Abstract
Climate change, driven by human activities and natural processes, has led to critical alterations in varying patterns during cropping seasons and is a vital threat to global food security. The climate change impose several abiotic stresses on crop production systems. These abiotic stresses include extreme temperatures, drought, and salinity, which expose agricultural fields to more vulnerable conditions and lead to substantial crop yield and quality losses. Plant hormones, especially salicylic acid (SA), has crucial roles for plant resiliency under unfavorable environments. This review explores the genetics and molecular mechanisms underlying SA's role in mitigating abiotic stress-induced damage in plants. It also explores the SA biosynthesis pathways, and highlights the regulation of their products under several abiotic stresses. Various roles and possible modes of action of SA in mitigating abiotic stresses are discussed, along with unraveling the genetic mechanisms and genes involved in responses under stress conditions. Additionally, this review investigates molecular pathways and mechanisms through which SA exerts its protective effects, such as redox signaling, cross-talks with other plant hormones, and mitogen-activated protein kinase pathways. Moreover, the review discusses potentials of using genetic engineering approaches, such as CRISPR technology, for deciphering the roles of SA in enhancing plant resilience to climate change related abiotic stresses. This comprehensive analysis bridges the gap between genetics of SA role in response to climate change related stressors. Overall goal is to highlight SA's significance in safeguarding plants and by offering insights of SA hormone for sustainable agriculture under challenging environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change refers to long-term alterations in weather patterns, predominantly attributed to the emission greenhouse gases originating from either natural processes or human activities (Mikhaylov et al. 2020; Fawzy et al. 2020; Skendžić et al. 2021; Shahid et al. 2021). Climate change events are considered the main threat to food security on a worldwide scale. Crop yields have decreased in many regions around the world as a consequence of climate change in the forms of increased drought and flooding events as well as extreme heat and cold waves (Agesa et al. 2019; Manabe 2019; Abdelrahman et al. 2021a)(Agesa et al. 2019; Manabe 2019; Abdelrahman et al. 2021a). It is predicted to influence total crop production by 9% and 23% in 2030s and 2050s, respectively, with huge differences across countries and crops (Haile et al. 2017. Recent estimates from the climate change predict increased water deficits and flash drought events by erratic rainfalls in the coming years (Wassmann et al. 2009; Mehana et al. 2021). Furthermore, drought intensity and frequencies are expected to increase (Haile et al. 2020). In 2022, Texas and Louisiana rice farmers in USA struggled with strong drought and intense heat issues and as a result some fields were left abandoned because farmers couldn’t keep water on their rice crop. This scenario may continue to happen as drought take serious hold during summer and Colorado river reservoirs have been depleted. According to adequate water risk atlas of the world resources institute (https://www.wri.org/insights/highest-water-stressed-countries), several more countries are already suffering from acute water scarcity such as Mexico, Pakistan, South Africa, and large parts of China and India as do most of the Near Middle East and North Africa countries.
Heat, cold, and drought are considered as the main abiotic stresses driven by climate change. Other abiotic stresses that happen as a consequence of the main climate change stressors includes increased soil salinity. These stressors are increasingly affecting crop productivity and threatening global food security with an estimated crop yield loss of 42% for the eight most frequently produced crops worldwide (Fahad et al. 2017; Wani et al. 2017). These stressors are continually affecting plants, and if they are neglected, they can trigger slow development and growth or even death, which could result in significant crop loss (Nazar et al. 2017; Wani et al. 2017). The evolving climate brings about daily environmental fluctuations that notably affect the molecular, cellular, physiological, and morphological aspects of plant vegetative growth which have profound implications for the sustainability of agriculture(Mehana et al. 2021; Baidya et al. 2022). Furthermore, it captivates global temperature rising, warming the ocean, decreasing snow covered area and rising of sea level. Due to this global warming, the heat waves is increasing in magnitude and occurrence frequency. As a consequence, it increases those abiotic stresses on a world scale, especially with the unevenness and unpredictable manner (Eckardt et al. 2023). Plants evoke different plant hormones as chemical messengers that act as signals for activating mechanisms that help the plants to survive during the stress events. (Raza et al. 2019; Malhi et al. 2021; Imran et al. 2021). Plant hormones, known as phytohormones, promote plant growth, and enable plants to perceive and respond to unfavorable environmental stimuli. Basically, those plant hormones modulate physiological and molecular responses of plants for its survival under abiotic stresses. Moreover, they function either at their site of production or elsewhere in plants based on their transport (Kundu et al. 2022). Among these plant hormones, cytokinin (CKs), abscisic acid (ABA), ethylene (ET), jasmonic acid (JA) and salicylic acid (SA) have been recognized to play a critical role in response to abiotic stresses (Zhao et al. 2021a; Mukherjee et al. 2022; EL Sabagh et al. 2022). SA reported to be involved in different defense responses in addition to its importance in regulation of plant growth and development as well as responses to abiotic stresses. Salicylic acid (SA, C7H6O3) is an endogenous phytohormone that has caught researcher's attention in recent years. It is 2-hydroxy benzoic acid, a phenolic derivative that was originally considered an insignificant byproduct named after the Latin name of the willow tree (Salix). It was found to be an important endogenously produced signaling molecule capable of defending plants against both biotic and abiotic stressors. SA has been linked to a variety of functions, including plant development, growth, and stress responses (Ahmad et al. 2019; Saleem et al. 2021; Khan et al. 2022; Boamah et al. 2023; Yang et al. 2023). Several internal physiological activities, such as photosynthesis, nitrogen metabolism, proline (Pro) metabolism, and glycine betaine (GB) production, are regulated by SA (Khan et al. 2015; Shemi et al. 2021). Furthermore, it involves complex signaling pathways that activate defense-related genes and proteins that lead to multiple events that respond to and resist stress (Kumar 2014).
SA is synthesized through phenylalanine ammonia-lyase (PAL) and isochorismate (ICS) pathways. Both pathways derive from chorismic acid, a byproduct of the shikimate pathway (Lefevere et al. 2020; Lone et al. 2020; Li et al. 2022a). The ICS pathway operates in stressed chloroplasts and involves chorismate conversion to isochorismate by isochorismate synthase 1 (ICS1), forming SA through amino acid conjugation. While the PAL pathway, is using phenylalanine, produces smaller SA amounts. The ICS pathway involves PAL and AIM1 are employed in this pathway to convert phenylalanine to SA via trans-cinnamic acid (tCA) and benzoic acid (BA) (Murphy et al. 2020; Sharma et al. 2020; Tyagi et al. 2022).
Climate-induced stresses regulate SA biosynthesis enzymes in stressed plants to act as a signaling molecule (Yang et al. 2023). SA mediates plant stress responses by regulating genes, enzymes, and molecules. SA receptors, nonexpresser of pathogenesis-related genes, NPR1 and NPR3/NPR4 activate or repress defense gene expression. NPR1 interacts with SA, influencing gene transcription and defense responses (Maier et al. 2011; Backer et al. 2019; Liu et al. 2020b). Although its mechanism is not fully understood, SA is a key regulator of physiological and biochemical processes throughout the plant's life cycle. It is essential for the regulation of many important plant processes, from photosynthesis to senescence (Zhang et al. 2023). For that reason, SA may be regarded as a ‘green guardian’ to plants, serving to protect plants from abiotic or biotic stressors by inducing their resistance and alleviating the effects of the stress, as well as regulating vital plant processes.
In recent years, significant discoveries have shed light on the importance of SA in enhancing plant resistance to biotic and climate change driven abiotic stresses. The role of SA in resistance to pathogen stresses has been extensively reviewed elsewhere (Benjamin et al. 2022). Several studies have been conducted on the biosynthesis pathway of SA in plants and its importance to abiotic stress resistance. However, some gaps are not yet fully understood in the mechanisms of SA in controlling abiotic tolerance in plants under the different scenarios of climate change. This review aims to emphasize the role of SA in abiotic stress resistance. We also discuss possible mechanisms by which SA employs its role in mitigating abiotic stresses. Further, we explored updates on the effect of SA on the different abiotic stresses in plants. This review article collects and describes recent published information to better understand the role of the SA crosstalk’s with other hormone signal system in plant stress resilience.
Biosynthesis of SA in plants
Plants biosynthesize SA through two distinct pathways: the isochorismate (ICS) pathway and the phenylalanine ammonia-lyase (PAL) pathway (Fig. 1A). The ICS pathway takes place in the plastids while the PAL pathway takes place in the cytosol. Both pathways rely on chorismate, which is a primary metabolite and the end-product of the shikimate pathway (Hao et al. 2018; Mishra and Baek 2021; Zhang et al. 2021b). It is produced within the plastid, specifically in the chloroplast, through a seven-step enzymatic process of the shikimate pathway. This compound is synthesized from the combination of phosphoenolpyruvate (PEP) and erythrose-4-phosphate (E4P) (Ding and Ding 2020). The ICS pathway is specific to Brassicaceae family genome such as in Arabidopsis, whereas the PAL pathway is more specific in many other trees and crops such as rice (Yuan et al. 2009; Holland et al. 2019; Torrens-Spence et al. 2019; Wu et al. 2023). It is also possible, as in the case of soybeans, for both pathways to contribute equally. Additionally, there may even be variations in the regulation of SA production within a single plant (Lefevere et al. 2020). However, not all SA biosynthesis-involved enzymes have been identified in plants.
ICS Pathway regulating SA biosynthesis
Genetic analysis in conjunction with biochemical techniques has paved the way for identifying and understanding the distinct stages involved in the biosynthesis of SA. Three main genes, enhanced disease resistance5 EDS5, ICS1, and PBS3 were recognized as encoding three enzymes that establish the fundamentals of the ICS pathway of SA biosynthesis. Chloroplastic AtICS1 (SA induction deficient 2, SID2) was identified by targeting plants with SA induction deficiency (Wildermuth et al. 2001). ICS1 and its closely related homolog ICS2 share homology with PchA, an isochorismate synthase (ICS) pivotal for the initial SA biosynthesis step in Pseudomonas aeruginosa. Both ICS1 and ICS2 play essential roles in SA biosynthesis, localizing within chloroplasts and catalyzing chorismate to isochorismate (IC) conversion in most plants (Wildermuth et al. 2001; Garcion et al. 2008a). While the OsICS gene's role in SA biosynthesis is less established, and alternative pathways might contribute to SA production (Table 1).
The pbs3 mutant Arabidopsis plants displayed low SA, SAG levels and increased susceptibility to avirulent Pseudomonas strains and virulent P. syringae strains, with PBS3 identified as a glycoside hydrolase 3 (GH3) protein family member and later confirmed as an isochorismate lyase (IPL) involved in SA biosynthesis (Li et al. 2023b). This function differs from bacterial PchB, with PBS3 exhibiting interaction with SA. PBS3 as an isochorismoyl-glutamate synthase (IGS), catalyzing IC adenylation and conjugation with Glu to yield isochorismoyl-9-glutamate (IC-9-Glu), which breaks down into SA and other compounds (Rekhter et al. 2019). Additionally, enhanced pseudomonas susceptibility 1 (EPS1), an enzyme enhancing SA production, was identified as an IC-9-Glu pyruvoyl-glutamate lyase (IPGL), indicating a distinctive SA biosynthesis pathway in plants like Arabidopsis compared to bacteria and other organisms (Chen et al. 2020).
In the bacterial system, the ICS pathway starts by converting chorismate to IC by the enzyme ICS. In Pseudomonas species, the PmsCEAB gene cluster, particularly the PmsC and PmsB genes, is vital for this pathway. PmsC shares sequence similarities with E. coli's ICS and catalyzes chorismate to IC conversion. PmsB encodes an IPL responsible for IC to SA conversion in a two-step process (Mercado-Blanco et al. 2001; Cosima et al. 2003). Interestingly, the number of ICS homologs in plant genomes is limited, with the primary enzymatic steps occurring in the chloroplast. Recent research has demonstrated variations in the SA synthesis pathway in Arabidopsis compared to bacteria. This involves amino acid conjugation of IC, catalyzed by the PBS3 gene, leading to the formation of SA. The ICS pathway significantly contributes to SA accumulation in Arabidopsis upon pathogen attack (Yang et al. 2015).
