplasmic shuttling and accumulation. Post-translational modification such as acetylation, phosphorylation, and methylation of multiple amino acid residues within nuclear localization sequences (NLSs) could result in HMGB1 translocation.172 HMGB1 acetylation is specifically regulated by histone deacetylase (HDAC) and histone acetylase (HAT).178 In several sepsis studies, both type I-IFN- and type II-IFN-mediated JAK-STAT were identified as the upstream promoting signal required for HMGB1 hyperacetylation.179,180,181 JAK-STAT signaling have been suggested to be involved with HMGB1 expression, hyperacetylation, and translocation in various activated immune cells (Fig. 1).179,182 As observed in ischemia-reperfusion injured-hepatocytes, the JAK/STAT-activated IRF physically interact with nuclear histone acetyltransferase enzyme p300 so as to regulate acetylation status of HMGB1,183 whereas a similar JAK-STAT-IRF-1 signaling was responsible for LPS-induced HMGB1 acetylation and essential for cytoplasmic accumulation.179
Phosphorylation of HMGB1 serve as another post-translational mechanism for active nucleocytoplasmic translocation. Concomitant phosphorylation on serine located at NLS1 and NLS2 sites might reduce its proximity and alter the three-dimensional structure required for KAP-a1 binding (a nuclear cargo-binding protein), therefore allowing cytoplasmic accumulation of HMGB1 and preventing relocation to nucleus.184 Calcium-mediated signaling might be the underlined mechanism for HMGB1 phosphorylation. Calcium/calmodulin-dependent protein kinase (CaMK) IV-dependent HMGB1 serine phosphorylation was known to mediate HMGB1 shuttling in LPS-stimulated macrophages,185 while in ischemic liver tissues, HMGB1 secretion adopt a regulated process facilitated by redox stress (ROS), which later induce CaMK signaling involved with CaMK IV and Camkk β.186 Moreover, CaMK I was shown to be involved in the release of HMGB1 via enhancing IFN-β signaling by a process of indirect phosphorylation of IRF3.187 As Camkk β catalyzes on its substrates (i.e., CaMK I and CaMK IV) and that CaMK signaling is upstream of HDAC inhibition, redox activation of CaMKs might trigger initial release of HMGB1 during sepsis.
Active release of HMGB1 from hepatocytes were identified as the major source of pro-inflammatory systemic HMGB1 in endotoxemia and CLP sepsis.174 The translocation of HMGB1 required co-activation of both TLR4 and caspase11/GSDMD signaling (Fig. 2).188 In line, increased TLR4 activation and intracellular uptake of LPS induce GSDMD cleavage via direct activation of caspase-11 found in cytosolic compartment of macrophages. However, signs of pyroptosis and lytic cell death was not observed in these models.189 Instead, activated GSDMD promote HMGB1 translocation and release into exosomes. Different from membrane GSDMD pores formed during pyroptosis, accumulation of cleaved GSDMD on ER facilitate free calcium leakage and promote calcium-dependent signaling by phosphorylation of Camkk β.188 Camkk β then act as upstream regulator of HDAC inhibition, which further lead to hyperacetylation and nucleocytoplasmic translocation of HMGB1 (Fig. 2),186 while extracellular release of HMGB1 requires receptor-specific TLR4 signals but independent of caspase-11 and GSDMD activation.
Previously, interaction of receptor for advanced glycation end products (RAGE) and HMGB1 in macrophages was shown to trigger pyroptosome formation, caspase-1 activation, and pyroptosis after endocytosis.190 Endocytosis of HMGB1 undergo a cascade of molecular events that result in release of cathepsin B from ruptured lysosomes, a key event for pyroptosome formation (Fig. 1). Cathepsin B is potent to directly interact with NLRP3 at the ER levels, resulting in pyroptosome formation and pro-caspase-1 activation.191 As demonstrated in sepsis model, neutrophil extracellular trap (NET)-derived HMGB1 was indicated as the distinct source for caspase-1-dependent macrophage pyroptosis associated with augmented pro-inflammatory activities.192
A recent study has unraveled the critical role of circulating HMGB1 in mediating lethal sepsis. It clearly illustrated the complete molecular mechanism of LPS-HMGB1 complex in initiating caspase-11-dependent pyroptosis.174 In response to PAMPs such as LPS and Poly(I:C), substantial level of HMGB1 was released by hepatocytes. Circulating HMGB1 promptly bound with LPS to form HMGB1-LPS complex and mediate translocation of extracellular LPS via RAGE. RAGE-mediated internalization of HMGB1-LPS into endo-lysosome serves as the critical step for cytosol delivery of LPS. The acidic pH provided by lysosomes subsequently enhance direct permeabilization of lysosome phospholipid bilayer by HMGB1, inducing transfer of LPS to cytosolic caspase and subsequent caspase-11-dependent pyroptosis for LPS lethality.
Though other studies have argued that HMGB1 itself could trigger ASC-dependent and caspase-11-independent pyroptosis,190 it was believed that the redox status of HMGB1 have contributed to these inconsistent observations. The three cysteines located in the A box and B box of HMGB1 was modified under different redox states.193 The all-thiol fully reduced form referred to the presence of reduced SH thiol groups at all cysteines. During early sepsis, HMGB1 is released in the all-thiol fully reduced form.194 It forms heterocomplex with CXCL12 that binds to receptor CXCR4 to elicit chemotactic activity but not TLR4-dependent cytokine release.195 In this redox state, HMGB1 serve as endotoxin delivery protein that bind and internalizes LPS into lysosome, an intermediate step required for LPS cytosol release and activation of non-canonical caspase-11-dependent pyroptosis (Fig. 2).174
As inflammation proceed and oxidative products accumulates, C23 and C45 in A box are close enough to be oxidized and form disulfide bond, while the third cysteine (C106) in B box remain reduced.196 This disulfide form of HMGB1 is capable in mediating massive inflammatory responses such as TNF-α release and NF-κB signaling by allowing binding of reduced C106 with TLR4-MD2.196,197 HMGB1 is also capable to interact on TLR2 and RAGE to elicit cytokine-like activity. The pro-inflammatory type of HMGB1 was predominantly detected at weeks 4–8 in sepsis survivors.194 Studies have claimed that pro-inflammatory effect largely depend on the B box due to its much lower dissociation rate with TLR4.198 Besides, disulfide form of HMGB1 alone could prime NLRP3 inflammasome activation for stimulating excessive cytokine production.199
Under massive oxidative stress, release of ROS from mitochondria abrogate both chemotactic and cytokine stimulating activity of HMGB1 and transited into the irreversible fully oxidized HMGB1. This inactive form of HMGB1 were found at weeks 8–12 after inflammation or during hepatic regeneration,194 therefore preventing from excessive inflammatory damages. Fully oxidized HMGB1 also function as an important element for induction of immunological tolerance in apoptotic cells. ROS production resulted from caspase cleavage of mitochondria substrate p75 neutralizes the stimulatory activity of HMGB1, thus avoiding the dangerous immune response elicited by the highly inflammatory HMGB1 once released from apoptotic cells.200,201 Redox modifications of HMGB1 might serve as a dynamic biological switch for its inflammatory activity in response to different magnitudes of oxidative stress.
Interactions of complements with hemostasis and pathogens
Initially, endothelial barrier damage by endotoxins and inflammatory cytokines result in exposure of endothelial collagen fiber and TF, which trigger platelet aggregation that activate thrombin release and fibrin formation. Once platelets are activated, the released serine/threonine-dependent protein kinase could phosphorylate residues of the C3d region of C3. Phosphorylation of other C3 fragments could prevent the cleavage degradation of C3b, resulting in persisted complement activation.202
Complement components reversely feedback to promote coagulation during activation. C5a induce the expression of TF and plasminogen-activator inhibitor 1 on various cell types, while shedding heparin sulfate on vascular endothelial.203,204 Similarly, membrane attack complex (MAC) induce the expression of TF in attached cell and adhesion molecule in endothelial cell lines.35,205 In platelets, MAC facilitate the expression of binding site and catalytic surface for the prothrombinase complex.206 Other complement components such as C1q and C3a enhance procoagulant activity and induce aggregation of platelets.207,208 An appropriate homeostasis between complement and coagulation system guaranteed the full performance of protective inflammatory response and pathogen clearance.
Pathogens could adapt and exploit host defense via interrupting with specific checkpoints of complement system, thus impairing the normal coupling between complements and TLR signaling pathway. Etiology-dependent mechanism has evolved as a decisive factor on the initiation of complement cascade, either via classical or MBL pathway. Certain microbial species have adopted specific mechanisms to evade complement attack. Porphyromonas gingivalis enzymatically cleaves C5 to generate high concentration of C5a for C5aR1 activation on neutrophils while coincidently detected by TLR2. This enhanced C5aR1–TLR2 cross-talk would lead to degradation of downstream signaling adapter MyD88, which is essential for bacterial clearance. After evasion of host immune surveillance, dysbiotic communities depends on host inflammatory products for metabolism and survival. The C5aR1–TLR2 cross-talk alternatively trigger activation of PI3K signaling that inhibit phagocytosis and induce inflammatory responses so as to provide a nutritionally favorable micro-environment that result in dysbiosis and disease development.209
Opsonized pathogens by C3b might end up in distinct intracellular outcomes depending on the activated signaling pathways. Certain non-enveloped bacteria opsonized extracellularly by C3 fragments escaped from phagosomes could still be detected by cytosolic C3 and trigger mitochondrial antiviral signaling, which robustly induce pro-inflammatory cytokines via upregulation of several transcription factor (NF-κB, AP-1, IRF-3, and IRF-5) and promote viral degradation.210 Conversely, uptake of C3-coated Francisella tularensis by C3R consequently activate Lyn kinase and AKT signaling, which in turn upregulate MAPK phosphatase-1 and inhibit MAPK-dependent pro-inflammatory responses downstream of TLR2, allowing persistent intracellular bacterial survival.209
Extracellular histones
Under various clinical scenarios (sepsis, trauma, cancer, and ischemia), substantial elevation of extracellular histones were detected after significant cell death.211,212,213 In the form of circulating nucleosomes or NETs, extracellular histones are established DAMPs that activate immune cells via TLR or NLR signaling pathways.214,215 Neutrophil-derived NETs function to trap pathogenic microbes and exhibit robust bactericidal effect.216 Within NETs, networks of DNA fibers and histones have provided scaffold for platelet aggregation, cell localization (neutrophils, erythrocytes), and activation, which further enhance formation of red blood cell-rich micro-thrombi.152 In comparison to DNA fibers, histones appear to have a more significant effect on clot formation by improving the mechanical stability in thrombi.217
However, excessive release of histones from dying cells and NETS during sepsis might be accompanied with cell cytotoxicity.218 Sub-lethal dose of histone causes early death in mice, presented with pathological features mimicking sepsis, such as neutrophil accumulation, vacuolated endothelial cells, and formation of macro- and micro-thrombi.219 Similarly, significant elevation of plasma histones were observed during sepsis deteriorating stages, accompanied with sepsis-related cytokines and highly correlated with mortality, organ dysfunction, and thrombocytopenia.220,221
In histone-mediated kidney and liver injuries, main pathological features include extensive cellular damage, hemostatic imbalance, and amplification of inflammatory responses.212,222 Both TLR2 and TLR4 were shown to have prominent role in histone-mediated cell toxicity, mediating signal transduction for MyD88, NF-κB, and MAPK pathways. Interestingly, genetic ablation of either TLR2 or TLR4 alone did not abrogate cytokine production, suggesting the requirement of both TLRs.222 In course of septic cardiomyopathy, both TLR3 and TLR9 were linked with histone-induced damage of cardiomyocytes. As demonstrated in TLR3 and TLR9 knockout animals, significant reduction in plasma levels of C5a, histones as well as cytokines in heart tissues were observed, confirming the detrimental roles of TLR3 and TLR9.223 Unexpectedly, study regarding the molecular events in complement-mediated lung injury identified a link between complement interactions with extracellular histones. Ligation of C5a with its receptors (C5aR and C5L2) triggered release of histones (H3 and H4) from activated neutrophils, which result in acute lung injury featured with intense inflammation, polymorphonuclear neutrophil accumulation, and alveolar epithelial cells damage (Fig. 3).224
Though numerous studies have established mechanism responsible for increased histone cytotoxicity, molecular events of extracellular histone-mediated endothelial dysfunction remains unclear. Recently, a dose-dependent mechanism of autophagy and apoptosis mediated by extracellular histones was confirmed in cultured human endothelial cell lines. Such responses were shown to be mediated via sestrin2/AMPK/ULK1-mTOR and Akt/mTOR pathways.225 In consistent with previous results, the type of histone-mediated cell death, either autophagy or apoptosis, is determined by concentration of extracellular histones (Fig. 3).226 Initially, low concentration of histones direct cells to autophagy via upregulation of sestrin2/AMPK/ULK1-mTOR pathways and decrease in Akt activation, while subsequent inactivation of mTOR and dephosphorylation of p70S6K (mTOR downstream target) also contribute to autophagy. As histone concentration increases, extracellular histones immediately induce a p53-dependent upregulation of Bax and result in apoptosis, which in turn inhibit the expression of autophagic protein Bcl-2. Involvement of both autophagic and apoptotic pathways in the dose-dependent mechanism of histone-related cytotoxicity have provided novel potential targets for therapeutic strategies.