SA has two inactive vacuolar storage forms, SA-β-D-Glucose Ester (SGE) and SA-2-O-β-D-glucoside (SAG) The accumulation of SAG and SGE in vacuoles is substantial, and these forms can be converted into active usable forms through hydrolysis (Dean and Delaney 2008; Allasia et al. 2018; Zeier 2021).In response to pathogen attack, the overall level of SA (including SA + SAG/SGE) significantly rises, triggering the activation of the systemic acquired resistance (SAR)-dependent defense pathway. Furthermore, methylation of SA leads to the production of a volatile variant called methyl salicylate (MeSA), which can be produced by esterification of SA leading to increased membrane permeability (Hayat et al. 2010 ; Wani et al. 2017). MeSA is stored in the cell until a large amount of SA is needed, thus acting as an inactive precursor to SA (Hayat et al. 2010 ; Saleem et al. 2020).
PAL Pathway regulating SA biosynthesis
In the PAL pathway, the synthesis of SA from phenylalanine (Phe) has been extensively explored. Several experiments demonstrated that plants can convert Phe into trans-cinnamic acid (tCA), a precursor for various phenolic compounds (Saunders and Olechno 1988; Rasmussen and Dixon 1999). This conversion is catalyzed by the enzyme phenylalanine ammonia-lyase (PAL), the initial enzyme in the phenylpropanoid pathway. Arabidopsis genome has four PAL genes, each encoding distinct enzyme variants (Huang et al. 2010b). PAL's role in SA production was confirmed through experiments reporting increased PAL expression accompanied by elevated SA levels during pathogen resistance. Suppression of PAL activity resulted in reduced pathogen-induced SA accumulation. Investigating the steps from tCA to BA revealed the possibility of SA synthesis through cinnamoyl-CoA β-oxidation, particularly in tobacco (Chong et al. 2001). Arabidopsis Aldehyde Oxidase 4 (AAO4) was identified as contributing to BA production in seeds, potentially acting as a precursor for SA synthesis (Ibdah et al. 2009). While PAL plays a part in SA biosynthesis, its functions extend beyond, affecting the production of various compounds potentially related to defense mechanisms. The conversion steps from Phe to SA are crucial, as prephenate can diverge into various biosynthetic routes PAL transforms Phe into tCA.
Moreover, the PAL pathway involves the multifunctional protein (MFP) family member Abnormal inflorescence meristem1 (AIM1), which was identified through mutant analysis in Arabidopsis. AIM1, present in both Arabidopsis and rice, participates in various metabolic processes, including fatty acid metabolism, hormone metabolism, and amino acid metabolism. AIM1's involvement in converting tCA into BA suggests a role in the PAL pathway. This enzyme has diverse substrates due to its beta-oxidation function. Although knockout plants involving the AIM1 gene complex are intricate to interpret, they remain valuable for studying SA biosynthesis and defense responses (Xu et al. 2017b; Jia et al. 2023). The hypothesis of a BA 2-hydroxylase (BA2H) enzyme converting BA to SA was suggested, supported by activity coinciding with SA buildup in tobacco (Staswick et al. 1995; Sawada et al. 2006; Steinwand 2017). However, the gene responsible for BA2H has not been definitively identified. To gain comprehensive insights into plant SA biosynthesis, further studies are required.
Regulation of salicylic acid biosynthesis
Mechanisms regulate SA concentration to maintain optimal levels is essential for plant function. Genes involved in SA biosynthesis are tightly controlled (Fig. 1b). Most of these genes are induced in response to stress, and SA accumulation can further stimulate its biosynthesis through transcriptional regulation. Phenomena like post-transcriptional regulation also impact SA biosynthesis which is a cornerstone of SA-mediated plant immunity (Zhong et al. 2021).
Positive transcriptional regulation
Positive transcriptional regulations are required to switch on the production of SA. Plants Calmodulin-binding TFs, such as CaM-Binding Protein 60 g (CBP60g) and Systemic Acquired Resistance Deficient 1 (SARD1), facilitate pathogen-induced SA synthesis by regulating the transcription of the key SA biosynthesis genes like ICS1 and EDS5 (Pokotylo et al. 2019; Huang et al. 2020; Santisree et al. 2020). WRKY TFs like WRKY28 and WRKY46 are involved in regulating ICS1 expression and PBS3 (van Verk et al. 2011; Chen et al. 2021). Furthermore, Teosinte branched1/cycloidea/proliferating cell factor (TCP) members TCP21, TCP8/TCP9, and NAC TF-like 9 (NTL9) have also been identified as activators of ICS1 during immune responses. AIM1, belonging to the MFP family, contributes to the conversion of tCA to BA in the PAL pathway. Also, the activation of SA biosynthesis is crucially influenced by calcium (Ca2+) signaling, which modulates the activities of CBP60g and the Ca2+/calmodulin-binding transcription factor CAMTA3 (Zhou and Zhang 2020; Peng et al. 2021; Yuan et al. 2021).
Negative transcriptional regulation
CBP60a, a homolog of CBP60g, negatively regulates ICS1 expression upon CaM-binding, suggesting a complex interplay between different factors. NAC TFs, including ANAC019/055/072, have been identified as negative regulators of SA biosynthesis (Bian et al. 2021). The abscisic acid-responsive NAC019 (ANAC019) directly binds to BA/SA carboxyl methyltransferase 1 (BSMT1) and ICS1 promoters, repressing SA production. Another SA negative regulator is DP-E2F-like1 (DEL1) and Ethylene Insensitive 3 (EIN3). DEL1 suppresses EDS5 expression, while EIN3 mediates the negative regulation of ICS1 expression (Chen et al. 2009; Eichmann and Schäfer 2015). Loss of function del1 mutant exhibits accumulated production of SA and increased disease resistance (Chandran et al. 2014).
Possible mechanisms for the actions of SA in mitigating abiotic stress
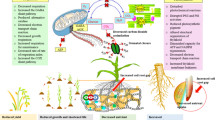
As a consequence of climate change plants are frequently exposed to abiotic stressors; likely more than one at the same time. Most frequently high temperature together with water deficit and increased soil salinity are the most common scenario. The events of abiotic stresses increase the accumulation of endogenous SA in plants during stress (Chieb and Gachomo 2023). Exogenous application of SA was found to regulate and mediate numerous plant responses to abiotic stress which, although not extensively documented or understood, are promising ways to alleviate the damage done by these stressors.
SA induces stomatal closure under stress conditions
SA's ability to promote stomatal closure was reported in experiments with SA exogenous treatment (Mori et al. 2001; Khokon et al. 2010; Panchal et al. 2016). Mutant Arabidopsis plants (carrying constitutive expresser of pathogenesis-related genes-5 (cpr5) and accelerated cell death 6(acd6)) exhibiting SA accumulation showed elevated drought tolerance and reduction in stomatal aperture (Miura et al. 2013). Furthermore, SA signaling in stomatal guard cells is mediated by its receptor NPR1 (Zeng and He 2010; Ding et al. 2018).
The SA signaling and its cross-talk with other signaling pathways in the guard cells are considered a cornerstone in conferring tolerance in plants. Recently, SA was reported to activate peroxidase-mediated reactive oxygen species (ROS) signal that is integrated into the Ca2+/CPK-dependent ABA signaling branch in the Arabidopsis guard cells. The model described by (Prodhan et al. 2018) explains the overlapping between both SA and ABA signaling in the guard cells (Fig. 3b). This mechanism is supported by the findings of Zhang et al. when conducted his studies on the PtrWRKY75 gene which acts upstream of PAL1 and promotes his expression by binding to its promoter under drought stress which promotes SA biosynthesis. SA increased ROS accumulation leading to smaller stomatal aperture (Zhang et al. 2020). Furthermore, the overexpressed PtrWRKY75 lines showed improved water-use efficiency under drought stress by reducing their stomatal conductance and transpiration rate.
SA regulation of osmolytes under stress conditions
Osmolytes are soluble organic compounds that play a role in regulating the amount of water in the cell via a process called osmoregulation (Saleem et al. 2021). Osmoregulation is a vital defense mechanism against various abiotic stressors including salinity, heavy metal toxicity, and cold stress (Saleem et al. 2020; Sharma et al. 2020). Osmolytes, such as proline and GB, accumulate in the plant to alleviate stress by reducing the number of ROS and maintaining cell turgor. It has been known that SA controls osmolytes as a metabolic process involved in stress signaling. Exogenous SA has been found to interact with osmolytes and induce their accumulation in plants to alleviate stress (Szegediensis et al. 2005; Sharma et al. 2019). This SA based modulation mainly through enhancing the expression pattern of key biosynthetic genes(La et al. 2019a; Singh et al. 2022a).
SA regulates proline production under stressful conditions
Proline is an amino acid that accumulates under stressful conditions and alleviates stress via many mechanisms including induction of stress-related enzymes, maintenance of membrane integrity, and chelation of heavy metals (Hayat et al. 2012; Saleem et al. 2021). Studies have shown that SA can increase the production of proline in plants under abiotic stresses, such as drought, salinity, and heat stress (Misra and Saxena 2009; Iqbal et al. 2014). Additionally, (Dawood et al. 2022) found that exogenous application of SA led to a significant increase in proline content in fava beans under simultaneous drought and salinity stress. It was proven that exogenous application of SA upregulates proline biosynthesis-related genes, e.g., P5CSA and P5CSB (pyrroline-5-carboxylate synthase A and B) while downregulating PDH (proline dehydrogenase) in drought stressed Brassica rapa (La et al. 2019b). At the same time, exogenous proline application was previously shown to induce SA production (mediated by NDR1-dependent signaling pathway) and was shown to modulate calcium (Ca2+)-mediated oxidative burst defense response in plants (Chen et al. 2001; La et al. 2019a).
SA induces the synthesis of glycine betaine (GB) under stressful conditions
GB is a quaternary ammonium compound that helps improve plant growth, reduce osmotic stress, and regulate nutrient uptake under stress (Ali et al. 2020; Sharma et al. 2020; Singh et al. 2022b; Shohani et al. 2023). GB helps to protect plants from stress by adjusting cell osmotic balance, which helps to prevent cells from shrinking or bursting (Raza et al. 2023), stabilizing membrane integrity, which helps to prevent the leakage of cellular contents (Khan et al. 2014), protecting Rubisco activity, which is the enzyme that converts carbon dioxide into sugar (Bharwana et al. 2013), detoxifying toxic ions (Jagendorf and Takabe 2001). SA has been reported numerous times to induce the synthesis of GB, thus enhancing plant growth under various stressors including cold stress, heat stress, and drought stress (Khan et al. 2015; Sharma et al. 2020). SA can induce GB accumulation in the range of 0.5–2.5 mM, which is sufficient to protect plants from stress (Jagendorf and Takabe 2001). The increase in GB level mediated by SA can improve overall plant growth (Misra and Misra 2012). (Khan et al. 2014) showed that the alleviation of salinity-inhibited photosynthesis and growth by SA in mungbean involves GB. SA can induce GB accumulation by increasing methionine content and suppressing ethylene formation. A similar effect was observed with the application of SA-analog, 2, 6, dichloro-isonicotinic acid, on GB accumulation and alleviation of salinity-induced adverse effects on photosynthesis and growth. More recently, Abbaspour and Ehsanpour (Abbaspour and Ehsanpour 2020) reported GB accumulation in response to exogenous SA treatment, this increase was not accompanied with an increase in BADH (GB processing enzyme) activity. Further studies are needed to reveal the mechanism of which GB is regulated by SA (Fig. 2).