Recently, a TLR9-dependent mechanism was identified for histone-mediated inflammation observed in I/R-injured Kupffer cells.227 Further investigation proposed novel role of histones in propagating I/R liver injury. Extracellular histones were hypothesized to directly interact and activate TLR9-mediated ROS generation, which further triggered NLRP3 inflammasome activation and recruitment of additional cell types, thus driving innate immune response that exacerbates I/R injury.228 In addition, stimulating histones in TLR-KO models have observed a dose-dependent increase in inflammasome activation within TLR2 and TLR4 KO cells but not in TLR9 KO cells, suggesting a significant role of TLR9 in histone-mediated inflammation.228 Other reports documented that TLR9 activation by histones was attributed to contamination,229,230 as TLR9 originally function for sensing intracellular DNA but not histones.231 This suggested that DNA-binding histones might have acted in conjunction to facilitate DNA-mediated TLR9 response.
Exclusively, extracellular histone promote thrombi generation via platelet-dependent mechanisms (Fig. 3)232 by directly modulating the clotting properties of platelet-induced polyphosphate (polyP), allowing its induction of thrombi generation with histones.233 Moreover, histone enhance procoagulant phenotype of platelets by upregulation of P-selectin, phosphatidylserine (PS), and FV/Va, partially in a TLR2- and TLR4-dependent mechanism.232 The enhanced platelet aggregation might contribute to formation of platelet-rich thrombi in sepsis. Direct histone–platelet interaction was also suggested as the potential mechanism for rapid profound thrombocytopenia.234 Histone preferentially bind with platelet plasma membrane via a charge-dependent manner. Subsequent induction of calcium influx promote activation of αIIbβ3 on platelets, together with specific binding to a platelet receptor or fibrinogen facilitated by histone, were responsible for the formation of large platelet aggregates that cause profound thrombocytopenia.
Platelets: crucial intermediators between inflammation and hemostasis
Upon extensive thrombus formation, platelets are sequentially activated and aggregated to the site of impaired vascular integrity, providing a perfect scaffold for coagulation cascades to take place.235 While during sepsis, rapid thrombocytopenia was associated with decreased platelets adhesion, increased bacterial load, and enhanced inflammatory cytokines, which further modulate coagulation–fibrinolysis system and impaired hemostasis process.236,237,238 In clinical sepsis, recruitment of LPS-induced platelets were detected in lung and liver microvascular thrombosis,239,240,241 in a TLR4-dependent manner. Supported with evidence of direct LPS receptor TLR4 expressed on platelet, a reciprocal relationship in LPS-induced platelet response was proposed.242 Discrepancy in antigenicity of LPS from different bacterial strains further determined its function on platelet responses. It was suggested that rough form of LPS directly interact with platelet, independent of soluble CD14 required by smooth LPS, might have participated in LPS-induced platelet activation.243
Several studies have investigated on the underlying signaling pathway during platelet activation. Akt, the downstream effector of PI3K pathway, as well as small GTPase (JNK, ERK, p38) implicated in the MAPK pathway, were observed to be activated in LPS-induced platelets in in vivo models.244,245 Elevated ROS was proven to be a required element for platelet activation, as administration of antioxidants and inhibition of relevant signaling pathway significantly reduce LPS-induced platelet function.244,245 To conclude, LPS-enhanced platelet activation is capable to initiate TLR4/PI3k/Akt-ERK1/2/PLA2 pathway that involves TXA2 and ROS generation. TXA2, the downstream product of PLA2 activation, is necessary for effective LPS action on platelets, while ROS is required to modulate LPS-TLR4 signaling at different levels.246 In stimulated platelets, PLA2 was activated in response to actions of Akt, thus stimulating a second wave of platelet aggregation. In addition, activation of the MAPK-ERK1/2 pathway is involved with platelet stimulation and ROS release under regulation of PI3K/Akt, in consistent with the broad biological effects of PI3K pathway reviewed previously.247
HMGB1 was also critical for the pathogenesis of coagulation abnormalities observed in trauma and hemorrhagic shock.248,249 In in vivo models of injury-induced thrombosis, elevated HMGB1 levels within thrombi was indicated as platelet-derived and had presumably contributed to inflammation and organ failure. By evaluating the molecular mechanism of HMGB1-driven platelet activation during thrombosis, critical role of HMGB1 was again established.250 Characterized with platelets activation, dense granule secretion, aggregation, and thrombus formation, HMGB1 potentially modulate the pro-thrombotic phenotype of platelets via complex formation of MyD88/GC and cGMP-dependent protein kinase (cGKI) pathway. It has been proven that platelets store and express HMGB1 on cell surface upon activation.251,252 Platelet-derived HMGB1 specifically interact with TLR4 to induce a MyD88-dependent GC recruitment toward platelet plasma membrane.253 Elevation of intracellular cGMP immediately induce downstream activation of cGKI, an essential signal required for TLR4/HMGB1-mediated thrombus formation and platelet aggregation.250
In interaction with adhesion protein such as von Willebrand factor (vWF), activation of platelet integrin affinity was enhanced via MAPK/ERK pathway in a cGKI mechanism, while MAPK-mediated activation of platelet integrin αIIbβ3 require signaling via platelet GPIb-IX receptor.254 In addition, insufficient degradation of vWF due to ADAMTS13 deficiency might have enhanced thrombosis in sepsis and mimic certain clinical presentation of thrombotic thrombocytopenia purpura, supported by evidence of low levels of ADAMTS13 and association with DIC severity.255,256
Coagulation: cross-talks between inflammasome, ROS, and TF
Bacterial-derived products trigger delivery of LPS into cytosol of host cells, which might be responsible for occurrence of DIC.257 Systemic activation of coagulation was recently revealed to be involved with caspase-11-dependent inflammasome activation.258 Demonstrated by monocytes and macrophages, activation of the LPS cytosolic receptor caspase-11 and formation of GSDMD pores initiate coagulation cascade via PS exposure (Fig. 1). The GSDMD pore-mediated Ca2+ influx promote exposure of PS on the outer leaflet of plasma membrane via activation of transmembrane protein 16F (TMEM16F), a calcium-dependent phospholipid scramblase. PS externalization allow activation of TF and assembly of cofactor–protease complexes during coagulation cascade. To date, molecules such as HMGB1 and bacterial outer membrane vesicles that are required for localization of LPS in cytosol might have taken advantage on this immune-thrombotic mechanism in triggering systemic coagulation during sepsis.259,260
In light of the advanced understanding of the role of caspase-11 and GSDMD during endotoxin-induced coagulation, this provided evidence for a previously undetermined relationship between type-I IFN signaling and intrinsic coagulation cascades. As coagulation process is intended for host to prevent dissemination of pathogenic bacteria,261 infection-induced type-I IFN serve as a crucial intermediator between innate immune response and coagulation.262 Induced by Gram-negative bacteria, LPS-mediated type-I IFN signaling amplify HMGB1 extracellular release via direct modification on acetylation levels at the nuclear location sequences. Extracellular HMGB1 has already proven to increase pro-coagulant activity of TF via promoting externalization of PS to the outer plasma membrane. While the corresponding caspase-11 activation converge as upstream signals for GSDMD-dependent PS exposure, other mechanism might have contributed to coagulation cascades such as thiol-disulfide exchange, NETosis, platelet activation, and disruption of endothelial Tie2 axis.263,264,265,266
TMEM173 (STING), a classic innate immune sensor that stimulate the expression of IFN in response to DAMP (i.e., cytosolic DNA),267 was identified to be involved with coagulation via triggering ER stress-induced GSDMD activation. TMEM173 were significantly activated during infection and inter-related with disease severity.268,269 Implication of excessive TMEM173 activation in sepsis pathogenesis have been widely discussed.27,269,270,271 TMEM173 interact with a predominant calcium channel ITPR1 to promote ER calcium efflux required for caspase-1/11/8-induced GSDMD cleavage and activation. The ER stress-mediated GSDMD pore formation then initiate pyroptosis and subsequent release of TF.272 Prolonged innate immune activation by TMEM173 had consistently promoted inflammasome coagulation process with the presence of GSDMD pores.
With the observation of extensive TF-driven coagulation events particularly in pyroptotic macrophages, other study agreed on an inflammasome-dependent mechanism that had supported a central role of TF in pathogenesis of pyroptotic cell death. In LPS-induced models, massive thrombosis and systemic coagulation triggered by increased TF activity were associated with inflammasome overactivation. Pyroptosis was mentioned as the trigger for lethal DIC not only via promoting TF-positive microvesicles formed by cell membrane fragment of pyroptotic macrophages but also triggering cell rupture required for extracellular TF release.273
Aside from post-transcriptional activation of TF via caspase-11-dependent PS exposure, stimulation of TF prothrombotic properties might require certain redox partners.263 Recently, generation of procoagulant microparticles (TF-bearing microparticles) in response to extracellular ATP signal was concluded to signal via caspase-1 pathway under regulation by Trx/TrxR system.274 The Trx/TrxR system was known to be associated with redox modification.275 Though experimental study had proposed a protective role of Trx,276 clinical studies have consistently observed elevation of plasma Trx levels in sepsis non-survivors, which is positively correlated with cytokines levels and lethal coagulation.275,277,278 Activated by P2X7 receptors, TrxR-dependent release of Trx and subsequent extracellular thiol-disulfide exchange result in progressive oxidation of extracellular Trx, which is analogous to the PDI-dependent TF activation mechanism. Intracellular depletion of Trx further lead to TXNIP-dependent inflammasome activation for IL-1β production and caspase-1 required for extracellular actin-dependent MP generation. Activation of caspase-1/calpain cysteine cascades then result in filamin degradation and release of TF for raft-dependent incorporation into PS-rich MPs. Besides, distinct composition such as TF and P-selectin levels have also contributed to the prothrombotic properties of these microparticles.279,280
Neutrophils, platelets, and coagulation
Several studies have demonstrated the crucial role of neutrophils in promoting thrombogenesis.281,282 Expression of TF in neutrophils was considered as an initiating event in coagulation cascade.283,284,285 In sepsis model, detection of TF-positive granulocytes and established regulatory mechanism for TF gene expression have provided evidences suggestive of in situ synthesis of TF by neutrophils.286 However, incapable to detect TF mRNA expression in TF-positive granuocytes,286 studies argue that neutrophil TF was not produced in situ but rather up-taken from cell vehicles such as monocyte-derived microparticles.287 In contrast, anaphylatoxin C5a was able to mediate in situ TF production by neutrophils via upregulation of TF gene,288 providing a convincing mechanism for de novo TF production by neutrophils (Fig. 3). Such C5a-dependent manner was also observed in other clinical situations, such as ARDS and end-stage renal disease.283,285
During sepsis, enhanced NET formation was observed subsequent to TLR4-mediated platelet binding to adherent neutrophils.289 TLR4 on platelet serve as a threshold switch for signaling pathogen stimuli to a neutrophil-mediated bacterial trapping mechanism. Comprised with DNA fibers and histones, NET is suffice to provide scaffold for fibrin deposition, platelet entrapment, and stabilization of thrombus essential for hemostasis (Fig. 3).152,290 However, overwhelmed platelet activation and DAMP released from NET compounds risk causing massive tissue and endothelial damages.241 As demonstrated in sepsis-induced hepatic injured tissues, NET-induced detachment of sinusoidal endothelial cells allows platelets to enter the space of Disse, where platelets bind with collagen-3 to initiate extravasated platelet aggregation, a thrombotic event leading to irreversible deterioration of hepatic function.
Platelet-derived HMGB1 was previously shown to be critical initiators of NET formation via interacting with RAGE.291 Extracellular delivery of platelet-derived HMGB1 to neutrophil serves as a causative signal to prime NET formation via promoting autophagy. Accumulation of TF and HMGB1 in acidified autophagosomes (LC3B-coated vacuoles) facilitate its delivery and localization to cytosolic NETs, which subsequently stimulate NETosis, release of TF, and initiation of coagulation cascade. Existence of an autophagic machinery during release of TF-bearing NETs suggested a novel secretory mechanism for membrane- or cytosolic-bound proteins toward NETs. This also emphasized that inflammatory mediators such as HMGB1 serve as crucial stimuli for post-transcriptional regulation of TF. More importantly, recruitment of neutrophils to damaged endothelial cells and release of TF-bearing NETs result in thrombus formation that could persistently activate inflammation responses via PAR signaling pathways.292
Thrombomodulin (TM), protein C, and endothelial protein C receptor (EPCR)
Thrombomodulin (TM) is an endothelial transmembrane glycoprotein that possess a central modulatory role in the natural anticoagulant system. Acting as a cofactor of thrombin, it reversibly formed TM–thrombin complex that blocks thrombin’s binding properties with pro-coagulant substrates, such as fibrinogen, protease-activated receptors, and coagulation factors V and VIII.293 Besides, binding of thrombin by TM inactivates its capacity for PAR-1 signaling and the downstream pro-inflammatory responses (Fig. 3).294 As the downstream target of PAR-1 signaling, decreased induction of ERK1/2 is also responsible for suppressed mitogenic effect of thrombin, via induction of NOS3 and generation of NO by TM–thrombin.295 TM–thrombin cross-talk with fibrinolytic cascade by accelerating activation of TAFI (thrombin-activatable fibrinolysis inhibitor), which render fibrin to be tolerant to plasminogen binding (Fig. 3).296 In consequence, TAFI effectively degrade pro-inflammatory mediators including bradykinin and anaphylatoxin complements (C3a and C5a).297,298 Independent of the anticoagulant property elicited by activation of protein C, the lectin-like domain of TM neutralize pro-inflammatory activity of thrombin via suppression of leukocyte adhesion,299 interference with complement activation,300,301 inactivation of HMGB1, histones,302 and bacterial endotoxin (Fig. 3).303
In transgenic mice expressing lectin-like domain-deleted TM, increased susceptibility to endotoxin shock is featured with enhanced expression of intercellular adhesion molecule and vascular cell adhesion molecule-mediated leukocytes. Recombinant lectin-domain of TM (rTMD1) was proven sufficient to restore normal leukocytes adhesion via suppressing TNF-α-induced ERK phosphorylation.299 Various studies had revealed molecular mechanism of the anti-inflammatory effect of TMD1. TMD1 structure within the thrombin–TM complex could specifically degrade HMGB1 to a less pro-inflammatory form that block its interaction with RAGE, while rTMD1 interfere with LPS-CD14 binding and inhibit subsequent LPS-induced inflammation via suppressing the MAPK and NF-κB signaling pathway as well as iNOS expression in macrophages.303,304 In animal models, recombinant TM significantly reduce LPS-induced sepsis mortality characterized with reduced levels of TNF-α and inflammatory cell infiltration in the lungs and livers.303,305
TM-dependent protein C activation augment the above-mentioned anti-coagulant and anti-inflammatory effects.306 Enhanced by cofactor protein S located on activated platelets and endothelial surface, the APC-PS complex proteolytically degrade coagulation factor Va and VIIIa to obtain anti-coagulant effect (Fig. 3).307 Efficacy of protein C activation could be further facilitated after interaction with EPCR.308 The resulting APC-ECPR complex downregulates NF-κB signaling and inflammatory cytokine production, via a PAR-1-dependent mechanism (Fig. 3).309,310
As a potent biomarker for reflecting severity of endothelial damages, downexpression of endothelial TM was vastly observed in the dermal microvasculature of patients with meningococcal sepsis.311 Similarly, the proteolytic degraded form namely “plasma-soluble TM” was distinctly observed in severe sepsis patients. Soluble TM was markedly elevated in septic patients with organ failure and in consistent with the serial changes of endothelial activation markers during the course of sepsis.312,313 Analogously, levels of soluble EPCR was shown correlated with poor outcomes in sepsis patients.314 Unexpectedly, formation of thrombin that generally result in upregulation of EPCR might also contribute to EPCR shedding during endotoxin shock.315,316 The elevated pro-inflammatory cytokines TNF-α and IL-1β during sepsis have enhanced EPCR shedding via activation of the MAPK pathway.317 In consistent, administration of APC showed limited protective effect in septic mice with low expression of EPCR, therefore emphasizing the requirement of EPCR- and PAR-1-dependent effect for APC’s efficacy in reversing sepsis-related mortality.318
Interaction with TM switch substrate specificity of thrombin from pro-coagulant (fibrin) to anti-coagulant (protein C).319 Following activation of protein-C, TM was potent to augment anti-inflammatory effects via APC-dependent mechanisms (Fig. 3).306 By interfering with NF-κB and AP-1 pathways, APC inhibited endotoxin-induced TNF-α production in human monocytes,320 which is parallel to results observed in endotoxemic mice. Recombinant APC was also reported to be therapeutically beneficial in severe sepsis patients presented with overt DIC.321 It is evident that APC interact with EPCR to produce enhanced anti-coagulant responses upon excessive thrombin generation.
Interestingly, thrombin exhibit bifunctional roles on endothelial barrier integrity via inducing dual effects of PAR-1 signaling in a thrombin concentration-dependent mechanism (Fig. 3). Both PAR-1 agonist peptide and low concentration of thrombin induce barrier-protective effect comparable to that observed in elevated APC condition. It was proposed that cross-talks of receptor systems have contributed to a balanced regulation of endothelial function in a dual-chamber system via PAR-1 signaling.322 Certainly, it is now clear that APC-EPCR complex interrupt with the effector response of thrombin by recoupling the PAR-1 to bind with alternative members of the G protein families responsible for multiple intracellular signaling pathways.323 In such response, occupancy of EPCR by APC induce dissociation of caveolin-1 from EPCR and recouple PAR-1 to the alternative Gi protein, thus preventing activation of the pro-inflammatory RhoA pathway related to cytoskeleton remodeling and exhibit an endothelial barrier protective effect.324 In addition, activated PAR-1 also improve endothelial function via inducing sphingosine-1-phosphate (S1P) generation and transactivation of S1P receptor type 1 (S1P1). Such response improves endothelial barrier stabilization via activating the Rac1-mediated cortical actin formation.325 Enhanced endothelial barrier mediates anti-inflammatory effect via reducing leukocyte extravasation to sites of inflammation (Fig. 3).322,326 EPCR-APC-dependent activation of PAR-1 signaling also promote barrier stabilization via activation of Ang/Tie2 system (Fig. 3).327,328 EPCR-dependent PAR-1 cleavage by low levels of thrombin increases the expression levels of Ang1 and Tie2 but downregulates Ang2, thus initiating a previously undefined protective mechanism by restoring endothelial integrity.324 Serving as potential strategies on endothelial barrier protection, both transactivation of S1P receptors and Ang1/Tie2 axis activation enhance barrier cell survival via the PI3K/Akt pathway.326,329
As a consequence of acute phase responses, increased plasma levels of C4bBP may account for the relative decrease in free protein S and reduced anti-coagulant activity observed in sepsis.330 In E. coli-infused baboons, inhibition of protein S by infusion of C4bBP result in a hypercoagulable state associated with enhanced lethality.331 Similar results were observed in condition of microvascular thrombotic disorder, such as hemolytic uremic syndrome.332 Inactivation of EPCR by neutralizing monoclonal antibody showed increased morbidity in E. coli baboon model while protein C administration protected against microvascular thrombotic response.332 Implicated from previous findings that interaction with cationic proteins potentially influence activities of TM–protein C system, extracellular histone, a late phase mediator for cell death and thrombin generation during sepsis, impair anti-coagulant activity via directly interacting with TM and protein C (Fig. 3). Additionally, co-factor activity of both soluble and endothelial TM were reduced.333 In view of this, preclinical trials using recombinant human form of TM (TM alfa) successfully restored equivalent anticoagulant effect. Clinically relevant concentration of TM alfa increased APC generation, leading to consequent cleavage of pro-coagulant histones and attenuated histone-induced endothelial cytotoxicity.334
Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) is a signaling lipid abundantly found in circulation and mainly bound to high-density lipoprotein (HDL)-associated apolipoprotein M and serum albumin.335 In PAR-1-activated endothelial cell, S1P is produced by phosphorylation of sphingosine by sphingosine kinase 1 (SphK1) and function to protect endothelial barrier integrity via enhancing cortical actin formation.336 In addition, S1P signaling recruits VE-cadherin for endothelial junction assembly via a Rac-1-dependent mechanism.337 In fact, S1P might have served as a bifunctional molecule. These contradicting functions were most likely due to the relative concentration and source of S1P production.338,339 In endotoxemia models, protein expression and enzymatic activity of SphK1 were observed to be elevated and responsible for hyper-inflammatory responses observed in macrophages.340,341 Recently, S1P was suggested as a DAMP involved with regulation of NLRP3 inflammasome activation in macrophages,342 while inhibition of SphK1 was shown to protect mice from sepsis-induced mortality. By suppressing NLRP3 inflammasome activation, this led to attenuated microvascular leakage via suppressing IL-1β-mediated adherens junction disassembly and endothelial cadherin internalization,343 though the involved signaling mechanism of NLRP3 inflammasome inhibition by SphK1 remains unknown. SphK1 was recently observed to regulate HMGB1 translocation by direct interaction with the CaMKII-δ. In sepsis-associated liver injury models, co-localization of SphK1 and CaMKII-δ in Kupffer cells provide signals for CaMKII-δ autophosphorylation. Activated CaMKII-δ serve as upstream regulator for HDAC4 phosphorylation, an inhibitory form of HDAC4 that favors hyperacetylation and translocation of HMGB1.344
In other studies, S1P signaling failed to exhibit endothelial protective effect over time due to the loss of endothelial responsiveness after prolonged exposure.345 Besides, the type of activating stimuli and S1P receptor coupled have determined whether a pro-inflammatory or barrier-enhancing feature would be exhibited by S1P. Still, S1P represents a potential therapeutic target with broad regulatory effect on endothelial barrier function and innate immune cells.325 In sepsis baboon models, plasma S1P was rapidly decreased within 6–8 h after E. coli injection.346 With particular significance for its regulatory role in endothelial barrier function, levels of serum S1P was dramatically decreased in end-stage severe sepsis patients, while similar changes were observed for the levels of S1P-binding molecules.347,348,349 Serum and plasma S1P levels were inversely correlated to disease severity in terms of septic shock incidence and higher SOFA scores.350
With ample evidences from several studies, it is rationale to claim that protective effect of PAR-1 signaling to be at least partially attributed to the anti-apoptotic effect of enhanced S1P-mediated responses.322 In early studies regarding endothelial function, APC is potent to inhibit staurosporine-induced apoptosis and modulate gene expression of the endothelial apoptotic pathway (Bcl-2, eNOS, and the IAP).351,352 Likewise, PAR-1 agonist had exhibited significant anti-apoptotic effect in human brain endothelium.353 As observed in embryonic stem cells, both Erk1/2 and PI3K/Akt signaling pathways were shown to be critical for the HDL/ApoM/S1P-mediated anti-apoptotic effect that promote stem cell survival.354,355 The EPCR-APC-mediated cell signaling was associated with cytoprotective and anti-inflammatory phenotype, featured with downregulation of vascular adhesion molecules, stabilization of endothelial barrier function, and inhibition of inflammatory and apoptotic signaling.356
Cross-talks of STING with immune pathways
Based on the pivotal role of STING in modulating innate immune response in sepsis, studies have focused to identify key regulators for the STING pathway. By screening potential pharmacological kinase inhibitors for STING-mediated type I IFN responses, a novel regulatory target for STING-mediated innate response was proposed—anapestic lymphoma receptor tyrosine kinase (ALK). Previously known as a tumor-associated receptor tyrosine kinase, expression of ALK was relatively low in healthy individuals. While components of both ALK and STING pathway were abundantly expressed and upregulated in innate immune cells during clinical sepsis, such observation was in accordance with the elevated DNA levels from invading pathogens and host damaged cells specifically observed in poor outcome patients.357,358
Pharmacological inhibition or genetic lockout of either ALK or STING protect mice from lethal sepsis by restricting excessive STING-mediated innate recognition of bacterial DNA and subsequent pro-inflammatory cytokine expression (Fig. 1). It was proposed that ALK might have interacted with other receptor tyrosine kinase (RTK) on cell surface so as to mediate signal transduction that interfere with STING-initiated signaling pathways. Mechanistically, ALK/EGFR binding was shown to trigger AKT-dependent STING activation in innate immune cells and provided evidence for a novel signaling pathway that have contributed to sepsis pathogenesis and septic shock (Fig. 1).359 In summary, ALK-EGFR-AKT pathway was proven as a critical modulator of lethal STING-mediated innate immune responses. This novel role of ALK in modulating inflammatory signaling pathway has opened new avenue for development of therapeutic targeting drugs.