SA signaling and its cross-talk with ABA. A SA was found to increase the amount of ABA in response to stressors such as chilling, drought, and salinity which in turn has led to stomatal closure. B Shows Salicylic acid (SA) signaling in guard cells integrated with abscisic acid (ABA) signaling via the calcium-dependent protein kinase (CPK) pathway. SA triggers a ROS signal that activates CPKs, which then phosphorylate S59 and S120 of SLAC1, a protein that regulates stomatal closure. ABA also requires CPKs for the activation of SLAC1, but it also requires OST1, a protein kinase that is not involved in SA signaling. The two MAPKs, MPK9 and MPK12, also function downstream of ROS and Ca2+ in guard cell SA signaling and regulate SLAC1 activity indirectly. The molecular mechanism underlying the interdependence of the CPK-dependent and MPK-dependent pathways for SLAC1 activation is unknown
SA regulated mineral uptake to elevate plant growth under stress
Plants require minerals to grow and survive. While abiotic stressors disrupt mineral uptake and lead to stunted growth and development. The mineral nutrient status of plants can affect their ability to tolerate stress. SA has been shown to regulate mineral uptake and metabolism in plants under stress. In addition, it has been shown to protect plant membranes and regulate the uptake (Alpaslan and Gunes 2001; Gunes et al. 2007). SA has been shown to improve photosynthesis in salt-stressed plants by decreasing cellular sodium (Na+) and chloride (Cl−) ions and increasing the content of nutrients. For example, SA supplementation strongly inhibited Na+ and Cl− accumulation but stimulated nitrogen, iron, and copper concentrations in salt-stressed corn (Gunes et al. 2007). However, the mechanism that SA uses to selectively promote the beneficial elements uptake while hampering the toxic ones is not yet fully understood (Kaya et al. 2023). Furthermore, SA priming improved plant growth under different abiotic stress conditions by improving water, secondary metabolite and nutrient uptake. Those abiotic stresses such as salt (Gondor et al. 2023), mainly through controlling the root-shoot system mineral nutrients by adapting the influx of Na+ and K+ ions. Another example was explained by improved grain filling and grain protein content in high temperature stresses Tritcum aestivum (Ihsan et al. 2022). and Cucumis Sativus (Basirat and Mousavi 2022) treated by SA Moreover, SA treatment increased nitrogen uptake in mustard (Brassica juncea) plants grown in water deficit with low nitrogen supply conditions (Iqbal et al. 2022).
SA induces major beneficial secondary metabolites in stressed plants
Secondary metabolites have been established as defense chemicals for plants under abiotic stress. There is extensive evidence that SA can directly and indirectly induce the synthesis of secondary metabolites in plants (Austen et al. 2019). A study reported that the exogenous application of SA to UV-B-exposed wheat plants increased the accumulation of anthocyanin and tocopherol, and also modulated the expression of pathogenesis-related (PR) proteins (Horváth et al. 2007a) Furthermore, the supplementation of SA in Capsicum chinense elicited the activity of PAL and caused subsequent increased vanillin production (Rodas-Junco et al. 2013). Numerous other studies have also reported an increase in PAL activity after the exogenous application of SA on plants including saffron (Tajik et al. 2019) and sage (Ejtahed et al. 2015); this led to an increase in the number of phenolic compounds in the plant. In another study by (Wen et al. 2008), it was shown that SA-mediated improved thermo-tolerance in grapes was a result of SA-induced accumulation of PAL mRNA, the synthesis of new PAL protein, and significant accumulation of phenolics.
SA application enhanced triterpenoid content in Centella asiatica through overexpression of triterpenoid biosynthesis-related genes, which led to enhanced anti-inflammatory activity (Buraphaka and Putalun 2020). In ginseng, SA induced accumulation of farensol, isochiapin B sesquiterpenoids, champhor, and cineole monoterpenoids by up-regulating genes corresponding to the enzymes involved in their syntheses such as farnesyl diphosphate synthase and isopentenyl pyrophosphate isomerise (Rahimi et al. 2014). In goji berries, the expression of chalcone isomerase gene and the total content of flavonoids were upregulated by exogenous SA treatment (Guan et al. 2014).
SA controls responsive genes involved in stress resistance
SA induces SAR, which generates signaling responses to abiotic stress. These signals stimulate the expression of stress-defensive genes. Defensive genes aid plants in alleviating abiotic stresses via eliciting internal physiological and molecular responses. Recently, stress-responsive genes; SOS1 (Salt overly sensitive 1) and NA+/H+ exchanger (NHX1) were reported to be significantly upregulated in SA seed-primed pea plants exposed to salt stress (Ahmad et al. 2022). Furthermore, SA was reported to influence heat shock proteins (HSPs), such as HSP70 and Hsp17.6 (Sangwan et al. 2022). HSPs act as molecular chaperons that repair denatured proteins and stabilize the cell proteins against heat stress damage (Mittler et al. 2012). Furthermore, SA pretreatment regulated the transcription of the antioxidant enzymes genes catalase (CAT) and ascorbate peroxidase (APX), APX, and GPX in barely any plants under Cd-induced oxidative stress. Lower APX activity coupled with higher sensitivity to drought stress, salt stress, or cold stresses were reported in rice plants with Osapx2 mutations. While plants with OsAPX2 overexpression showed higher APX activity and stress tolerance were reported (Zhang et al. 2013). The elevation in the expression level of Pathogenesis-related (PR) genes and the endogenous levels of salicylic acid (SA) coincide during the activation of disease resistance in plants. Studies have shown that these genes are regulated by abiotic stresses and SA, suggesting that they serve a specific function during abiotic stress (Miura et al. 2013). In rice, the PR genes PR4c and PR4d were induced by SA and improved drought tolerance (Wang et al. 2011). The transcriptional coregulator NPR1 is a key gene in the activation of SA-dependent stress defense genes. It was reported that SA-based oxidative and salt stress tolerance happens through NPR1- dependent pathway (Pan et al. 2006; Jayakannan et al. 2015). SA is also involved in several in upregulation of genes of several signaling pathways such as ABA and Jasmonic Acid (JA). Wang et al. reported an elevated expression of NCED1 and NCED2, two important genes involved in ABA synthesis, followed by a subsequent increase in endogenous ABA concentration after exogenous SA treatment (Wang et al. 2018b). ABA accumulation appears to be a signaling process that follows SA sensing. The expression of the ABA biosynthesis genes, SlZEP1, SlNCED1, SlAO1, and SlAO2, was increased following SA treatment in salt-stressed tomato roots (Szepesi et al. 2009).
Furthermore, SA has a direct role in activating several transcription factors which involved in enhancing plant tolerance to abiotic stresses (Sakuma et al. 2006). In Arabidopsis thaliana, DREB2A, a transcription factor with a negative regulatory domain in its middle region, plays an important role in heat stress response. Deletion of the negative regulatory domain in DREB2A transforms it into a constitutively active form (DREB2A CA). Overexpression of DREB2A CA in transgenic Arabidopsis enhanced tolerance to heat/drought stress. The relationship between DREB2A and SA is complex and not fully understood. However, they work together to promote plant stress tolerance. Proteomic analysis investigations revealed that some transcription factors are differentially regulated via SA under abiotic stresses such as 14–3-3, NAC domain-containing proteins, and SET domain proteins (Kang et al. 2012).
SA regulating redox signaling and antioxidant defense system
SA plays a fundamental role in regulating redox homeostasis and regulating the antioxidant defense system. This redox regulation is happen to occur by enhancing expression of redox regulating genes (such as, TRXh5 and GRXC9) coupled with enhancing NPR1- dependent signaling pathway (La et al. 2019a). This redox modulation through transcriptional regulation enhanced abiotic stress tolerance, such as drought (La et al. 2019a). For instance, SA treatment on young grape leaves reduced the levels of thiobarbituric acid reactive substances (TBARS) and relative electrolyte leakage and increased the activity of antioxidant enzymes (Wang and Li 2006). Furthermore, SA contributed to cellular redox homeostasis through upregulating proline biosynthesis under drought stress conditions as well as the redox status including glutathione cycle enzymes and the antioxidant defense(La et al. 2019b). The antioxidant defense system comprises a complex network of genes and proteins that collectively orchestrate the production and elimination of ROS which acts as oxidant molecules under abiotic stress. These ROS, such as superoxide radicals (\({O}_{2}^{-}\)) and hydrogen peroxide (H2O2H2O2), possess high reactivity and can disrupt a wide array of biochemical metabolic pathways, including enzyme functions, DNA integrity, membrane permeability, and protein synthesis. Typically, plant cells have mechanisms in place to regulate ROS levels, ensuring they remain within manageable limits (Swanson and Gilroy 2010; Mhamdi and Van Breusegem 2018; Mansoor et al. 2022). In plant cells, this system involves approximately 152 genes that contribute to the synthesis of enzymes and non-enzymatic compounds responsible for both generating and eliminating ROS (Hideg et al. 2013; Dumanović et al. 2021).
ROS primarily act as metabolic by-products stemming from oxygen utilization, representing a potential source of cellular toxicity. Also, ROS are utilized as key signaling molecules in various physiological processes. These include growth and development, hormone signaling, cell apoptosis, cell cycle regulation, as well as responses to both biotic and abiotic stressors. This dual role of ROS highlights their significance as molecular messengers in plant biology (Miller et al. 2008; Nadarajah 2020; Sachdev et al. 2021).
Since H2O2 holds a vital role in plants' defense against abiotic stressors, the SA-induced H2O2 surge can potentially enable plants to withstand subsequent abiotic stressors as well (Faizan et al. 2021). This has been particularly evident in experiments involving exposure to heavy metals when rice roots were treated with SA before exposure to Cd stress, it initially increased the presence of H2O2, alongside higher levels of antioxidant molecules and enzyme activities. This enhanced antioxidant system effectively minimized the oxidative damage caused by Cd stress (Guo et al. 2007, 2009).
ROS, including some genes play a critical role in plants' responses to abiotic stresses. Transcription factors like FtbZIP5 and GarWRKY5 may enhance drought and salt tolerance through ROS-related pathways, potentially involving ROS scavenging genes, JA and SA-related genes (Guo et al. 2019; Gechev and Petrov 2020). The relationship between SA and ROS-scavenging enzymes like catalases (CATs) and ascorbate peroxidase (APX) is complex. CATs are highly efficient enzymes that rapidly convert H2O2 into water and oxygen (Azarabadi et al. 2017). Application of SA inhibited CAT activity, leading to a self-amplifying loop of ROS production. This, in turn, triggers the expression of defense-related proteins like PR1 and culminates in SAR (Khokon et al. 2011; Mejía-Teniente et al. 2013). Similarly, APX, which is an enzyme with a strong affinity for H2O2, can also be influenced by SA. Supplementation with SA can enhance APX activity, aiding in the detoxification of ROS. This is particularly evident under conditions of stress, such as exposure to heavy metals or waterlogging (Fig. 3) (Torun 2019).
Furthermore, the antioxidant molecules ascorbate peroxidase (ASA), glutathione (GSH), malondialdehyde (MDA) and Glutathione persulfide (GSSH) play critical roles in mitigating oxidative damage caused by abiotic stresses. GSSH is involved in redox signaling through its association with H2S signaling pathways, ASA plays a role in maintaining redox balance by reducing H2O2, and MDA serves as an indicator of oxidative damage in cells. Genetic studies have indicated a linkage between GSH biosynthesis and endogenous SA signaling. SA levels have been correlated with changes in GSH levels in mutants deficient in catalase activity (Herrera-Vásquez et al. 2015; Chaouch et al. 2010). Several enzymes involved in GSH metabolism, such as glutathione synthetase (GSHS), glutathione reductase (GR), glutathione S-transferases (GST), and glutathione peroxidase (GPX), also show relationships with SA signaling (Njålsson and Norgren 2005; Nazar et al. 2011; Mostofa et al. 2015).