As a Food and Drug Administration (FDA)-approved second-generation ALK inhibitor, LDK378 exhibit STING-dependent anti-inflammatory effect in innate immune cells that rendered mice more resistant to lethal sepsis. Recent studies have demonstrated that LDK378 alleviate sepsis-induced pathological changes in heart, lung, and kidney. LDK378 improve micro- and macro-circulation via regulating STING-mediated cytokine release and microcirculatory dysfunction.270 However, some scientists argued that off-target effect of ALK inhibition might have contributed to the anti-inflammatory protection observed.
Recent work using LDK378 have identified novel function regarding host immune response. LDK378 was shown to interfere with LPS-mediated CCR2 upregulation that is required for recruitment of myeloid-derived suppressor cells (MDSCs) to peripheral lymph organs. Such effect was blocked via inhibiting the phosphorylation of p38 and subsequent G protein-coupled receptor kinase-2 in bone marrow MDSCs, which subsequently lead to CCR2 internalization and desensitization. Here LDK378 was proven to interact with p38-MAPK pathway in which the p38/GRK/CCR2 pathway plays pivotal role in chronic immunosuppression. During sepsis, high levels of circulating MDSCs abundantly found in the spleen, lymph nodes and bone marrows were correlated with increased nosocomial infection and poorer functional status at hospital discharge.360 MDSC is known as a type of suppressive innate immune cells that mainly function via secretion of ROS and NO, induction of T cell suppression, and selective polarization of Th-2 cells. By partially inhibiting recruitment of MSDC, LDK378 relieves the immunosuppression and inflammatory responses induced in lethal sepsis.
In the previous study, acquired mutation of STING was proven to disrupt ER calcium homeostasis and prime T cell to be hyper-responsive to TCR signaling-induced ER stress. This STING-mediated chronic elevation of ER stress was potent to prime T cell death by apoptosis.361 Expression of STING and GSDMD correlates with severity of disseminated intravascular coagulation and mortality in sepsis patients. Such STING-dependent systemic coagulation was independent from the classic STING-induced pathway that have already been mentioned in the previous section. Based on the multiple impacts on immune homeostasis, coagulation, and inflammation, STING pathway has been recently proposed as another potent therapeutic target for sepsis.
Ang/Tie2 pathway and regulatory mechanism
Ang/Tie2 pathway was regulated and linked with a complexity of signaling pathways (Fig. 3). In the context of sepsis, stimuli of inflammatory cytokines, coagulants, or VEGF allow secretion of Ang2 from WPB.162,362 Ang2 itself serve as a rapidly acting regulator of the endothelium through an autocrine loop mechanism.363 In the presence of LPS or infection signals, ectodomain cleavage of Tie1 block Tie2 phosphorylation and contribute to loss of agonist property of Ang2 as well as weakened Ang1 activity.158,161 This suggest the indispensable role of Tie1 in Tie2 signaling. LPS-mediated Ang2 expression was found to be regulated by NADPH oxidase 2 (Nox2) via the IKKb/NF-κB and MAPK/AP-1 signaling pathways.364 The specific structure of Ang2 that prevent scattered Tie2 molecules from clustering to form multimers365 serve as the core mechanism in Ang2/Tie2 antagonism. In endothelial cells, Tie2 inactivation promotes nuclear translocation of FOXO1 and inhibition of KLF2, which enhance transcription of target genes, such as Ang2, VEGFR2, and ET-1,366,367 while increased expression of VEGFR2 activate downstream pathway such as eNOS, PI3K/Akt and Src, resulting in disruption of adhesion molecules (degradation of VE-cadherin and S-nitrosylation of beta-catenin).368,369
Reduction of Tie2 expression is another distinct feature observed in sepsis models and could further contribute to microvascular leakage. The VEGF-mediated PI3K/Akt pathway subsequently cross-interact with a p38 MAPK-dependent protease (ADAM) that is capable in cleaving Tie2 ectodomain into soluble Tie2 (sTie2).370 Likewise, changes in the levels of sTie2 were observed in critically ill sepsis patients.371 Under hypoxia condition, increased levels of VE-PTP was associated with Tie2 and act as a negative feedback mechanism by limiting the Tie2 downstream activity.372 In addition, decline Tie2 mRNA levels was known to be induced by decreased endothelial shear stress in a NF-κB-dependent manner.373
From gene transduction by recombinant virus to recombinant human antibodies targeting on Ang/Tie2 axis, several studies have proposed effective therapeutic strategies in restoring endothelial stability in sepsis murine models. Via augmenting Ang1, inhibiting Ang2, and promoting Tie2 activation, preclinical studies have demonstrated promising results in different sources of endothelial cells under various settings. Rh-Ang1 protein is capable to prevent pulmonary capillary leakage and preserve integrity of blood–brain barrier (BBB) after intravenous administration.374,375 Rosiglitazone, a PPAR-γ agonist that result in higher brain and plasma levels of Ang1 and lower Ang2/Ang1 ratios, is able to restore BBB integrity, achieve better neurocognitive outcomes, and improved survivals in malaria-infected mice.376
Considerable elevated levels of Ang2 in response to infectious signals makes it an ideal pharmaceutical target for restoring Tie2 signaling during sepsis. The anti-Ang2 antibody, ABTAA demonstrated a novel mechanism in stabilizing Ang/Tie2 axis during sepsis. It specifically targets on the deleterious Ang2 clusters by converting it into a high molecular complex that bind and act as agonist of Tie2.377 Tie2 activation protect sepsis progression by strengthening the endothelial glycocalyx, amelioration of cytokine storm, and vascular leakage in targeted organs.377 Other compounds such as rh-Ang1 variant (COMP-Ang1, MAT.Ang1), inhibitors of VE-PTP (AKB-9778), and synthetic Tie2 agonist (Vasculotide) directly modified the functional state of their reciprocal targets so as to preserve endothelial barrier integrity.374,378,379,380
Besides, Ang2 is capable in inducing RHoA activation via SHP-2-dependent dephosphorylation of RHoGAP (GTP activation protein), which activate downstream Rho kinase (ROCK).381 Such signaling contribute to cytoskeleton rearrangement (stress fiber assembly, cell contact destabilization) and endothelial junction breakdown (internalization of adhesion and tight junctions).382 Besides, increased secretion of thrombin and TNF-α during sepsis favors stress fiber assembly and cell contraction by RhoA-mediated activation of ROCK and myosin light chain kinase.383 As the downstream target of RhoA, ROCK-mediated regulation in vascular permeability was suggested to be a critical mechanism in sepsis-induced lung injuries.384
Actin-binding proteins (ABPs) are another group of regulating proteins involved in modulation of actin and myosin function during cytoskeleton remodeling.385 ABPs maintain endothelial stabilization by directly binding to actin/myosin and induce actin remodeling via polymerization, branching, and severing.385 Besides, ABPs cross-interact with complexity of intracellular signaling pathways via activation of small GTPases (Rac1, Rap1). Expression of NF-κB, MyD88, and p38 MAPK were also found to be involved in ABP regulatory signaling machinery.
Intervention therapies in sepsis
Conventional management interventions such as early antibiotics, fluid resuscitation, and hemodynamic support by vasopressors have been effective for early sepsis resuscitation and significantly improve overall clinical outcomes. Yet, treatment targeting the underlying causes that had incited to devastating manifestation seems pivotal but remains unclear. Recognized as the host-mediated systemic inflammatory responses to infection, numerous clinical studies have observed evidences of immune cell activation and dysregulated host responses in severe sepsis patients that coincide with much of the established molecular mechanisms, together with the development of therapeutic monoclonal antibodies proven with safe profile in clinical sepsis patients. These provide strong rationales for further investigation on larger sepsis population.
Over the past two decades, multi-center randomized controlled trials (RCTs) have failed to obtain successful results using therapeutic strategies targeting on inhibition of specific components involved with inflammatory responses (i.e., IL-1, IL-6, TNF-α; Table 1) and coagulation cascades (Table 2). Discrepancy in sepsis clinical definition as well as multifaceted manifestations observed during clinical course have made such syndrome a more complicated clinical situation to investigate on. Besides, inclusion criteria of patients and observed clinical endpoints showed inconsistency between trials. Some of the measuring indicators seems inappropriate for clinical implication and incapable to address their full performance on clinical efficacy, which added up to the inherent limitation in these studies. Meta-analysis concluded low-quality levels of evidences from previous RCTs and subtle values for clinical translation.
Lately, post hoc studies have observed encouraging results after stratification and subgroup analysis (Tables 1 and 2). In common, these recent studies have taken advantages on revised inclusion criteria based on novel biomarkers or scoring system consisted of numerous clinical variables, therefore emphasizing the importance of precise therapy and effectiveness of biomarker-guided trials. Before that, several studies have already proposed potential biomarkers for endothelial injuries, including fragments of membrane-bound molecules generated after proteolytic cleavage by neutrophils. Administration of recombinant (i.e., TM) or activated form (i.e., APC) of molecules showed uplifting results in early clinical trials (Table 2); however, have also risen certain concerns on lethal adverse events, such as bleeding complications. Monitoring of vast group of biomarkers associated with innate immune responses, receptor expression, vascular barrier integrity, and tissue/organ injuries could provide indirect evidences informing the real-time magnitude and depth of dysregulated host responses. Dynamically reflecting the immune homeostasis status relevant to clinical course are essential for elucidating sepsis pathophysiology. Innate immune response biomarker such as DAMPs, PAMPs, chemokines, and cytokines are capable for guidance of therapeutic strategies targeting on removal and inhibition of respective molecular stimuli. These approaches might be beneficial for early reversal of detrimental events. With the help of other novel biomarkers, continuous monitoring levels of immune cell activity help to identify patients who might benefit most from a specific intervention. Such approach is vital especially when majority of sepsis therapeutic strategies might be harmful to patients who express immunoparalysis or a more dysregulated proinflammatory host response. Monitoring of biomarkers related to vascular, tissue, and organ failure could early identify patients suspected of severe sepsis and refractory septic shock. These measures have provided rationales for future biomarker-guided therapy and personalized, tailored therapy.
Concurrently, significant number of studies have identified previously unknown components and novel relationship between signaling pathways that serve as key drivers for enhanced or dysregulated inflammatory responses. These studies inspired therapeutic approaches that target on a wider scope of host responses and shed light on the development of novel intervention therapies. Asides from the well-known PAMPs such as LPS and mannose, identification of a substantial group of DAMPs showed superior role in sepsis pathogenesis. Inhibition of ligands or receptors could prevent activation of devastating downstream events. These referred to the use of monoclonal antibodies, downregulation of DAMP expression, and inactivation of the intrinsic binding interaction (Table 3 and Fig. 5).
Systemic removal of pro-inflammatory mediators is another effective therapeutic approach that has been extensively applied in clinical scenarios. Extracorporeal removal of inflammatory mediators or toxic substances consists of conventional and novel blood purification techniques. Essential information of each of these techniques were compared and presented in table form (Table 4 and Fig. 4). In terms of flow volume and modality, these could be classified into high volume hemofiltration and continuous hemofiltration,386 while more studies shed light on the type of CRRT membrane or adsorption cartridges incorporated,387 or the use of coupled plasma filtration adsorption or double adsorption.388 Clinically, polymyxin B-immobilized hemoperfusion serves as an adjuvant therapy for septic shock by removing circulating endotoxin via absorption.389 CytoSorb, a CE-approved cytokine adsorber, was recently under great concerns due to its safe rescuing effect on SARS-CoV-2 infected multiple organ dysfunction syndrome patients.390 As an extracorporeal cytokine hemoadsorption device, CytoSorb showed significant improvements on norepinephrine requirements and PCT but failed to obtain benefits on clinical outcomes.391
Recently, the novel ALK-EGFR-AKT pathway that serve as potential regulator on STING-dependent inflammatory responses was suggested to be useful therapeutic target during sepsis. LDK378, a USA-FDA approved ALK inhibitor, was shown to exhibit anti-inflammatory effect and potent to confer protection against lethal endotoxemia and sepsis in mice.359 Other approach that interfere with interaction between toxins/alarmins and their reciprocal receptor also result in attenuated cytokine gene expression and inflammatory responses. CD28 homodimer is proven to prevent access of superantigen to CD28 expressed on T cells,392 while TN-domain-specific mAb blocking the harmful interaction between TN and HMGB1 has prevented macrophage pyroptosis and rescued animals from lethal sepsis393 (Table 3 and Fig. 5).
Novel intervention therapeutic strategies in sepsis. In terms of the signaling pathways or molecules targeted, potential therapeutic strategies for sepsis that have been proposed so far could be classified into six categories: (1) targeting DAMPs (including host cell stress), (2) targeting PAMPs, (3) targeting inflammatory mediators (anti-inflammatory), (4) immune checkpoint modulation, (5) endothelial barrier stabilization, and (6) restoration of vascular anticoagulant properties. Nanoparticles therapy is a promising novel strategy that have broad therapeutic effects in sepsis experimental studies. Titles in italic refer to the specific targeted mechanism (strategies). Dot-labeled subtitles refer to the specific therapeutic techniques or bioactive molecules proposed
Biomedical nanoparticles posing specific binding affinity and catalytic property are powerful therapeutic agents that have broad therapeutic effect on sepsis. Decorated or coated with specific ligands, these nanoparticles were designed to have preferential effect on various targeted substrates. Nanobeads coated with human opsonin mannose-binding protein captured pathogen and toxin, which would in turn minimize immune cell infiltration in multiple organs,394 while ceria–zirconia nanoparticles and silica nanoparticles that serve as specific scavengers for ROS and nucleic acid, respectively, were targeted to reduce stress-induced injuries and cfDNA-induced inflammation.395,396 Nanoparticles also serve as an ideal carrier for targeted drug delivery to infectious microenvironments (IME). Co-delivery of antibiotics and anti-inflammatory agent to IME markedly reduce inflammatory responses and improve survival rate397 (Table 3 and Fig. 5).
Future prospectives and conclusion
Immunological pathologies and pathogenesis of sepsis is becoming gradually clear; however, the complete outlook of these mechanisms still could not reach agreement due to the difficulties to identify and clarify clinical significance of observed host responses. This situation could be even more complex as heterogeneous outcomes were observed between individuals or among different host organs. Sepsis remains a huge challenge to intensive care specialist with a lot of unsolved issues. Mutual goal of sepsis management is to early identify and provide rapid intervention, so as to prevent development of irreversible septic shock and multiple organ dysfunction syndrome. However, complexity of this syndrome makes it impossible to develop a precise golden standard for routine use. With breakthroughs in identifying sepsis-specific biomarkers and involved signaling pathways, more precise and accurate diagnostic measures are required to rule out predisposed sepsis patients and to allocate precise therapeutics in advance, before shifting the equilibrium to irreversible detrimental outcomes. Future studies should focus on molecular or biomarker-based therapy. Also, recent studies have proposed the observed heterogeneous host responses to be related to genomic profile between individuals, which is referred to as sepsis endotypes.398 Pronounced alteration of RNA transcripts related to inflammation and mitochondria dysfunction was distinctly observed in sepsis patients and independent of the source and causative pathogens.399,400 Such altered genomic profile was also found in burn injuries, traumatic, and critically ill patients.401 Genetic variation might have determined the leukocyte transcriptome and degree of immunosuppression in sepsis patients.
This review focused on dysregulated/altered signaling pathways and molecules involved with sepsis pathogenesis. Any statements of perspectives were based on information from hitherto described sepsis-related signaling pathways in previous studies. Potential molecular mechanism for sepsis pathogenesis as well as cross-talks between these pathways were also discussed in detail. Proposed outlook of these activated host responses and therapeutic potentials were illustrated for better understanding. Lastly, informative tables were used to compare clinical efficacy of RCTs and valuable studies related to conventional or novel therapeutic strategies targeting on signaling pathways and molecules.
References
Levy, M. M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31, 1250–1256 (2003).
Dellinger, R. P. et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 32, 858–873 (2004).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Goldstein, B., Giroir, B., Randolph, A. & International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8 (2005).
Davis, A. L. et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit. Care Med. 45, 1061–1093 (2017).
Weiss, S. L. et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 21, e52–e106 (2020).
Fleischmann, C. et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 (2016).
Becker, J. U. et al. Surviving sepsis in low-income and middle-income countries: new directions for care and research. Lancet Infect. Dis. 9, 577–582 (2009).
van den Boogaard, W., Manzi, M., Harries, A. D. & Reid, A. J. Causes of pediatric mortality and case-fatality rates in eight Medecins Sans Frontieres-supported hospitals in Africa. Public Health Action 2, 117–121 (2012).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020).
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 6, 223–230 (2018).
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018).
Lukacs, S. L. & Schrag, S. J. Clinical sepsis in neonates and young infants, United States, 1988-2006. J. Pediatr. 160, 960.e1–965.e1 (2012).
Sgro, M. et al. Early-onset neonatal sepsis: rate and organism pattern between 2003 and 2008. J. Perinatol. 31, 794–798 (2011).
Weiss, S. L. et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 191, 1147–1157 (2015).
Weiss, S. L. et al. The epidemiology of hospital death following pediatric severe sepsis: when, why, and how children with sepsis die. Pediatr. Crit. Care Med. 18, 823–830 (2017).
Lehmann-Werman, R. et al. Monitoring liver damage using hepatocyte-specific methylation markers in cell-free circulating DNA. JCI Insight 3, e120687 (2018).
Zemmour, H. et al. Non-invasive detection of human cardiomyocyte death using methylation patterns of circulating DNA. Nat. Commun. 9, 1443 (2018).
Nakahira, K. et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 10, e1001577 (2013).
Yoshida, H. et al. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6, 49–56 (2005).
Laukova, L. et al. Exogenous deoxyribonuclease has a protective effect in a mouse model of sepsis. Biomed. Pharmacother. 93, 8–16 (2017).
Ahn, J. & Barber, G. N. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr. Opin. Immunol. 31, 121–126 (2014).
Kato, Y. et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 77, 1507–1515 (2018).
Banete, A. et al. On taking the STING out of immune activation. J. Leukoc. Biol. https://doi.org/10.1002/JLB.2MIR0917-383R (2018).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Li, N. et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 24, 101215 (2019).
Riedemann, N. C. et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 18, 370–372 (2004).
Wrann, C. D. et al. The phosphatidylinositol 3-kinase signaling pathway exerts protective effects during sepsis by controlling C5a-mediated activation of innate immune functions. J. Immunol. 178, 5940–5948 (2007).
Huber-Lang, M. S. et al. Complement-induced impairment of innate immunity during sepsis. J. Immunol. 169, 3223–3231 (2002).
Riedemann, N. C. et al. Regulation by C5a of neutrophil activation during sepsis. Immunity 19, 193–202 (2003).
Jones, C. N. et al. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS ONE 9, e114509 (2014).
Bermejo-Martin, J. F. et al. Defining immunological dysfunction in sepsis: a requisite tool for precision medicine. J. Infect. 72, 525–536 (2016).
Borkowska, S. et al. Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs). Leukemia 28, 2148–2154 (2014).
Markiewski, M. M. et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 28, 184–192 (2007).
Huber-Lang, M. et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 12, 682–687 (2006).
Lachmann, P. J., Pangburn, M. K. & Oldroyd, R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J. Exp. Med. 156, 205–216 (1982).
Ghebrehiwet, B. et al. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J. Clin. Investig. 71, 1450–1456 (1983).
Wiggins, R. C., Giclas, P. C. & Henson, P. M. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J. Exp. Med. 153, 1391–1404 (1981).
Garlanda, C., Bottazzi, B., Bastone, A. & Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 23, 337–366 (2005).
Abderrazak, A. et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 4, 296–307 (2015).
Zhao, S. et al. Reactive oxygen species interact with NLRP3 inflammasomes and are involved in the inflammation of sepsis: from mechanism to treatment of progression. Front. Physiol. 11, 571810 (2020).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Kowaltowski, A. J., de Souza-Pinto, N. C., Castilho, R. F. & Vercesi, A. E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 47, 333–343 (2009).
Zhou, R. et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
von Moltke, J. et al. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013).
Liu, L. & Sun, B. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol. Life Sci. 76, 2031–2042 (2019).
Esquerdo, K. F. et al. Inflammasome gene profile is modulated in septic patients, with a greater magnitude in non-survivors. Clin. Exp. Immunol. 189, 232–240 (2017).
Arbore, G. & Kemper, C. A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur. J. Immunol. 46, 1563–1573 (2016).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Yazdi, A. S. et al. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc. Natl Acad. Sci. USA 107, 19449–19454 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Piccini, A. et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc. Natl Acad. Sci. USA 105, 8067–8072 (2008).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Munoz-Planillo, R. et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
He, Y. et al. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Walev, I. et al. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 14, 1607–1614 (1995).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Case, C. L. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl Acad. Sci. USA 110, 1851–1856 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Baker, P. J. et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 45, 2918–2926 (2015).
Ruhl, S. & Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 45, 2927–2936 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Chen, X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016).
Yu, J. et al. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl Acad. Sci. USA 111, 15514–15519 (2014).
Gupta, S. C. et al. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal 16, 1295–1322 (2012).
Grijalba, M. T., Vercesi, A. E. & Schreier, S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry 38, 13279–13287 (1999).
Durand, A. et al. Involvement of mitochondrial disorders in septic cardiomyopathy. Oxid. Med. Cell. Longev. 2017, 4076348 (2017).
Larche, J. et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J. Am. Coll. Cardiol. 48, 377–385 (2006).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109, 11282–11287 (2012).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Zhang, Z., Han, N. & Shen, Y. S100A12 promotes inflammation and cell apoptosis in sepsis-induced ARDS via activation of NLRP3 in fl ammasome signaling. Mol. Immunol. 122, 38–48 (2020).
Horng, T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 35, 253–261 (2014).
Silke, J., Rickard, J. A. & Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 16, 689–697 (2015).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 (2017).
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 (2000).
He, S., Liang, Y., Shao, F. & Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl Acad. Sci. USA 108, 20054–20059 (2011).
Kaiser, W. J. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
McComb, S. et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc. Natl Acad. Sci. USA 111, E3206–E3213 (2014).
Thapa, R. J. et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl Acad. Sci. USA 110, E3109–E3118 (2013).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012).
Geserick, P. et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 187, 1037–1054 (2009).
Silke, J. & Brink, R. Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ. 17, 35–45 (2010).
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Dondelinger, Y. et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 20, 1381–1392 (2013).
Wang, B. et al. Necroptosis regulated proteins expression is an early prognostic biomarker in patient with sepsis: a prospective observational study. Oncotarget 8, 84066–84073 (2017).
Sharma, A. et al. Receptor-interacting protein kinase 3 deficiency inhibits immune cell infiltration and attenuates organ injury in sepsis. Crit. Care 18, R142 (2014).
Chen, H. et al. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 27, 2568–2585 (2020).
Bolognese, A. C. et al. Inhibition of necroptosis attenuates lung injury and improves survival in neonatal sepsis. Surgery https://doi.org/10.1016/j.surg.2018.02.017 (2018).
Hansen, L. W. et al. Deficiency of receptor-interacting protein kinase 3 (RIPK3) attenuates inflammation and organ injury in neonatal sepsis. J. Pediatr. Surg. 53, 1699–1705 (2018).
Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011).
Sureshbabu, A. et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 3, e98411 (2018).
Jo, E. K., Yuk, J. M., Shin, D. M. & Sasakawa, C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front. Immunol. 4, 97 (2013).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Tang, D., Kang, R., Zeh, H. J. 3rd & Lotze, M. T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal 14, 1315–1335 (2011).
Yanai, H. et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc. Natl Acad. Sci. USA 110, 20699–20704 (2013).
Tang, D. et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 190, 881–892 (2010).
Sun, Q. et al. Redox-dependent regulation of hepatocyte absent in melanoma 2 inflammasome activation in sterile liver injury in mice. Hepatology 65, 253–268 (2017).
Kang, R. et al. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy 6, 1209–1211 (2010).
Liu, L. et al. HMGB1-DNA complex-induced autophagy limits AIM2 inflammasome activation through RAGE. Biochem. Biophys. Res. Commun. 450, 851–856 (2014).
Zhu, X. et al. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J. Clin. Investig. 125, 1098–1110 (2015).
Sun, Y. et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 138, 2247–2262 (2018).
Sun, Y., Cai, Y. & Zang, QS. Cardiac autophagy in sepsis. Cells 8, 141 (2019).
Mao, K. et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 23, 201–212 (2013).