However, while the positive impacts of SA signaling have been extensively explored, some studies have yielded conflicting results. For instance, while SA-induced H2O2 production can be beneficial, it has also been associated with potentially detrimental outcomes, such as oxidative damage (Choudhury and Kumar Panda 2004; Hayat et al. 2010). Similarly, the effects of SA mutants on ROS responses can vary depending on the specific genetic context. While our understanding of these interactions has progressed significantly, there are still aspects that require further exploration to understand the complexities of plant-ROS interactions.
SA cross-talks with other plant hormones
In addition to ROS, other types of plant hormones play a role in the signaling pathway involving SA in plants. Various research studies have observed a connection between SA and ABA, SA and nitric oxide (NO), and SA with JA, especially during times of stress (Cao et al. 2011). All of these cross-talks aim to increase abiotic stress tolerance, Fig. 4.
The relationship between SA and ABA mostly is directly proportional and this cross-talk has a positive impact on tolerating abiotic stress. It was evident that when plants are treated with SA, which triggers an increase in ABA levels in plants such as in barley and tomato (Torun et al. 2020). This increase is mainly due to enhancing in expressing of ABA biosynthesis genes. Wang et al., (Wang et al. 2018b) reported elevated NCED1 and NCED2 gene expression in wheat leading to increased ABA content and reduced freezing stress. Szepesi et al. (Szepesi et al. 2009) reported increased in ABA biosynthesis genes (SlZEP1, SlNCED1, SlAO1, and SlAO2) expression after SA treatment in tomato under salt stress. However, when leaves of A. thaliana are exposed to ABA, it has an inhibitory effect on the transmission of SA signals through the SAR signaling pathway (Yasuda et al. 2008). It is suggested that the biosynthesis of ABA is a downstream event in the signaling process, influenced by the sensing of SA (Khan et al. 2019). Moreover, in response to drought stress, ABA facilitates cuticular wax biosynthesis through the transcription factor MYB96, which stimulates the production of SA as the super-active myb96-1d mutant shows increased expression of sid2 the crucial enzyme in SA biosynthesis pathway. Also, the SUMO (small ubiquitin-like modifier) E3 Ligase 1 (SIZ1) negatively regulates the ABA-responsive gene ABI3, resulting in increased ABI3 protein levels in the siz1 mutant (Miura et al. 2009). SIZ1 also negatively modulates SA levels, leading to higher SA accumulation. (Corina Vlot et al. 2009; Miura et al. 2013). These effects can be reversed by introducing the NahG gene (Xu et al. 2015). More recently it was reported that SA and ABA signaling are integrating in stomatal guard cells under drought stressed condition (Prodhan et al. 2018; Li et al. 2023c); this integration is mediated by CBK pathway. The interaction between SA and ABA is still not fully understood, but it is clear that these two hormones play important roles in plant stress tolerance. Further research into this interaction is likely to lead to new strategies for improving plant resilience to stress.
Both SA and NO play crucial roles in maintaining redox balance in plants during responses to abiotic stresses (Rai et al. 2020). For instance, Applying SA and a NO-releasing compound significantly enhances the tolerance of certain plants to heat stress and nickel toxicity. Both NO and SA participates in the signaling pathway that leads to stomatal closure, with NO levels increasing through enzymes like those activated by SA (Wendehenne et al. 2004). Their combination effectively mitigates nickel toxicity in Brassica napus by facilitating proline accumulation, reducing lipid peroxidation, and enhancing chlorophyll content (Kamran et al. 2020; Ahmad et al. 2021). On the other hand, the NO-SA combination in Arachis hypogaea increases Cd concentration in the cell wall, thereby safeguarding organelles from its toxic effects (Shah et al. 2019). Moreover, the NO-SA interaction synergistically alleviates salt stress by enhancing divalent cations absorption (Verma et al. 2022).
The cross-talk between SA and JA is indirect and mostly it involves the interaction of other phytochromes. In a study involving heat-stressed soybean plants inoculated with B. tequilensis, the levels of endogenous JA and SA were significantly elevated, while the endogenous ABA level was markedly reduced (Aloo et al. 2023). Also, Salt stress on plants leads to an increase in the content of JA and ABA, but a decrease in the levels of other plant hormones like indole-3-acetic acid (IAA), gibberellic acid (GA), and SA in species like Iris hexagona and soybean (Wang et al. 2001).
SA inducing mitogen-activated protein kinase
Mitogen-activated protein kinase (MAPK) is a specific type of protein kinase that targets threonine and serine amino acids. MAPKs play crucial roles in various cell functions and plant survival under a wide range of abiotic stress (Ji et al. 2017; Zhou et al. 2017). It was reported that MAPK cascade mediated are regulating H2O2 metabolism in tobacco and Arabidopsis (Xing et al. 2008).Research on Arabidopsis thaliana has indicated that MAPK cascade signaling is influenced by SA. Across various species, MAPK cascades have been implicated in signaling pathways triggered by abiotic stresses such as cold, salt, heat, UV, osmotic shock, heavy metals, and more (Raja et al. 2017). The components of MAPK cascades involved in different abiotic stresses.
SA-deficient mutants demonstrated a notable decrease, about 50%, compared with the wild-type expression level, in AtMPK3 activity (Pál et al. 2013; Lin et al. 2021). SA activation led to the identification of a 48-kDa MAPK in tobacco, primarily phosphorylating myelin basic protein (MBP) (Lebrun-Garcia et al. 1998; Zhang and Klessig 1998). In turn, MAPKs influence SA levels in stressed plants. Remarkably, SA treatment boosted TaMAPK4 transcripts in wheat during avirulent pathogen attacks, while downregulating the TaMAPK4 gene led to decreased SA accumulation (Wang et al. 2018a). On the other hand, the StMKK1 protein negatively influenced SA-related signaling pathways during pathogen defense in potatoes. Although the mpk4 mutant showed excessive SA accumulation, this wasn't solely responsible for its severe dwarf phenotype, as knocking down the ICS1 gene failed to restore normal growth (Li et al. 2022b, 2023a). Furthermore, the connection between MPK4 accumulation and SA-regulated redox balance remains unclear and necessitates further investigation. Moreover, recently it was reported that SA triggers ROS signals in the stomatal guard cells which activate MPK genes-dependent pathway (MPK9 and MPK12) to regulate genes responsible for stomatal closure in Arabidopsis (Prodhan et al. 2018).
Role of salicylic acid in mitigating abiotic stress in plants
Effect of SA on plants under drought stress
Drought is one of the most important natural abiotic stresses that can negatively impact plant growth and development(Dai 2010). From an agricultural and physiological perspective, drought stress occurs when the available water in the soil is not enough to meet the needs of plants, which can eventually lead to a further decrease in soil moisture (Farooq et al. 2009b).
The exogenous application of SA at 100 mg/L significantly mitigated the negative effects of drought stress on Brassica napus plants (Ali et al. 2023). The application of SA reduced the effect of drought on leaf area chlorophyll, and gaseous exchange rate, (Table 2). It also reduced MDA contents, proline content, and sugar content, (indicators of lipid peroxidation and oxidative stress. The application of SA also minimized the deterioration ultrastructure caused by drought within the leaves (Ali et al. 2023). Feng et al. (Feng et al. 2023) reported that drought stress reduced the photosynthetic rate, stomatal conductance, and transpiration rate in flue-cured tobacco variety K326. However, SA spraying at 0.3 mM improved these parameters. SA also increased the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) enzymes, and proline content, while decreasing MDA content (Table 2).
In Arabidopsis thaliana, SA-accumulating mutants (cpr5 and acd6) exhibited stomatal closure and improved drought tolerance. This was attributed to SA-mediated induced expression of PR genes, such as PR1, PR2, and PR5 (Liu et al. 2013). In wheat, 78 proteins were identified that were potentially involved in SA signaling under drought stress. These proteins were involved in major physiological functions, such as photosynthesis, carbohydrate metabolism, protein metabolism, stress and defense, energy production, signal transduction, and toxin metabolism (Kang et al. 2012).
Effect of SA under temperature stress
One of the most detrimental stressors on plants and a major side effect of global warming is extreme temperatures. Whether high temperature (HT) or chilling, both extremes are sure to stunt the growth and development of the plant.
Role of SA in heat stress tolerance
Heat stress (HT) is a significant environmental stress that can hinder plant growth, metabolism, and productivity (Zhao et al. 2020). Global temperatures increasing by 1.5 degrees Celsius by 2050 and 2 to 4 degrees Celsius by 2100 accordingly HT is a growing concern for crop production (Zhao et al. 2020). HT can induce physiological, biochemical, and molecular changes, disrupting membrane integrity and protein denaturation (Fig. 5) (Levitt 1980).
SA is thought to induce heat tolerance in plants by activating many genes involved in stress response, including genes encoding antioxidant enzymes and heat shock proteins (Suzuki et al. 2008). MBF1c, a highly conserved transcriptional coactivator, is upregulated by heat stress (Nazar et al. 2017). Suzuki et al. (Suzuki et al. 2011) reported that MBF1c is a key regulator of basal thermotolerance and provided evidence for the existence of a coordinated heat stress response network involving SA signaling pathways under the control of MBF1c. MBF1c is induced by heat stress and binds to the promoter of a gene encoding SA. Furthermore, in Arabidopsis ATMBF1c recognizes and regulate stress-responsive gene dehydration-responsive element (DRE)-binding protein 2A (DREB2A). It is evident that expression of PaMBF1c of Antarctic Moss) enhanced heat stress tolerance in Arabidopsis (Alavilli et al. 2017).
SA is thought to help plants tolerate HT stress by activating genes involved in stress tolerance. (Khan et al. 2013) found that SA treatment under heat stress increased the production of proline, which increased the osmotic potential of the plants. This enabled the plants to uptake more water, which had a positive influence on stomatal aperture and photosynthetic machinery. In cucumber plants, foliar spraying with 1 mM SA induced heat tolerance, as evidenced by the lower electrolyte leakage parameter, lower H2O2 levels, higher catalase activity, and lipid peroxide levels, and higher Fv/Fm chlorophyll a fluorescence value (Shi et al. 2006). SA has been found to increase the photosynthetic rate in grape leaves under heat stress (Wang et al. 2010). It can also alleviate heat-induced damage in plants by upregulating the antioxidant system (Fig. 5) (Wang and Li 2006).
Several investigations had been conducted with the aim of deciphering the effect of SA on heat tolerance from the molecular and biochemical point of view. SA stabilizes the trimerization of heat shock transcription factors (HSFs) and helps them bind to the heat shock element (HSE) in the promoter of heat shock protein (HSP) genes (Andrási et al. 2021; Haider et al. 2022; Wang et al. 2010) found that SA did not affect the level of heat shock protein 21 (HSP21) in grape leaves before heat stress. However, the HSP21 defense signal increased in both SA-treated and control leaves during heat stress. (Wang and Li 2006) found that spraying a 0.1 mM solution of SA on young grape leaves increased heat tolerance by reducing the levels of thiobarbituric acid reactive substances (TBARS) and relative electrolyte leakage and increased the activity of antioxidant enzymes. These results suggest MBF1c, a highly conserved transcriptional coactivatthat SA can induce intrinsic heat tolerance in plants. (Pan et al. 2006) found that blocking SA synthesis in plants under heat stress decreased both the endogenous SA content and the level of heat tolerance.