Kim, M. J., Yoon, J. H. & Ryu, J. H. Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep. 49, 529–535 (2016).
Kang, R. et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24, 97.e4–108.e4 (2018).
Park, Y. J. et al. TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis. PLoS Pathog. 9, e1003646 (2013).
Fortunato, F. et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 137, 350–360 (2009).
Kim, M. J. et al. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 12, 1272–1291 (2016).
Bae, S. H. et al. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 17, 73–84 (2013).
Rhee, S. G. & Bae, S. H. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic. Biol. Med 88, 205–211 (2015).
Yang, L. et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436 (2014).
Peyssonnaux, C. et al. Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J. Immunol. 178, 7516–7519 (2007).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Hsu, P. P. & Sabatini, D. M. Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008).
Muller, H. J. & Boos, J. Use of L-asparaginase in childhood ALL. Crit. Rev. Oncol. Hematol. 28, 97–113 (1998).
Evankovich, J. et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J. Biol. Chem. 285, 39888–39897 (2010).
Xie, M. et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 7, 13280 (2016).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
He, Y., Franchi, L. & Nunez, G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur. J. Immunol. 43, 1147–1152 (2013).
Yim, H. C. et al. The kinase activity of PKR represses inflammasome activity. Cell Res. 26, 367–379 (2016).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131 (2010).
Kang, R. et al. A novel PINK1- and PARK2-dependent protective neuroimmune pathway in lethal sepsis. Autophagy 12, 2374–2385 (2016).
Lin, Q. et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 26, 101254 (2019).
Wang, Y. et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 38, 101767 (2021).
Levi, M. & Ten Cate, H. Disseminated intravascular coagulation. N. Engl. J. Med. 341, 586–592 (1999).
Gando, S. et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit. Care 17, R297 (2013).
Gando, S. et al. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit. Care 17, R111 (2013).
Franco, R. F. et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood 96, 554–559 (2000).
Pawlinski, R. et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116, 806–814 (2010).
Delabranche, X. et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 39, 1695–1703 (2013).
Tang, H. et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am. J. Pathol. 171, 1066–1077 (2007).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA 107, 15880–15885 (2010).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017).
Liaw, P. C. et al. DAMP and DIC: the role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev. 30, 257–261 (2016).
Drake, T. A., Cheng, J., Chang, A. & Taylor, F. B. Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am. J. Pathol. 142, 1458–1470 (1993).
Pawlinski, R. & Mackman, N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb. Res. 125, S70–S73 (2010).
Chen, V. M. Tissue factor de-encryption, thrombus formation, and thiol-disulfide exchange. Semin. Thromb. Hemost. 39, 40–47 (2013).
Kim, M. et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 126, 3511–3525 (2016).
Kumpers, P. et al. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit. Care 13, R64 (2009).
Davis, J. S. et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit. Care 14, R89 (2010).
Korhonen, E. A. et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J. Clin. Investig. 126, 3495–3510 (2016).
Fiedler, U. et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103, 4150–4156 (2004).
Matsushita, K. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139–150 (2003).
Huang, H. et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology 59, 1984–1997 (2014).
Lotze, M. T. & Tracey, K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 (2005).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Sunden-Cullberg, J. et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 33, 564–573 (2005).
Nakamura, T. et al. Suppression of high-mobility group box-1 and receptor for advanced glycation end-product axis by polymyxin B-immobilized fiber hemoperfusion in septic shock patients. J. Crit. Care 26, 546–549 (2011).
Karlsson, S. et al. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 34, 1046–1053 (2008).
Deng, M., Scott, M. J., Fan, J. & Billiar, T. R. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J. Leukoc. Biol. 106, 161–169 (2019).
Qin, S. et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 203, 1637–1642 (2006).
Andersson, U. et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192, 565–570 (2000).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Deng, M. et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 49, 740.e7–753.e7 (2018).
Kaczmarek, A., Vandenabeele, P. & Krysko, D. V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Frank, D. & Vince, J. E. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114 (2019).
Bonaldi, T. et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22, 5551–5560 (2003).
Lu, B. et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl Acad. Sci. USA 111, 3068–3073 (2014).
Kim, J. H. et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J. Immunol. 182, 2458–2466 (2009).
Rendon-Mitchell, B. et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 170, 3890–3897 (2003).
Liu, H. et al. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock 27, 55–60 (2007).
Dhupar, R. et al. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock 35, 293–301 (2011).
Youn, J. H. & Shin, J. S. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J. Immunol. 177, 7889–7897 (2006).
Zhang, X. et al. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J. Immunol. 181, 5015–5023 (2008).
Tsung, A. et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 (2007).
Ma, L., Kim, S. J. & Oh, K. I. Calcium/calmodulin-dependent protein kinase is involved in the release of high mobility group box 1 via the interferon-beta signaling pathway. Immune Netw. 12, 148–154 (2012).
Li, W. et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 11, 229 (2020).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35.e6–44.e6 (2018).
Xu, J. et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 21, 1229–1239 (2014).
Chevriaux, A. et al. Cathepsin B is required for NLRP3 inflammasome activation in macrophages, through NLRP3 interaction. Front. Cell Dev. Biol. 8, 167 (2020).
Chen, L. et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 9, 597 (2018).
Antoine, D. J. et al. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol. Med. 20, 135–137 (2014).
Valdes-Ferrer, S. I. et al. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors. J. Intern. Med. 274, 381–390 (2013).
Schiraldi, M. et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 209, 551–563 (2012).
Venereau, E. et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 209, 1519–1528 (2012).
Yang, H. et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol. Med. 18, 250–259 (2012).
He, M. et al. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol. Med. 24, 21 (2018).
Frank, M. G. et al. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain Behav. Immun. 55, 215–224 (2016).
Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Kazama, H. et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29, 21–32 (2008).
Ekdahl, K. N. & Nilsson, B. Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J. Immunol. 154, 6502–6510 (1995).
Muhlfelder, T. W. et al. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J. Clin. Invest 63, 147–150 (1979).
Platt, J. L. et al. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur. J. Immunol. 21, 2887–2890 (1991).
Tedesco, F. et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J. Exp. Med. 185, 1619–1627 (1997).
Sims, P. J., Faioni, E. M., Wiedmer, T. & Shattil, S. J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 263, 18205–18212 (1988).
Peerschke, E. I., Reid, K. B. & Ghebrehiwet, B. Platelet activation by C1q results in the induction of alpha IIb/beta 3 integrins (GPIIb-IIIa) and the expression of P-selectin and procoagulant activity. J. Exp. Med. 178, 579–587 (1993).
Polley, M. J. & Nachman, R. L. Human platelet activation by C3a and C3a des-arg. J. Exp. Med. 158, 603–615 (1983).
Maekawa, T. et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15, 768–778 (2014).
Tam, J. C., Bidgood, S. R., McEwan, W. A. & James, L. C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070 (2014).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Xu, J. et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 187, 2626–2631 (2011).
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
Allam, R., Darisipudi, M. N., Tschopp, J. & Anders, H. J. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur. J. Immunol. 43, 3336–3342 (2013).
Marsman, G., Zeerleder, S. & Luken, B. M. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis. 7, e2518 (2016).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Longstaff, C. et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 288, 6946–6956 (2013).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7, e32366 (2012).
Xu, J. et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 (2009).
Ekaney, M. L. et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 18, 543 (2014).
Zeerleder, S. et al. Elevated nucleosome levels in systemic inflammation and sepsis. Crit. Care Med. 31, 1947–1951 (2003).
Allam, R. et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 23, 1375–1388 (2012).
Fattahi, F. et al. Harmful roles of TLR3 and TLR9 in cardiac dysfunction developing during polymicrobial sepsis. Biomed. Res. Int. 2018, 4302726 (2018).
Bosmann, M. et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 (2013).
Ibanez-Cabellos, J. S. et al. Extracellular histones activate autophagy and apoptosis via mTOR signaling in human endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3234–3246 (2018).
Abrams, S. T. et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 187, 160–169 (2013).
Huang, H. et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 54, 999–1008 (2011).
Huang, H. et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 191, 2665–2679 (2013).
Gowda, N. M., Wu, X. & Gowda, D. C. The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs. PLoS ONE 6, e20398 (2011).
Parroche, P. et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl Acad. Sci. USA 104, 1919–1924 (2007).
Pisetsky, D. S. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin. Immunol. 144, 32–40 (2012).
Semeraro, F. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118, 1952–1961 (2011).
Muller, F. et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139, 1143–1156 (2009).
Fuchs, T. A., Bhandari, A. A. & Wagner, D. D. Histones induce rapid and profound thrombocytopenia in mice. Blood 118, 3708–3714 (2011).
Versteeg, H. H., Heemskerk, J. W., Levi, M. & Reitsma, P. H. New fundamentals in hemostasis. Physiol. Rev. 93, 327–358 (2013).
de Stoppelaar, S. F. et al. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 124, 3781–3790 (2014).
Vincent, J. L., Yagushi, A. & Pradier, O. Platelet function in sepsis. Crit. Care Med. 30, S313–S317 (2002).
Yin, H. et al. Role for platelet glycoprotein Ib-IX and effects of its inhibition in endotoxemia-induced thrombosis, thrombocytopenia, and mortality. Arterioscler. Thromb. Vasc. Biol. 33, 2529–2537 (2013).
Cleary, S. J. et al. LPS-induced lung platelet recruitment occurs independently from neutrophils, PSGL-1, and P-selectin. Am. J. Respir. Cell Mol. Biol. 61, 232–243 (2019).
Shibazaki, M. et al. Complement-dependent accumulation and degradation of platelets in the lung and liver induced by injection of lipopolysaccharides. Infect. Immun. 67, 5186–5191 (1999).
Sakurai, K. et al. Role for neutrophil extracellular traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo 31, 1051–1058 (2017).
Andonegui, G. et al. Platelets express functional Toll-like receptor-4. Blood 106, 2417–2423 (2005).
Kappelmayer, J. et al. Distinct effects of Re- and S-forms of LPS on modulating platelet activation. J. Thromb. Haemost. 11, 775–778 (2013).
Feng, G. et al. LPS enhances platelets aggregation via TLR4, which is related to mitochondria damage caused by intracellular ROS, but not extracellular ROS. Cell Immunol. 328, 86–92 (2018).
Lopes Pires, M. E., Clarke, S. R., Marcondes, S. & Gibbins, J. M. Lipopolysaccharide potentiates platelet responses via toll-like receptor 4-stimulated Akt-Erk-PLA2 signalling. PLoS ONE 12, e0186981 (2017).
Powers, K. A. et al. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 203, 1951–1961 (2006).
Manukyan, M. C. et al. The phosphoinositide-3 kinase survival signaling mechanism in sepsis. Shock 34, 442–449 (2010).
Fan, J. et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 178, 6573–6580 (2007).
Levy, R. M. et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1538–R1544 (2007).
Vogel, S. et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Investig. 125, 4638–4654 (2015).
Maugeri, N. et al. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity 45, 584–587 (2012).
Vogel, S. et al. Activated platelets interfere with recruitment of mesenchymal stem cells to apoptotic cardiac cells via high mobility group box 1/Toll-like receptor 4-mediated down-regulation of hepatocyte growth factor receptor MET. J. Biol. Chem. 289, 11068–11082 (2014).
Zhang, G. et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 182, 7997–8004 (2009).
Li, Z., Xi, X. & Du, X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J. Biol. Chem. 276, 42226–42232 (2001).
Booth, K. K., Terrell, D. R., Vesely, S. K. & George, J. N. Systemic infections mimicking thrombotic thrombocytopenic purpura. Am. J. Hematol. 86, 743–751 (2011).
Kremer Hovinga, J. A. et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J. Thromb. Haemost. 5, 2284–2290 (2007).
Hatada, T. et al. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb. Haemost. 94, 975–979 (2005).
Yang, X. et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity 51, 983.e6–996.e6 (2019).
Peng, Y. et al. Bacterial outer membrane vesicles induce disseminated intravascular coagulation through the caspase-11-gasdermin D pathway. Thromb. Res. 196, 159–166 (2020).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Yang, X. et al. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood 135, 1087–1100 (2020).
Furlan-Freguia, C. et al. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J. Clin. Investig. 121, 2932–2944 (2011).
Chen, KW. et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3, eaar6676 (2018).
Stark, K. et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128, 2435–2449 (2016).
Higgins, S. J. et al. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J. Clin. Investig. 128, 1471–1484 (2018).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Heipertz, E. L., Harper, J. & Walker, W. E. STING and TRIF contribute to mouse sepsis, depending on severity of the disease model. Shock 47, 621–631 (2017).
Ge, W. et al. LDK378 improves micro- and macro-circulation via alleviating STING-mediated inflammatory injury in a sepsis rat model induced by cecal ligation and puncture. J. Inflamm. 16, 3 (2019).
Hu, Q. et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine 41, 497–508 (2019).
Zhang, H. et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe 27, 556.e6–570.e6 (2020).
Wu, C. et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 50, 1401.e4–1411.e4 (2019).
Rothmeier, A. S. et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J. Clin. Investig. 125, 1471–1484 (2015).
Hofer, S. et al. Thioredoxin in human and experimental sepsis. Crit. Care Med. 37, 2155–2159 (2009).
Chen, G. et al. Thioredoxin-1 increases survival in sepsis by inflammatory response through suppressing endoplasmic reticulum stress. Shock 46, 67–74 (2016).
Leaver, S. K. et al. Increased plasma thioredoxin levels in patients with sepsis: positive association with macrophage migration inhibitory factor. Intensive Care Med. 36, 336–341 (2010).
Shao, R. et al. The expression of thioredoxin-1 and inflammatory cytokines in patients with sepsis. Immunopharmacol. Immunotoxicol. 42, 280–285 (2020).
Falati, S. et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 197, 1585–1598 (2003).
Hrachovinova, I. et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat. Med. 9, 1020–1025 (2003).
von Bruhl, M. L. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 (2012).
Darbousset, R. et al. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 120, 2133–2143 (2012).
Kambas, K. et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J. Immunol. 180, 7368–7375 (2008).
Rafail, S. et al. Leptin induces the expression of functional tissue factor in human neutrophils and peripheral blood mononuclear cells through JAK2-dependent mechanisms and TNFalpha involvement. Thromb. Res. 122, 366–375 (2008).
Kourtzelis, I. et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood 116, 631–639 (2010).
de Waard, V. et al. Differential expression of tissue factor mRNA and protein expression in murine sepsis. The role of the granulocyte revisited. Thromb. Haemost. 95, 348–353 (2006).
Egorina, E. M., Sovershaev, M. A., Olsen, J. O. & Osterud, B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood 111, 1208–1216 (2008).
Ritis, K. et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 177, 4794–4802 (2006).
Caudrillier, A. et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 122, 2661–2671 (2012).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Maugeri, N. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 (2014).
Kambas, K. et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS ONE 7, e45427 (2012).
Fuentes-Prior, P. et al. Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex. Nature 404, 518–525 (2000).
Coughlin, S. R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814 (2005).
David-Dufilho, M. et al. Endothelial thrombomodulin induces Ca2+ signals and nitric oxide synthesis through epidermal growth factor receptor kinase and calmodulin kinase II. J. Biol. Chem. 280, 35999–36006 (2005).
Bajzar, L., Morser, J. & Nesheim, M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J. Biol. Chem. 271, 16603–16608 (1996).
Campbell, W. D., Lazoura, E., Okada, N. & Okada, H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol. Immunol. 46, 131–134 (2002).
Myles, T. et al. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J. Biol. Chem. 278, 51059–51067 (2003).
Conway, E. M. et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J. Exp. Med. 196, 565–577 (2002).
Delvaeye, M. et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 345–357 (2009).
Van de Wouwer, M. et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J. Thromb. Haemost. 4, 1813–1824 (2006).
Ito, T., Kakihana, Y. & Maruyama, I. Thrombomodulin as an intravascular safeguard against inflammatory and thrombotic diseases. Expert Opin. Ther. Targets 20, 151–158 (2016).
Shi, C. S. et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood 112, 3661–3670 (2008).
Ito, T. et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler. Thromb. Vasc. Biol. 28, 1825–1830 (2008).
Nagato, M. et al. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit. Care Med. 37, 2181–2186 (2009).
Esmon, C. T. The regulation of natural anticoagulant pathways. Science 235, 1348–1352 (1987).
Dahlback, B. & Villoutreix, B. O. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler. Thromb. Vasc. Biol. 25, 1311–1320 (2005).
Stearns-Kurosawa, D. J. et al. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc. Natl Acad. Sci. USA 93, 10212–10216 (1996).
Joyce, D. E. & Grinnell, B. W. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit. Care Med. 30, S288–S293 (2002).
White, B. et al. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br. J. Haematol. 110, 130–134 (2000).
Faust, S. N. et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N. Engl. J. Med. 345, 408–416 (2001).
Gando, S. et al. Serial changes in neutrophil-endothelial activation markers during the course of sepsis associated with disseminated intravascular coagulation. Thromb. Res. 116, 91–100 (2005).
Borgel, D. et al. A comparative study of the protein C pathway in septic and nonseptic patients with organ failure. Am. J. Respir. Crit. Care Med. 176, 878–885 (2007).
Guitton, C. et al. Early rise in circulating endothelial protein C receptor correlates with poor outcome in severe sepsis. Intensive Care Med. 37, 950–956 (2011).
Gu, J. M. et al. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood 95, 1687–1693 (2000).
Gleeson, E. M., O’Donnell, J. S. & Preston, R. J. The endothelial cell protein C receptor: cell surface conductor of cytoprotective coagulation factor signaling. Cell Mol. Life Sci. 69, 717–726 (2012).
Menschikowski, M., Hagelgans, A., Eisenhofer, G. & Siegert, G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp. Cell Res. 315, 2673–2682 (2009).
Kerschen, E. J. et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J. Exp. Med. 204, 2439–2448 (2007).
Adams, T. E. & Huntington, J. A. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler. Thromb. Vasc. Biol. 26, 1738–1745 (2006).
Okajima, K. Regulation of inflammatory responses by activated protein C: the molecular mechanism(s) and therapeutic implications. Clin. Chem. Lab. Med. 42, 132–141 (2004).
Bernard, G. R. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 (2001).
Feistritzer, C. & Riewald, M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105, 3178–3184 (2005).
Bae, J. S., Yang, L., Manithody, C. & Rezaie, A. R. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood 110, 3909–3916 (2007).
Bae, J. S. & Rezaie, A. R. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb. Haemost. 100, 101–109 (2008).
Goldenberg, N. M., Steinberg, B. E., Slutsky, A. S. & Lee, W. L. Broken barriers: a new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25 (2011).
Finigan, J. H. et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 280, 17286–17293 (2005).
Bae, J. S. & Rezaie, A. R. Thrombin upregulates the angiopoietin-Tie2 Axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. J. Thromb. Haemost. 8, 1107–1115 (2010).
Minhas, N., Xue, M., Fukudome, K. & Jackson, C. J. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 24, 873–881 (2010).
Bae, J. S., Kim, Y. U., Park, M. K. & Rezaie, A. R. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J. Cell Physiol. 219, 744–751 (2009).
Garcia de Frutos, P. et al. Differential regulation of alpha and beta chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood 84, 815–822 (1994).
Taylor, F. et al. C4b-binding protein exacerbates the host response to Escherichia coli. Blood 78, 357–363 (1991).
Taylor, F. B. Jr. et al. Role of free protein S and C4b binding protein in regulating the coagulant response to Escherichia coli. Blood 86, 2642–2652 (1995).
Ammollo, C. T. et al. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 9, 1795–1803 (2011).
Osada, K. et al. Thrombomodulin alfa attenuates the procoagulant effect and cytotoxicity of extracellular histones through the promotion of protein C activation. Thromb. Res. 160, 51–57 (2017).
Winkler, M. S. et al. Sphingosine-1-Phosphate: A Potential Biomarker And Therapeutic Target For Endothelial Dysfunction And Sepsis? Shock 47, 666–672 (2017).
Garcia, J. G. et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 108, 689–701 (2001).
Mehta, D., Konstantoulaki, M., Ahmmed, G. U. & Malik, A. B. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J. Biol. Chem. 280, 17320–17328 (2005).
Camerer, E. et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 119, 1871–1879 (2009).
Klarenbach, S. W. et al. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ. Res. 92, 272–278 (2003).
Lufrano, M., Jacob, A., Zhou, M. & Wang, P. Sphingosine kinase1 mediates endotoxemiainduced hyperinflammation in aged animals. Mol. Med. Rep. 8, 645–649 (2013).
Zhang, T. et al. Apigenin attenuates heart injury in lipopolysaccharide-induced endotoxemic model by suppressing sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Chem. Biol. Interact. 233, 46–55 (2015).
Luheshi, N. M., Giles, J. A., Lopez-Castejon, G. & Brough, D. Sphingosine regulates the NLRP3-inflammasome and IL-1beta release from macrophages. Eur. J. Immunol. 42, 716–725 (2012).
Zhong, M. et al. Inhibition of sphingosine kinase 1 attenuates sepsis-induced microvascular leakage via inhibiting macrophage NLRP3 inflammasome activation in mice. Anesthesiology 132, 1503–1515 (2020).
Tian, T. et al. Sphingosine kinase 1 regulates HMGB1 translocation by directly interacting with calcium/calmodulin protein kinase II-delta in sepsis-associated liver injury. Cell Death Dis. 11, 1037 (2020).
Shea, B. S. et al. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 43, 662–673 (2010).
Frej, C. et al. Sphingosine 1-phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. J. Cell Mol. Med. 20, 1170–1181 (2016).
Nicholson, J. P., Wolmarans, M. R. & Park, G. R. The role of albumin in critical illness. Br. J. Anaesth. 85, 599–610 (2000).
Morin, E. E., Guo, L., Schwendeman, A. & Li, X. A. HDL in sepsis - risk factor and therapeutic approach. Front. Pharmacol. 6, 244 (2015).
Kumaraswamy, S. B., Linder, A., Akesson, P. & Dahlback, B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit. Care 16, R60 (2012).
Winkler, M. S. et al. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care 19, 372 (2015).
Joyce, D. E. et al. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 276, 11199–11203 (2001).
Mosnier, L. O. & Griffin, J. H. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 373, 65–70 (2003).
Cheng, T. et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 9, 338–342 (2003).
Wong, R. C. et al. Anti-apoptotic effect of sphingosine-1-phosphate and platelet-derived growth factor in human embryonic stem cells. Stem Cells Dev. 16, 989–1001 (2007).
Ruiz, M., Okada, H. & Dahlback, B. HDL-associated ApoM is anti-apoptotic by delivering sphingosine 1-phosphate to S1P1 & S1P3 receptors on vascular endothelium. Lipids Health Dis. 16, 36 (2017).
Mohan Rao, L. V., Esmon, C. T. & Pendurthi, U. R. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood 124, 1553–1562 (2014).
Saukkonen, K. et al. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin. Chem. 54, 1000–1007 (2008).
Rhodes, A. & Cecconi, M. Cell-free DNA and outcome in sepsis. Crit. Care 16, 170 (2012).
Zeng, L. et al. ALK is a therapeutic target for lethal sepsis. Sci. Transl. Med. 9, eaan5689 (2017).
Mathias, B. et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann. Surg. 265, 827–834 (2017).
Wu, J. et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 216, 867–883 (2019).
Mandriota, S. J. & Pepper, M. S. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ. Res. 83, 852–859 (1998).
Scharpfenecker, M., Fiedler, U., Reiss, Y. & Augustin, H. G. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 118, 771–780 (2005).
Menden, H. et al. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells. J. Biol. Chem. 290, 5449–5461 (2015).
Kim, K. T. et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J. Biol. Chem. 280, 20126–20131 (2005).
Daly, C. et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev. 18, 1060–1071 (2004).
Sako, K. et al. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J. Biol. Chem. 284, 5592–5601 (2009).
Thibeault, S. et al. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol. Cell 39, 468–476 (2010).
Gavard, J. & Gutkind, J. S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223–1234 (2006).
Findley, C. M., Cudmore, M. J., Ahmed, A. & Kontos, C. D. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler. Thromb. Vasc. Biol. 27, 2619–2626 (2007).
van der Heijden, M., van Nieuw Amerongen, G. P., van Hinsbergh, V. W. & Groeneveld, A. B. The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 33, 263–268 (2010).
Winderlich, M. et al. VE-PTP controls blood vessel development by balancing Tie-2 activity. J. Cell Biol. 185, 657–671 (2009).
Kurniati, N. F. et al. The flow dependency of Tie2 expression in endotoxemia. Intensive Care Med. 39, 1262–1271 (2013).
Kumpers, P. et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit. Care 15, R261 (2011).
Davis, S. et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat. Struct. Biol. 10, 38–44 (2003).
Serghides, L. et al. PPARgamma agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog. 10, e1003980 (2014).