Liu et al. (Liu et al. 2008) found that SA can induce heat tolerance in plants by regulating the activities of plasma membrane H+-ATPase and Ca2+-ATPase. Furthermore, SA-induced thermotolerance is associated with the Ca2+-calmodulin (CaM) system in grape plants. SA increased the activity of H+-ATPase in grape leaves, which helped to maintain the stability of the plasma membrane and protect cells from heat damage. The increase in the Ca2+ level in the cellswas the reason behind Ca2+-CaM system activation (LIU and HUANG 2005). This further clarifies the role of SA in stimulating the activity of Ca2+-ATPase, which helped to regulate the Ca2+ level in the cytoplasm and prevent heat injury.
The role of the genetics underlying SA biosynthesis and functions in plants facing heat stress is still under study. However, According to (Nazar et al. 2017) recent studies, SA plays an important role in heat stress tolerance by activating genes that encode proteins that help the plant cope with heat stress, such as HSPs and antioxidant enzymes. The genes that encode SA are also involved in the regulation of heat stress response, and their overexpression can improve the heat tolerance of plants. More research is needed to fully understand the role of the genetics of salicylic acid in heat stress tolerance, but these studies suggest that it is a promising target for the development of new crop varieties that are more tolerant to heat stress.
Role of SA in chilling stress tolerance
Extremely low temperatures could result in chilling injury to the plant. A chilling temperature denotes a temperature as low as possible without freezing (Kang and Saltveit 2002). Plants exposed to these low temperatures are at risk of suffering from slower seed germound that SA can induce heat tolerance in plants by regulating the aination and growth, a slower rate of photosynthesis, as well as stiffening and deterioration of the plasma membrane (Saleem et al. 2020). Cold stress also decreases water and nutrient uptake, hence putting the plant at risk of starvation (Saleem et al. 2020). The low temperatures lead to decreased plasma membrane fluidity due to disruption of the ratio and composition of plasma membrane lipids and proteins (Saleem et al. 2020). This causes the membrane to go from fluid-like to gel-like. The most visible symptom of chilling injury manifests itself as internal browning and softening of the plant (Zhao et al. 2021b).
Exogenous SA has been proven in numerous investigations to be effective in mitigating cold stress and maintaining fruit quality (weight loss, discoloration, softening, etc.). This might be because SA can promote protein synthesis and stimulate enzyme activity, which could wake the plant from its dormant state (Liu et al. 2022). SA also can enhance water and nutrient uptake in plants, although the mechanism under chilling stress is still uncovered (Saleem et al. 2020). Furthermore, SA has been shown to delay the accumulation of ethylene (ET) in fruit, which delay fruit senescence, a common symptom of chilling injury in plants (Chen et al. 2023). (Song et al. 2022) showed that soaking post-harvest Hami melons in SA improved their cold tolerance and extended their storage time, (Zhao et al. 2021b) reported that SA treatment delayed the internal browning of the peaches. Overall, evidence of the ability of SA to mitigate cold stress, whether it was applied pre-harvest or post-harvest has been documented heavily. (Zhao et al. 2021b) reported that SA treatment delayed the internal browning of the peaches and mitigated the overall effects of chilling injury by increasing the total soluble sugars via increasing the synthesis of sucrose and the expression of genes related to sucrose synthesis, such as Neutral Invertase 2 (NINV2), Sucrose Phosphate Synthase 4 (SPS4), Sucrose Synthase 2 (SuSy2), and Sucrose Transporter 1 (SUT1). SA treatment also increased the expression of genes involved in cold-stress response, DREB1A and DREB2A (Zhao et al. 2021b). Exogenous SA treatment was also involved in the upregulation of genes involved in the cold signaling pathway, such as C-repeat Binding Factor 1 (CBF1), and Inducer of CBF expression 1 (ICE1) in cucumber plants (Fu et al., 2021). Overall, evidence of the ability of SA to mitigate cold stress, whether it was applied pre-harvest or post-harvest has been documented heavily. Table 2 displays recent examples of SA treatment and its effects on plants under chilling stress.
Effect of SA on plants under salinity stress
Salinity is regarded as one of the most detrimental abiotic stressors to plants and crops. Salinity denotes an elevated concentration of soluble salts present in the soil (Singh and Gautam 2013). Plants exposed to salinity are threatened with various deleterious effects such as osmotic stress, reduced chlorophyll amounts, altered metabolic activity, decreased size, and shortened height of the plant (Wani et al. 2017; Liu et al. 2022). Salinity has also been linked to the formation of ROS, leading to oxidative stress and eventually DNA damage, as well as lipid peroxidation and enzyme inactivation (Hayat et al. 2010; Wani et al. 2017). Salinity has also been shown to decrease endogenous SA content in plants, e.g., tomatoes (Molina et al. 2002; Liu et al. 2022).
SA mainly support plants to tolerate salinity tolerance by modulating key salinity stress responsive genes. SA was found to increase the transcript of 1-aminocyclo propnae 1-carboxylic acid synthase (ACS), salt overly sensitive 1 (sos1), sodium-hydrogen exchanger (NHX1), and high-affinity K+ transporter (HKT1;2) under salinity stressed tomato plants. Furthermore, those SA treated plants exhibited reduced electrolyte leakage and sodium ion (Na+) content and increased endogenous proline and potassium ion (K+) content, and K+/Na+ ratio (Szepesi et al. 2009). In addition, SA prevented K+ loss via guard cell outward-rectifying K+ (GORK) channels. This reduction is NPR1-dependent as npr1-5 mutant is unable to control K+ leak (Jayakannan et al. 2013). Moreover H+ ATPase activity was increased in SA pretreated Arabidopsis plants subsequently there were a reduction in NaCL-induced K+ efflux through KOR channels resulting in tolerance to salinity stress (Jayakannan et al. 2013).
As a vital regulator of sodium influx and efflux, SA helps alleviate salt stress (Liu et al. 2022). Omidi et al., (Omidi et al. 2022) demonstrated that foliar application of SA in concentrations as little as 0.5 mM was still able to neutralize the salt stress and increase the expression of antioxidant genes in the damask rose. When (Hongna et al. 2021) soaked the seeds of false wheatgrass (Leymus chinensis) in SA solution and then subjected them to salt stress, the seeds exhibited improved germination and viability, as well as alleviated plasma membrane damage and ROS modulation. Although not as efficient as foliar pretreatment, treating tomato roots using SA still resulted in alleviated salt stress (Souri and Tohidloo 2019). Table (2) displays a summary of recent investigations reporting the effect of SA on various plants that were subjected to salt stress.
SA was also found to increase the amount of osmolytes (proline) present in plants, which acts on in mitigating salt stress as osmolyte accumulation represents a vital adaptive response to stress in plants (Hayat et al. 2010; Singh and Gautam 2013). Proline represents a very important stress amino acid that accumulates under salt-stressed conditions. This is because it plays a vital role in regulating osmotic balance, which could help in alleviating salt stress (Kang and Saltveit 2002). Furthermore, exogenous application of SA was found to maintain membrane stability and integrity as well as increase the rate of photosynthesis (Hayat et al. 2010; Singh and Gautam 2013).
Effect of SA on plant response to other abiotic stresses
Sunlight, essential for plant growth, can be harmful to plants, particularly UV light, which can damage DNA and proteins (Müller-Xing et al. 2014). Climate change's frequent heatwaves have increased UV radiation levels (Bernhard et al. 2020). UV radiation is divided into UVA, UVB, and UVC regions, with UVC being the most harmful. SA can modulate antioxidant levels, detoxify superoxide radicals, prevent oxidative damage, increase photosynthetic rate, pollen viability, leaf phenolic concentration, and yield in UV-B-stressed plants (Karioti et al. 2008; Mohammed and Tarpley 2013; Li et al. 2014). The SA-mediated modulation of UV-induced oxidative stress was thought to be largely influenced by the activation of antioxidant enzymes in Capsicum annuum leaves ((Khan et al. 2015)). Increased photosynthetic rate, pollen viability, leaf phenolic concentration, and yield have also been reported in UV-B-stressed rice plants (Mohammed and Tarpley 2013; Khan et al. 2015). SA has been shown to decrease the level of chromosome aberrations caused by UV-B radiation in meristematic root tip cells (Rančelienė and Vyšniauskienė 2012; Khan et al. 2015). Exogenously applied SA has been reported to significantly improve photosynthetic function and its related variables in plants exposed to UV-B radiation ((Karioti et al. 2008; Khan et al. 2015). UV-C radiation has also been shown to upregulate the transcription of the SA induction deficient 2 gene, which codes for the SA biosynthetic ICS1 enzyme (Martínez et al. 2003; Khan et al. 2015). Additionally, the SA-dependent pathway has been reported to control the upregulation of the PR proteins (PR-1, PR-2, and PR-5) in UV-B-exposed transgenic NahG A. thaliana plants (Surplus et al. 1998; Khan et al. 2015).
Ozone, a strong oxidizing agent, penetrates mesophyll cells in plants through the leaf stomata; this leads to the generation of ROS after reacting directly with proteins and lipids, a process that could take mere minutes (Hara et al. 2012; Hasan et al. 2021; Leisner et al. 2022). The ozone-induced ROS then leads to the formation of an oxidative burst in the apoplast, wherein the plant undergoes lipid peroxidation and apoptosis, leading to leaf necrosis (Hara et al. 2012; Leisner et al. 2022; Ramya et al. 2023). The result of the necrosis disrupts the rate of photosynthesis, reproductive capacity, stomatal conductance, and the amount of chlorophyll in plants (Hasan et al. 2021; Ramya et al. 2023). Thereby affecting the growth rate, yield, and quality of crops (Ramya et al. 2023). Additionally, ozone-exposed plants are more vulnerable to biotic stressors (Leisner et al. 2022).
Ozone exposure has been shown to cause SA accumulation in plants (Horváth et al. 2007b; Hara et al. 2012). SA does not work alone in defending against ozone stress; instead, it acts both synergistically and antagonistically with other phytohormones belonging to the same signaling network including, ET and JA (Hara et al. 2012; Hasan et al. 2021; Leisner et al. 2022). Although ET responds faster relative to SA and JA (Grulke and Heath 2019). ET, much like SA, has been shown to defend plants against both biotic and abiotic stressors, although their role in mitigating ozone stress is not entirely understood. ET has been shown to increase SA accumulation via mediated regulation of the PAL gene (Khan et al. 2015). Whereas SA-treated plants showed increased ET production (Hara et al. 2012). On the other hand, JA impeded the oxidative burst caused by ozone upstream of SA accumulation (Leisner et al. 2022).
Genome editing and advanced genetic engineering approaches for understanding the role of SA and abiotic resistance
To explore underlying mechanism of SA to regulate climate change induced abiotic stresses, more investigations at physiological and molecular levels are required. Genetic engineering considers a great tool in revealing and filling the gaps in plant SA biochemical pathways. Genome editing holds a great opportunity to decipher and enhance our knowledge regarding the genetics involved in the role of SA. Genome editing is a technology that can accurately pinpoint a specific genomic sequence and alter that sequence for genetic improvement of biological studies (Zhang et al. 2021a). Mainly to knock out or in the function of a gene or a sequence (Abdelrahman and Zhao 2020; Tang et al. 2021; Wei et al. 2021; Abdelrahman et al. 2021b). Many genome editing techniques have been used on crops to enhance their stress tolerance and quality. Among others, clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR/Cas9), has proven to be the most versatile and highly effective (Abdelrahman and Zhao 2020; Abdelrahman et al. 2021b). Also, transformed plants with foreign genes play an important role in understanding gene function either through overexpression or being inserted as a sole copy in a test genetic background.
The NPR1 gene is the SA receptor. Its role in plant immunity to pathogens has been well described (Wu et al. 2012). Yet, its role in regulating the plant response to abiotic stress is poorly understood. To decipher its role under drought, CRISPR/Cas9 was utilized to knock out the NPR1 gene in tomatoes. Mutant plants exhibited sensitivity to drought stress and lower levels of drought-related gene transcripts, including SlGST, SlDHN, and SlDREB (Li et al. 2019). SlNPR1 knocked out mutants were sensitive to drought stress with increased H2O2 and MDA content, developed oxidative damage and suppressed antioxidant genes.