Han, S. et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci. Transl. Med. 8, 335ra355 (2016).
Alfieri, A. et al. Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Crit. Care 16, R182 (2012).
Kim, D. H. et al. COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int. 76, 1180–1191 (2009).
Frye, M. et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 212, 2267–2287 (2015).
Bregeon, J., Loirand, G., Pacaud, P. & Rolli-Derkinderen, M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 297, C1062–C1070 (2009).
Dorland, Y. L. & Huveneers, S. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol. Life Sci. 74, 279–292 (2017).
Marcos-Ramiro, B., Garcia-Weber, D. & Millan, J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb. Haemost. 112, 1088–1102 (2014).
Cinel, I. et al. Involvement of Rho kinase (ROCK) in sepsis-induced acute lung injury. J. Thorac. Dis. 4, 30–39 (2012).
Garcia-Ponce, A. et al. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 113, 20–36 (2015).
Ankawi, G. et al. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit. Care 22, 262 (2018).
Honore, P. M. et al. Newly designed CRRT membranes for sepsis and SIRS-a pragmatic approach for bedside intensivists summarizing the more recent advances: a systematic structured review. ASAIO J. 59, 99–106 (2013).
Livigni, S. et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open 4, e003536 (2014).
Nakamura, Y. et al. Potential survival benefit of polymyxin B hemoperfusion in patients with septic shock: a propensity-matched cohort study. Crit. Care 21, 134 (2017).
Alharthy, A. et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: a case-series. Artif. Organs 45, E101–E112 (2021).
Hawchar, F. et al. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J. Crit. Care 49, 172–178 (2019).
Ramachandran, G. et al. CD28 homodimer interface mimetic peptide acts as a preventive and therapeutic agent in models of severe bacterial sepsis and gram-negative bacterial peritonitis. J. Infect. Dis. 211, 995–1003 (2015).
Chen, W. et al. Identification of tetranectin-targeting monoclonal antibodies to treat potentially lethal sepsis. Sci. Transl. Med 12, eaaz3833 (2020).
Kang, J. H. et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat. Med. 20, 1211–1216 (2014).
Soh, M. et al. Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 56, 11399–11403 (2017).
Dawulieti, J. et al. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci. Adv. 6, eaay7148 (2020).
Zhang, C. Y., Gao, J. & Wang, Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 30, e1803618 (2018).
Davenport, E. E. et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 4, 259–271 (2016).
van Vught, L. A. et al. Comparative analysis of the host response to community-acquired and hospital-acquired pneumonia in critically ill patients. Am. J. Respir. Crit. Care Med. 194, 1366–1374 (2016).
Burnham, K. L. et al. Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am. J. Respir. Crit. Care Med. 196, 328–339 (2017).
Xiao, W. et al. A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 (2011).
Fisher, C. J. Jr et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 (1994).
Meyer, N. J. et al. Mortality benefit of recombinant human interleukin-1 receptor antagonist for sepsis varies by initial interleukin-1 receptor antagonist plasma concentration. Crit. Care Med. 46, 21–28 (2018).
Opal, S. M. et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 25, 1115–1124 (1997).
Shakoory, B. et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44, 275–281 (2016).
Francois, B. et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3, e98960 (2018).
Fisher, C. J. Jr et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 334, 1697–1702 (1996).
Abraham, E. et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 29, 503–510 (2001).
Opal, S. M. et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309, 1154–1162 (2013).
Bernard, G. R. et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-alpha fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study. Crit. Care Med. 42, 504–511 (2014).
Vincent, J. L. et al. Phase II multicenter clinical study of the platelet-activating factor receptor antagonist BB-882 in the treatment of sepsis. Crit. Care Med. 28, 638–642 (2000).
Poeze, M. et al. Decreased organ failure in patients with severe SIRS and septic shock treated with the platelet-activating factor antagonist TCV-309: a prospective, multicenter, double-blind, randomized phase II trial. TCV-309 Septic Shock Study Group. Shock 14, 421–428 (2000).
Schuster, D. P. et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit. Care Med. 31, 1612–1619 (2003).
Opal, S. et al. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit. Care Med. 32, 332–341 (2004).
Rice, T. W. et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 38, 1685–1694 (2010).
Tidswell, M. et al. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit. Care Med. 38, 72–83 (2010).
Watanabe, E. et al. Pharmacokinetics, pharmacodynamics, and safety of nivolumab in patients with sepsis-induced immunosuppression: a multicenter, open-label phase 1/2 study. Shock 53, 686–694 (2020).
Dhainaut, J. F. et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J. Thromb. Haemost. 2, 1924–1933 (2004).
Vincent, J. L. et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit. Care Med. 33, 2266–2277 (2005).
Abraham, E. et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 353, 1332–1341 (2005).
Levi, M. et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am. J. Respir. Crit. Care Med. 176, 483–490 (2007).
Nadel, S. et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369, 836–843 (2007).
Annane, D. et al. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 187, 1091–1097 (2013).
Ranieri, V. M. et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 366, 2055–2064 (2012).
Marti-Carvajal, A. J. et al. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst. Rev. 12, CD004388 (2012).
Abraham, E. et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 290, 238–247 (2003).
Wunderink, R. G. et al. Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. Am. J. Respir. Crit. Care Med. 183, 1561–1568 (2011).
Saito, H. et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 5, 31–41 (2007).
Aikawa, N. et al. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock 35, 349–354 (2011).
Vincent, J. L. et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit. Care Med. 41, 2069–2079 (2013).
Hagiwara, A. et al. Can recombinant human thrombomodulin increase survival among patients with severe septic-induced disseminated intravascular coagulation: a single-centre, open-label, randomised controlled trial. BMJ Open 6, e012850 (2016).
Vincent, J. L. et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA 321, 1993–2002 (2019).
Yamakawa, K., Murao, S. & Aihara, M. Recombinant human soluble thrombomodulin in sepsis-induced coagulopathy: an updated systematic review and meta-analysis. Thromb. Haemost. 119, 56–65 (2019).
Levi, M. et al. Effect of a recombinant human soluble thrombomodulin on baseline coagulation biomarker levels and mortality outcome in patients with sepsis-associated coagulopathy. Crit. Care Med. 48, 1140–1147 (2020).
Warren, B. L. et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286, 1869–1878 (2001).
Kienast, J. et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 4, 90–97 (2006).
Iba, T. et al. Efficacy and bleeding risk of antithrombin supplementation in patients with septic disseminated intravascular coagulation: a third survey. Clin. Appl. Thromb. Hemost. 23, 422–428 (2017).
Allingstrup, M. et al. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 42, 505–520 (2016).
Wiedermann, C. J. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: meta-analyses revisited. J. Thromb. Haemost. 16, 455–457 (2018).
Jaimes, F. et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (The HETRASE Study). Crit. Care Med. 37, 1185–1196 (2009).
Umemura, Y. et al. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J. Thromb. Haemost. 14, 518–530 (2016).
Yamakawa, K. et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit. Care 20, 229 (2016).
Yatabe, T. et al. The anticoagulant treatment for sepsis induced disseminated intravascular coagulation; network meta-analysis. Thromb. Res. 171, 136–142 (2018).
Liu, X. et al. Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment. Br. J. Pharmacol. 162, 1274–1290 (2011).
Wang, Q. et al. Xuebijing ameliorates sepsis-induced lung injury by downregulating HMGB1 and RAGE expressions in mice. Evid. Based Complement. Altern. Med. 2015, 860259 (2015).
Arad, G. et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 9, e1001149 (2011).
Bulger, E. M. et al. A novel immune modulator for patients with necrotizing soft tissue infections (NSTI): results of a multicenter, phase 3 randomized controlled trial of reltecimod (AB 103). Ann. Surg. 272, 469–478 (2020).
Spence, S. et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 7, 303ra140 (2015).
Geven, C. et al. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2). BMJ Open 9, e024475 (2019).
Pickkers, P. et al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 320, 1998–2009 (2018).
Cornejo, R. et al. High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med. 32, 713–722 (2006).
Network, V. N. A. R. F. T. et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359, 7–20 (2008).
Joannes-Boyau, O. et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 39, 1535–1546 (2013).
Clark, E. et al. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit. Care 18, R7 (2014).
Borthwick, E. M. et al. High-volume haemofiltration for sepsis in adults. Cochrane Database Syst. Rev. 1, CD008075 (2017).
Payen, D. et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit. Care Med. 37, 803–810 (2009).
Morgera, S. et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit. Care Med. 34, 2099–2104 (2006).
Haase, M. et al. Hemodialysis membrane with a high-molecular-weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am. J. Kidney Dis. 50, 296–304 (2007).
Chelazzi, C. et al. Hemodialysis with high cut-off hemodialyzers in patients with multi-drug resistant Gram-negative sepsis and acute kidney injury: a retrospective, case-control study. Blood Purif. 42, 186–193 (2016).
Cruz, D. N. et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 301, 2445–2452 (2009).
Payen, D. M. et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 41, 975–984 (2015).
Dellinger, R. P. et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA 320, 1455–1463 (2018).
Klein, D. J. et al. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 44, 2205–2212 (2018).
Fujii, T. et al. Polymyxin B-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 44, 167–178 (2018).
Yamato, M. et al. Effective combination therapy of polymyxin-B direct hemoperfusion and recombinant thrombomodulin for septic shock accompanied by disseminated intravascular coagulation: a historical controlled trial. Ther. Apher. Dial. 17, 472–476 (2013).
Mochizuki, K. et al. Beneficial effect modification on survival outcome of sepsis between ART-123 and polymyxin Bimmobilised haemoperfusion: a nationwide Japanese registry study. Ann. Intensive Care 10, 57 (2020).
Nakada, T. A. et al. Continuous hemodiafiltration with PMMA Hemofilter in the treatment of patients with septic shock. Mol. Med. 14, 257–263 (2008).
Matsuda, K. et al. Comparison of efficacy between continuous hemodiafiltration with a PMMA high-performance membrane dialyzer and a PAN membrane hemofilter in the treatment of septic shock patients with acute renal failure. Contrib. Nephrol. 173, 182–190 (2011).
Shiga, H. et al. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter in patients with septic shock: a preliminary report. Blood Purif. 38, 211–218 (2014).
Doi, K., Iwagami, M., Yoshida, E. & Marshall, M. R. Associations of polyethylenimine-coated AN69ST membrane in continuous renal replacement therapy with the intensive care outcomes: observations from a claims database from Japan. Blood Purif. 44, 184–192 (2017).
Broman, M. E., Hansson, F., Vincent, J. L. & Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS ONE 14, e0220444 (2019).
Schwindenhammer, V. et al. oXiris(R) use in septic shock: experience of two French centres. Blood Purif. 47, 1–7 (2019).
Hazzard, I., Jones, S. & Quinn, T. Coupled plasma haemofiltration filtration in severe sepsis: systematic review and meta-analysis. J. R. Army Med. Corps 161, i17–i22 (2015).
Mariano, F. et al. Coupled-plasma filtration and adsorption for severe burn patients with septic shock and acute kidney injury treated with renal replacement therapy. Burns 46, 190–198 (2020).
Friesecke, S. et al. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J. Artif. Organs 20, 252–259 (2017).
Schadler, D. et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS ONE 12, e0187015 (2017).
Paul, R. et al. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb((R))) in patients with sepsis and septic shock. World J. Crit. Care Med. 10, 22–34 (2021).
Acknowledgements
This study was supported by grants from the Shanghai Natural Science Foundation of China (grant No. 19ZR1432900) and Shanghai Translational Medicine Collaborative Innovation Center (grant No. TM202012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Yy., Ning, Bt. Signaling pathways and intervention therapies in sepsis. Sig Transduct Target Ther 6, 407 (2021). https://doi.org/10.1038/s41392-021-00816-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-021-00816-9
- Springer Nature Limited
This article is cited by
-
Activation of senescence in critically ill patients: mechanisms, consequences and therapeutic opportunities
Annals of Intensive Care (2024)
-
Mitochondrial quality control in human health and disease
Military Medical Research (2024)
-
CD5L as a promising biological therapeutic for treating sepsis
Nature Communications (2024)
-
A synthetic bioactive peptide of the C-terminal fragment of adhesion protein Fibulin7 attenuates the inflammatory functions of innate immune cells in LPS-induced systemic inflammation
Inflammation Research (2024)
-
MicroRNA-483-3p Inhibitor Ameliorates Sepsis-Induced Intestinal Injury by Attenuating Cell Apoptosis and Cytotoxicity Via Regulating HIPK2
Molecular Biotechnology (2024)