Furthermore, the NPR1-dependent SA signaling pathway was proved to be crucial for alleviating salt and oxidative stresses in A. thaliana (Jayakannan et al. 2015). Expression of AtNPR1 in transgenic tobacco plants enhanced the tolerance to oxidative stress. The tolerance was associated with a constitutive upregulation in PR1, PR2, PR5, APX, and SOD genes (Srinivasan et al. 2009). In contrast, its heterologous expression, AtNPR1, in rice plants exhibited high sensitivity to salt and drought stresses accompanied by negative regulation salt and drought stress responsive genes; (rab21, salT, and dip1) (Quilis et al. 2008). Further, molecular and physiological studies are required to explain these contradictory results questioning the role of the NPR1 gene. While overexpression of the PR1 gene enhanced the tolerance of the transgene tobacco plants to heavy metals (Sarowar et al. 2005). Another study concluded that transgenic plants where the AhSIPR10 gene was overexpressed showed tolerance to heavy metal, salt, and drought stress (Jain et al. 2012).
Furthermore, in tomatoes the expression rates of SA-related genes, SlPR1 and SlPR2, were increased in SlHyPRP1 edited plants. These edited plants showed multiple abiotic stress tolerance (Tran et al. 2023). Disrupting the OsCYP71A1 blocked serotonin biosynthesis and greatly increased salicylic acid levels in knocked-out rice plants.
CRISPR-Cas9 technology has facilitated the enhancement of plant stress tolerance through targeted gene modifications. In the context of drought stress, genes associated with stress response have been edited in various crops, such as the knockout of ABA signaling-related genes like OST2, miR169a, AVP1, AREB1, and TRE1 in Arabidopsis (Table 3) (Osakabe et al. 2014; Park et al. 2017; Roca Paixão et al. 2019), and genes involved in drought-responsive pathways like DERF1, PMS3, MSH1, MYB5, SPP, SRL1, SRL2, and ERA1 in rice (Shi et al. 2017; Liao et al. 2019; Ogata et al. 2020; Usman et al. 2020).
CRISPR/Cas9 based genome editing has been utilized successfully in different research investigations for several abiotic stress responsive genes. The heat stress response has also been augmented through CRISPR-Cas9-mediated gene editing. Heat-inducible rice mutants have been created, and heat stress sensitivity has been increased in tomatoes by editing genes such as MAPK3 and agamous-like 6 (Nandy et al. 2019; Bouzroud et al. 2020). Overexpression of the HSPS gene, which increases osmolyte levels and prevents cell protein damage, can also be used to make plants more resistant to heat (Debbarma et al. 2019). The protein kinase SAPK6 and the transcription factor OsbZIP46CA1 in rice have also been shown to increase the capacity for responding to heat stress (Chang et al. 2017). Cold stress mitigation has been achieved through CRISPR-mediated knockout of genes like OsPRP1 and OsMYB30 in rice, as well as modulation of the CBF genes in Arabidopsis and tomato (Li et al. 2022c).
Salinity stress tolerance has been improved by targeting genes like OsRR22 and OsPQT3 in rice, involved in cytokinin metabolism and salt stress response respectively (Table 4) (Takagi et al. 2015; Zhang et al. 2019; Alfatih et al. 2020). Furthermore, CRISPR-Cas9 technology has been employed to address heavy metal stress. Genes associated with heavy metal transport and detoxification, including oxp1 in Arabidopsis and OsNRAMP1 in rice, have been edited to enhance plant resistance to heavy metals like Cd and lead (Wang et al. 2017; Hasanuzzaman et al. 2019; Chu et al. 2022).
While there have been a handful of studies to genetically amplify the amount of SA in the plant to induce resistance against pathogens (Ma et al. 2018; Tezuka et al. 2021; Liu et al. 2023), little or no studies have been published to explore the possibility of inducing resistance against abiotic stressors using novel genetic techniques. This leaves the field open to exploration and potential new findings that may breed new plants with an enhanced tolerance for abiotic stressors.
Conclusion and future perspectives
This comprehensive review provides information about the genetic and physiological basis of SA's in supporting plants to withstand abiotic stress in plants. The review highlights SA's significance as a versatile safeguard for plants against various abiotic stressors. It has the potential to enhance plant resilience in the face of challenging environmental and climatic stress conditions. By exploring the complexities of SA production pathways, this review sheds light on the promising mechanisms underlying the SA enhanced plant resistance to climate change related abiotic stresses, such as cross-talks with other phytohormones, orchestrating plant responses to stress, and employing multiple mechanisms to enhance stress tolerance. It is evident that SA pathway is sensitive to the alteration of climatic factors. SA biosynthetic pathways mediated by both isochorismate synthase (ICS) and phenylalanine ammonia lyase (PAL) are regulated by abiotic derived from changes in the climate. The SA transcriptional coactivator NPR1 is positively regulated by chilling condition and suppressed by drought and high humidity(Rossi et al. 2023). The changing of abiotic stress impacts the key component of SA pathway through its biosynthesis levels, signaling, metabolism and transport in plants.
While significant knowledge has been obtained on the biosynthesis of SA and its role in stress tolerance, as it is only a recent discovery, there are still significant knowledge gaps that necessitate further investigation. For example, there is a hypothesis suggesting the involvement of a BA2H enzyme in converting BA into SA, but the precise identification of the responsible gene remains elusive. Over the years, multiple genetic mechanisms responsible for the role of SA in mitigating stress have been uncovered. Despite what we know, many mechanisms have yet to be fully understood and uncovered. For instance, how does exogenous SA interact with endogenous SA? What is the exact mechanism by which SA interacts with other phytohormones? Those and many other questions require further research to unravel the precise mechanisms underlying SA-mediated climate change mitigation and explore its potential applications in agriculture and ecosystem management.SA has been shown to alleviate the adverse effects caused by various climate change-related abiotic stressors. Exogenous SA treatment was found to induce tolerance to abiotic stressors via the induction of various signal transducers, leading to the activation of the broad-spectrum stress response, SAR, ultimately leading to the generation of signaling responses to stress (Fig. 6). While the physiological role of SA in mitigating abiotic stress has been documented broadly, few have explored the proteomic, transcriptomic, and genomic sides. Perhaps utilizing novel bioinformatics tools could help reveal the complexity of the SA signaling network and its defensive role.
Further future investigations should be employed by combining the different fields of research. Transcriptomics analysis of different genotypes under different stress conditions compared to normal conditions together with exogenous application of SA should be employed. Furthermore, comparative analysis of SA pathway involved genes in different plant species should be conducted to decipher the differences of the SA effect under different stresses in those species. This should be based on the available bioinformatic tools and online databases. The output of these research investigations should be examined by the new biotechnological tools of genome editing to study the effect of knocking in/out different genes on the genetic response of different SA linked genes. There is a great potential to use novel genetic approaches, such as genome editing, to reveal new discoveries in the genetic pathway of SA. Genome editing has been utilized successfully to breed plants for diverse effects (Abdelrahman et al. 2021a and Abdelrahman et al. 2021b). Genome editing via CRISPR/Cas9 provides a versatile tools and mechanisms to develop plants better equipped to withstand climate challenges and reduce the threat of food insecurity also remains unexplored.
Availability of data and materials
Not applicable.
Abbreviations
- SA:
-
Salicylic acid
- CKs:
-
Cytokinin
- ABA:
-
Abscisic acid
- ET:
-
Ethylene
- JA:
-
Jasmonates
- Pro:
-
Proline
- GB:
-
Glycine betaine.
- PAL:
-
Phenylalanine ammonia-lyase pathway
- ICS:
-
Isochorismate pathway
- ICS1:
-
Isochorismate synthase 1 gene
- tCA:
-
Trans-cinnamic acid
- BA:
-
Benzoic acid
- NPR:
-
Nonexpresser of pathogenesis-related Genes
- PEP:
-
Phosphoenolpyruvate
- E4P:
-
Erythrose-4-phosphate
- EDS 5:
-
enhanced disease resistance5 Gene
- PBS3:
-
avrPPHB susceptible 3 Gene
- GH3:
-
Glycoside hydrolase 3
- EPS:
-
enhanced pseudomonas susceptibility Genes
- SGE:
-
SA-β-D-Glucose Ester
- SAG:
-
SA-2-O-β-D-glucoside
- SAR:
-
Systemic acquired resistance
- MeSA:
-
Methyl salicylate
- AtAIM1 :
-
Abnormal inflorescence meristem 1
- Ba2H:
-
BA 2-hydroxylase enzyme
- SARD:
-
Systemic Acquired Resistance Deficient
- TCP:
-
Teosinte branched1/cycloidea/proliferating cell factor.
- CBP:
-
CaM-Binding Protein.
- EIN:
-
Ethylene Insensitive
- CPR:
-
constitutive expresser of pathogenesis-related Genes
- ACD:
-
accelerated cell death gene
- ROS:
-
Reactive oxygen species
- P5CSA:
-
pyrroline-5-carboxylate synthase A
- P5CSA:
-
pyrroline-5-carbonlate synthase B
- CAT:
-
catalase
- APX:
-
Ascorbate peroxidase
- ACS:
-
1-aminocyclo propane 1-carboxylic acid synthase
- SOSI:
-
salt overly sensitive 1
- NHX:
-
sodium-hydrogen exchanger
- HKT:
-
high-affinity K+ transporter
- ACS:
-
1-aminocyclo propnae 1-carbonxylic acid synthase
- CRISPR-Cas:
-
Clustered regularly interspaced short palindromic repears)-Cas (CRISPR-associated) nucleases system
References
Abbaspour J, Ehsanpour AA (2020) Sequential expression of key genes in proline, glycine betaine and artemisinin biosynthesis of Artemisia aucheri Boiss using salicylic acid under in vitro osmotic stress. Biologia (bratisl) 75:1251–1263. https://doi.org/10.2478/S11756-020-00507-W/FIGURES/5
Abdelrahman M, Zhao K (2020) Genome Editing and Rice Grain Quality. In: Costa de Oliveira A et al (eds) The Future of Rice Demand: Quality Beyond Productivity. Springer, Cham, p 395–422. https://doi.org/10.1007/978-3-030-37510-2_17
Abdelrahman M, Selim ME, Elsayed MA et al (2021a) Developing novel rice genotypes harboring specific qtl alleles associated with high grain yield under water shortage stress. Plants 10. https://doi.org/10.3390/plants10102219
Abdelrahman M, Wei Z, Rohila JS et al (2021b) Multiplex Genome-Editing Technologies for Revolutionizing Plant Biology and Crop Improvement. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.721203
Agesa BL, Onyango CM, Kathumo VM et al (2019) Climate change effects on crop production in Yatta sub-County: Farmer perceptions and adaptation strategies. Afr J Food Agric Nutr Dev 19:14010–14042. https://doi.org/10.18697/AJFAND.84.BLFB1017
Ahmad F, Kamal A, Singh A et al (2022) Salicylic Acid Modulates Antioxidant System, Defense Metabolites, and Expression of Salt Transporter Genes in Pisum sativum Under Salinity Stress. J Plant Growth Regul 41:1905–1918. https://doi.org/10.1007/S00344-020-10271-5/METRICS
Ahmad F, Singh A, Kamal A (2019) Salicylic acid-mediated defense mechanisms to abiotic stress tolerance. In: Plant Signaling Molecules: Role and Regulation under Stressful Environments. Elsevier, p 355–369
Ahmad A, Khan WU, Ali Shah A et al (2021) Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 262. https://doi.org/10.1016/j.chemosphere.2020.128384
Akasha A, Ashraf M, Shereen A et al (2019) Heat Tolerance Screening Studies and Evaluating Salicylic Acid Efficacy against High Temperature in Rice (Oryza sativa L.) Genotypes. https://doi.org/10.35248/2329-9029.19.7.235
Alavilli H, Lee H, Park M et al (2017) Antarctic moss multiprotein bridging factor 1c overexpression in arabidopsis resulted in enhanced tolerance to salt stress. Front Plant Sci 8:271055. https://doi.org/10.3389/FPLS.2017.01206/BIBTEX
Alfatih A, Wu J, Jan SU et al (2020) Loss of rice PARAQUAT TOLERANCE 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ 43:2743–2754. https://doi.org/10.1111/pce.13856
Ali S, Abbas Z, Seleiman MF et al (2020) Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 9:1–23. https://doi.org/10.3390/PLANTS9070896
Ali E, Hussain S, Jalal F et al (2023) Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front Plant Sci 14. https://doi.org/10.3389/fpls.2023.1187260
Allasia V, Industri B, Ponchet M et al (2018) Quantification of Salicylic Acid (SA) and SA-glucosides in Arabidopsis thaliana. Bio Protoc 8. https://doi.org/10.21769/bioprotoc.2844
Aloo BN, Dessureault-Rompré J, Tripathi V et al (2023) Signaling and crosstalk of rhizobacterial and plant hormones that mediate abiotic stress tolerance in plants. Front Microbiol 14. https://doi.org/10.3389/fmicb.2023.1171104
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128. https://doi.org/10.1023/a:1011931831273
Andrási N, Pettkó-Szandtner A, Szabados L (2021) Diversity of plant heat shock factors: regulation, interactions, and functions. J Exp Bot 72:1558–1575. https://doi.org/10.1093/jxb/eraa576
Austen N, Walker HJ, Lake JA et al (2019) The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions Between Heat Shock and Elevated CO2. Front Plant Sci 10:476199. https://doi.org/10.3389/FPLS.2019.01463/BIBTEX
Azarabadi S, Abdollahi H, Torabi M et al (2017) ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur J Plant Pathol 147:279–294. https://doi.org/10.1007/s10658-016-1000-0
Backer R, Naidoo S, van den Berg N (2019) The NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) and related family: Mechanistic insights in plant disease resistance. Front Plant Sci 10:102. https://doi.org/10.3389/fpls.2019.00102
Baidya A, Anwar Ali M, Atta K (2022) Climate Change and Abiotic Stresses in Plants. In: Josphert Ngui K (ed) Advances in Plant Defense Mechanisms. IntechOpen, Rijeka. https://doi.org/10.5772/intechopen.105575
Baninaiem E, Dastjerdi AM (2023) Enhancement of storage life and maintenance of quality in tomato fruits by preharvest salicylic acid treatment. Front Sustain Food Syst 7:1180243. https://doi.org/10.3389/FSUFS.2023.1180243/BIBTEX
Basirat M, Mousavi SM (2022) Effect of Foliar Application of Silicon and Salicylic Acid on Regulation of Yield and Nutritional Responses of Greenhouse Cucumber Under High Temperature. J Plant Growth Regul 41:1978–1988. https://doi.org/10.1007/S00344-021-10562-5/METRICS
Benjamin G, Pandharikar G, Frendo P (2022) Salicylic Acid in Plant Symbioses: Beyond Plant Pathogen Interactions. Biology 11:861. https://doi.org/10.3390/BIOLOGY11060861
Bernhard GH, Neale RE, Barnes PW et al (2020) Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem Photobiol Sci 19:542-584. https://doi.org/10.1039/d0pp90011g
Bharwana SA, Ali S, Farooq MA et al (2013) Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res 21:717–731. https://doi.org/10.1007/s11356-013-1920-6
Bian Z, Gao H, Wang C (2021) NAC transcription factors as positive or negative regulators during ongoing battle between pathogens and our food crops. Int J Mol Sci 22:1–21. https://doi.org/10.3390/ijms22010081
Boamah S, Ojangba T, Zhang S et al (2023) Evaluation of salicylic acid (SA) signaling pathways and molecular markers in Trichoderma-treated plants under salinity and Fusarium stresses. A Review Eur J Plant Pathol 166:259–274. https://doi.org/10.1007/s10658-023-02660-9
Bouzroud S, Gasparini K, Hu G et al (2020) Down Regulation and Loss of Auxin Response Factor 4 Function Using CRISPR/Cas9 Alters Plant Growth, Stomatal Function and Improves Tomato Tolerance to Salinity and Osmotic Stress. Genes 11:272. https://doi.org/10.3390/genes11030272
Buraphaka H, Putalun W (2020) Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind Crops Prod 146:112171. https://doi.org/10.1016/J.INDCROP.2020.112171
Bussell JD, Reichelt M, Wiszniewski AAG et al (2014) Peroxisomal ATP-Binding Cassette Transporter COMATOSE and the Multifunctional Protein ABNORMAL INFLORESCENCE MERISTEM Are Required for the Production of Benzoylated Metabolites in Arabidopsis Seeds. Plant Physiol 164:48–54. https://doi.org/10.1104/PP.113.229807
Cao FY, Yoshioka K, Desveaux D (2011) The roles of ABA in plant-pathogen interactions. J Plant Res 124:489–499. https://doi.org/10.1007/s10265-011-0409-y
Chakma R, Biswas A, Saekong P et al (2021) Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci Hortic 280:109904. https://doi.org/10.1016/j.scienta.2021.109904
Chandran D, Rickert J, Huang Y et al (2014) Atypical E2F Transcriptional Repressor DEL1 Acts at the Intersection of Plant Growth and Immunity by Controlling the Hormone Salicylic Acid. Cell Host Microbe 15:506–513. https://doi.org/10.1016/j.chom.2014.03.007
Chang YK, Nguyen BH, Xie Y et al (2017) Co-overexpression of the Constitutively Active Form of OsbZIP46 and ABA-Activated Protein Kinase SAPK6 Improves Drought and Temperature Stress Resistance in Rice. Front Plant Sci 8:1102. https://doi.org/10.3389/fpls.2017.01102
Chaouch S, Queval G, Vanderauwera S et al (2010) Peroxisomal Hydrogen Peroxide Is Coupled to Biotic Defense Responses by ISOCHORISMATE SYNTHASE1 in a Daylength-Related Manner. Plant Physiol 153:1692–1705. https://doi.org/10.1104/pp.110.153957.
Chen H, Hou W, Kuć J et al (2001) Ca2+-dependent and Ca2+-independent excretion modes of salicylic acid in tobacco cell suspension culture. J Exp Bot 52:1219–1226. https://doi.org/10.1093/jexbot/52.359.1219
Chen H, Xue L, Chintamanani S et al (2009) ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21:2527–2540. https://doi.org/10.1105/tpc.108.065193
Chen J, Clinton M, Qi G et al (2020) Reprogramming and remodeling: Transcriptional and epigenetic regulation of salicylic acid-mediated plant defense. J Exp Bot 71:5256–5268. https://doi.org/10.1093/jxb/eraa072
Chen S, Ding Y, Tian H et al (2021) WRKY54 and WRKY70 positively regulate SARD1 and CBP60g expression in plant immunity. Plant Signal Behav 16:1932142. https://doi.org/10.1080/15592324.2021.1932142
Chen C, Sun C, Wang Y et al (2023) The preharvest and postharvest application of salicylic acid and its derivatives on storage of fruit and vegetables: A review. Sci Hortic 312:111858. https://doi.org/10.1016/J.SCIENTA.2023.111858
Chieb M, Gachomo EW (2023) The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biology 2023 23:1 23:1–23. https://doi.org/10.1186/S12870-023-04403-8
Chong J, Pierrel M-A, Atanassova R et al (2001) Free and Conjugated Benzoic Acid in Tobacco Plants and Cell Cultures. Induced Accumulation upon Elicitation of Defense Responses and Role as Salicylic Acid Precursors. Plant Physiol 125:318-328. https://doi.org/10.1104/pp.125.1.318
Choudhury S, Panda SK (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol 30:95-110.
Chu C, Huang R, Liu L et al (2022) The rice heavy-metal transporter OsNRAMP1 regulates disease resistance by modulating ROS homoeostasis. Plant Cell Environ 45:1109–1126. https://doi.org/10.1111/pce.14263
Corina Vlot A, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. https://doi.org/10.1146/annurev.phyto.050908.135202
Cosima P, Daniela B, Jürgen H (2003) Irp9, Encoded by the High-Pathogenicity Island of Yersinia enterocolitica, Is Able To Convert Chorismate into Salicylate, the Precursor of the Siderophore Yersiniabactin. J Bacteriol 185:5648–5653. https://doi.org/10.1128/jb.185.18.5648-5653.2003
Dai A (2010) Drought under global warming: a review. Wiley Interdiscip Rev Clim Change 2:45–65. https://doi.org/10.1002/wcc.81
Dawood MFA, Zaid A, Latef AAHA (2022) Salicylic Acid Spraying-Induced Resilience Strategies Against the Damaging Impacts of Drought and/or Salinity Stress in Two Varieties of Vicia faba L. Seedlings J Plant Growth Regul 41:1919–1942. https://doi.org/10.1007/S00344-021-10381-8
Dean JV, Delaney SP (2008) Metabolism of salicylic acid in wild-type, ugt74f1 and ugt74f2 glucosyltransferase mutants of Arabidopsis thaliana. Physiol Plant 132:417–425. https://doi.org/10.1111/j.1399-3054.2007.01041.x
Debbarma J, Sarki YN, Saikia B et al (2019) Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol Biotechnol 61:153–172. https://doi.org/10.1007/s12033-018-0144-x
Ding P, Ding Y (2020) Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci 25:549–565. https://doi.org/10.1016/j.tplants.2020.01.004
Ding Y, Sun T, Ao K et al (2018) Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 173:1454-1467.e15. https://doi.org/10.1016/j.cell.2018.03.044
Dumanović J, Nepovimova E, Natić M et al (2021) The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front Plant Sci 11:552969. https://doi.org/10.3389/fpls.2020.552969
Eckardt NA, Cutler S, Juenger TE et al (2023) Focus on climate change and plant abiotic stress biology. Plant Cell 35:1–3. https://doi.org/10.1093/PLCELL/KOAC329
Eichmann R, Schäfer P (2015) Growth versus immunity-a redirection of the cell cycle? Curr Opin Plant Biol 26:106–112. https://doi.org/10.1016/j.pbi.2015.06.006
Ejtahed RS, Radjabian T, Hoseini Tafreshi SA (2015) Expression Analysis of Phenylalanine Ammonia Lyase Gene and Rosmarinic Acid Production in Salvia officinalis and Salvia virgata Shoots Under Salicylic Acid Elicitation. Appl Biochem Biotechnol 176:1846–1858. https://doi.org/10.1007/S12010-015-1682-3/FIGURES/7
Fahad S, Bajwa AA, Nazir U et al (2017) Crop production under drought and heat stress: Plant responses and management options. Front Plant Sci 8:147. https://doi.org/10.3389/fpls.2017.01147
Faizan M, Sehar S, Rajput VD et al (2021) Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 10. https://doi.org/10.3390/plants10112254
Farooq M, Basra SMA, Wahid A et al (2009a) Improving the Drought Tolerance in Rice (Oryza sativa L.) by Exogenous Application of Salicylic Acid. J Agron Crop Sci 195:237–246. https://doi.org/10.1111/J.1439-037X.2009.00365.X
Farooq M, Wahid A, Kobayashi N et al (2009b) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212. https://doi.org/10.1051/agro:2008021
Fawzy S, Osman AI, Doran J et al (2020) Strategies for mitigation of climate change: a review. Environ Chem Lett 18:2069–2094. https://doi.org/10.1007/s10311-020-01059-w
Feizi H, Moradi R, Pourghasemian N et al (2021) Assessing saffron response to salinity stress and alleviating potential of gamma amino butyric acid, salicylic acid and vermicompost extract on salt damage. S Afr J Bot 141:330–343. https://doi.org/10.1016/J.SAJB.2021.04.036
Feng Y, Zhao Y, Li Y et al (2023) Improving photosynthesis and drought tolerance in Nicotiana tabacum L. by foliar application of salicylic acid. All Life 16. https://doi.org/10.1080/26895293.2023.2224936
Garcion C, Lohmann A, Lamodière E et al (2008a) Characterization and biological function of the Isochorismate Synthase2 gene of Arabidopsis. Plant Physiol 147:1279–1287. https://doi.org/10.1104/pp.108.119420
Gechev T, Petrov V (2020) Reactive oxygen species and abiotic stress in plants. Int J Mol Sci 21:1–5. https://doi.org/10.3390/ijms21207433
Gomes EP, Vanz Borges C, Monteiro GC et al (2021) Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’ table grape. Postharvest Biol Technol 176:111505. https://doi.org/10.1016/J.POSTHARVBIO.2021.111505
Gondor OK, Maqsood MF, Shahbaz M et al (2023) Enhancing Wheat Growth and Yield through Salicylic Acid-Mediated Regulation of Gas Exchange, Antioxidant Defense, and Osmoprotection under Salt Stress. Stresses 3:372–386. https://doi.org/10.3390/STRESSES3010027
Grulke NE, Heath RL (2019) Ozone effects on plants in natural ecosystems. Plant Biol 22:12–37. https://doi.org/10.1111/PLB.12971
Guan C, Song X, Ji J et al (2014) Salicylic acid treatment enhances expression of chalcone isomerase gene and accumulation of corresponding flavonoids during fruit maturation of Lycium chinense. Eur Food Res Technol 239:857–865. https://doi.org/10.1007/S00217-014-2282-0/FIGURES/7
Gunes A, Inal A, Alpaslan M et al (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol 164:728–736. https://doi.org/10.1016/j.jplph.2005.12.009
Guo B, Liang YC, Zhu YG et al (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749. https://doi.org/10.1016/j.envpol.2006.09.007
Guo B, Liang Y, Zhu Y (2009) Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J Plant Physiol 166:20–31. https://doi.org/10.1016/j.jplph.2008.01.002
Guo Q, Zhao L, Fan X et al (2019) Transcription factor garwrky5 is involved in salt stress response in diploid cotton species (Gossypium aridum L.). Int J Mol Sci 20. https://doi.org/10.3390/ijms20215244
Haider S, Raza A, Iqbal J et al (2022) Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: a brief appraisal. Mol Biol Rep 49:5771–5785. https://doi.org/10.1007/s11033-022-07190-x
Haile MG, Wossen T, Tesfaye K et al (2017) Impact of Climate Change, Weather Extremes, and Price Risk on Global Food Supply. Econ Disasters Clim Chang 1:55–75. https://doi.org/10.1007/S41885-017-0005-2
Haile GG, Tang Q, Hosseini-Moghari SM et al (2020) Projected Impacts of Climate Change on Drought Patterns Over East Africa. Earth Future 8:e2020EF001502. https://doi.org/10.1029/2020EF001502
Hao Q, Wang W, Han X et al (2018) Isochorismate-based salicylic acid biosynthesis confers basal resistance to Fusarium graminearum in barley. Mol Plant Pathol 19:1995–2010. https://doi.org/10.1111/mpp.12675
Hara M, Furukawa J, Sato A et al (2012) Abiotic Stress and Role of Salicylic Acid in Plants. In: Ahmad P, Prasad M (eds) Abiotic Stress Responses in Plants. Springer, New York. https://doi.org/10.1007/978-1-4614-0634-1_13
Hasan MM, Rahman MA, Skalicky M et al (2021) Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int J Mol Sci 22:6304. https://doi.org/10.3390/IJMS22126304
Hasanuzzaman M, Bhuyan MHM, Anee TI et al (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hayat Q, Hayat S, Irfan M et al (2010) Effect of exogenous salicylic acid under changing environment: A review. Environ Exp Bot 68:14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
Hayat S, Hayat Q, Alyemeni MN et al (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456-1466. https://doi.org/10.4161/PSB.21949
Herrera-Vásquez A, Salinas P, Holuigue L (2015) Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front Plant Sci 6:1–9. https://doi.org/10.3389/fpls.2015.00171
Hideg É, Jansen MA, Strid Å (2013) UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci 18:107–115. https://doi.org/10.1016/j.tplants.2012.09.003
Holland CK, Westfall CS, Schaffer JE et al (2019) Brassicaceae-specific Gretchen Hagen 3 acyl acid amido synthetases conjugate amino acids to chorismate, a precursor of aromatic amino acids and salicylic acid. J Biol Chem 294:16855–16864. https://doi.org/10.1074/jbc.RA119.009949
Hongna C, Leyuan T, Junmei S et al (2021) Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ Exp Bot 188:104498. https://doi.org/10.1016/J.ENVEXPBOT.2021.104498
Horváth E, Szalai G, Janda T (2007b) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300. https://doi.org/10.1007/S00344-007-9017-4/FIGURES/1
Horváth E, Pál M, Szalai G et al (2007a) Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biologia Plantarum 51:480–487. https://doi.org/10.1007/S10535-007-0101-1
Huang J, Gu M, Lai Z et al (2010a) Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol 153:1526–1538. https://doi.org/10.1104/PP.110.157370
Huang W, Wang Y, Li X et al (2020) Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity. Mol Plant 13:31–41. https://doi.org/10.1016/j.molp.2019.12.008
Ibdah M, Chen YT, Wilkerson CG et al (2009) An aldehyde oxidase in developing seeds of arabidopsis converts benzaldehyde to benzoic acid. Plant Physiol 150:416–423. https://doi.org/10.1104/pp.109.135848
Ihsan MZ, Khaliq A, Siddiqui MH et al (2022) The Response of Triticum aestivum Treated with Plant Growth Regulators to Acute Day/Night Temperature Rise. J Plant Growth Regul 41:2020–2033. https://doi.org/10.1007/S00344-022-10574-9/METRICS
Imran QM, Falak N, Hussain A et al (2021) Abiotic stress in plants, stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy 11:1579. https://doi.org/10.3390/agronomy11081579
Iqbal N, Umar S, Khan NA et al (2014) A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ Exp Bot 100:34–42. https://doi.org/10.1016/j.envexpbot.2013.12.006
Iqbal N, Fatma M, Gautam H et al (2022) Salicylic Acid Increases Photosynthesis of Drought Grown Mustard Plants Effectively with Sufficient-N via Regulation of Ethylene, Abscisic Acid, and Nitrogen-Use Efficiency. J Plant Growth Regul 41:1966–1977. https://doi.org/10.1007/S00344-021-10565-2/METRICS
Jagendorf AT, Takabe T (2001) Inducers of Glycinebetaine Synthesis in Barley. Plant Physiol 127:1827–1835. https://doi.org/10.1104/pp.010392
Jain S, Kumar D, Jain M et al (2012) Ectopic overexpression of a salt stress-induced pathogenesis-related class 10protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell Tiss Organ Cult 109:19–31. https://doi.org/10.1007/s11240-011-0069-6
Jayakannan M, Bose J, Babourina O et al (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64:2255–2268. https://doi.org/10.1093/JXB/ERT085
Jayakannan M, Bose J, Babourina O et al (2015) The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J Exp Bot 66:1865–1875. https://doi.org/10.1093/JXB/ERU528
Mercado-Blanco J, van der Drift KM, Olsson PE et al (2001) Analysis of the pmsCEAB Gene Cluster Involved in Biosynthesis of Salicylic Acid and the Siderophore Pseudomonine in the Biocontrol Strain Pseudomonas fluorescensWCS374. J Bacteriol 183:1909–1920. https://doi.org/10.1128/jb.183.6.1909-1920.2001
Ji T, Li S, Huang M et al (2017) Overexpression of cucumber phospholipase D alpha gene (CsPLDα) in tobacco enhanced salinity stress tolerance by regulating Na+-K+ balance and lipid peroxidation. Front Plant Sci 8:248312. https://doi.org/10.3389/FPLS.2017.00499/BIBTEX
Jia X, Wang L, Zhao H et al (2023) The origin and evolution of salicylic acid signaling and biosynthesis in plants. Mol Plant 16:245–259. https://doi.org/10.1016/j.molp.2022.12.002
Kamran M, Xie K, Sun J et al (2020) Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol Environ Saf 188:109877. https://doi.org/10.1016/j.ecoenv.2019.109877
Kang G, Li G, Zheng B et al (2012) Proteomic analysis on salicylic acid-induced salt tolerance in common wheat seedlings (Triticum aestivum L.). BBA-Proteins Proteomics 1824:1324–1333. https://doi.org/10.1016/j.bbapap.2012.07.012
Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576. https://doi.org/10.1034/J.1399-3054.2002.1150411.X
Karioti A, Kitsaki CK, Zygouraki S et al (2008) Occurrence of flavonoids in Ophrys (Orchidaceae) flower parts. Flora Morphol Distrib Funct Ecol Plants 203:602–609. https://doi.org/10.1016/j.flora.2007.09.009
Kaya C, Ashraf M, Alyemeni MN et al (2020) The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol Biochem 147:10–20. https://doi.org/10.1016/J.PLAPHY.2019.11.040
Kaya C, Ugurlar F, Ashraf M et al (2023) Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol Biochem 196:431–443. https://doi.org/10.1016/J.PLAPHY.2023.02.006
Khan MIR, Iqbal N, Masood A et al (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8:e26374–e26374. https://doi.org/10.4161/psb.26374
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74. https://doi.org/10.1016/j.plaphy.2014.03.026
Khan MIR, Fatma M, Per TS et al (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:135066. https://doi.org/10.3389/FPLS.2015.00462/BIBTEX
Khan MIR, Reddy PS, Ferrante A et al (eds) (2019) Plant signaling molecules: role and regulation under stressful environments. Woodhead Publishing. https://doi.org/10.1016/C2017-0-03384-0
Khan MIR, Poor P, Janda T (2022) Salicylic Acid: A Versatile Signaling Molecule in Plants. J Plant Growth Regul 41:1887–1890. https://doi.org/10.1007/S00344-022-10692-4/METRICS
Khokon MDAR, Okuma E, Hossain MA et al (2010) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443. https://doi.org/10.1111/j.1365-3040.2010.02253.x
Khokon MAR, Okuma E, Hossain MA et al (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443. https://doi.org/10.1111/j.1365-3040.2010.02253.x
Kumar D (2014) Salicylic acid signaling in disease resistance. Plant Sci 228:127–134. https://doi.org/10.1016/j.plantsci.2014.04.014
Kundu P, Nehra A, Gill R et al (2022) Unraveling the importance of EF-hand-mediated calcium signaling in plants. S Afr J Bot 148:615–633. https://doi.org/10.1016/J.SAJB.2022.04.045
La VH, Lee BR, Islam MT et al (2019a) Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ Exp Bot 157:1–10. https://doi.org/10.1016/J.ENVEXPBOT.2018.09.013
La VH, Lee BR, Zhang Q et al (2019b) Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic Environ Biotechnol 60:31–40. https://doi.org/10.1007/S13580-018-0099-7/FIGURES/4
Lebrun-Garcia A, Ouaked F, Chiltz A et al (1998) Activation of MAPK homologues by elicitors in tobacco cells. . Plant J 15:773-781. https://doi.org/10.1046/j.1365-313x.1998.00269.x
Lefevere H, Bauters L, Gheysen G (2020) Salicylic Acid Biosynthesis in Plants. Front Plant Sci 11:338. https://doi.org/10.3389/fpls.2020.00338
Leisner CP, Potnis N, Sanz-Saez A (2022) Crosstalk and trade-offs: Plant responses to climate change-associated abiotic and biotic stresses. Plant Cell Environ. https://doi.org/10.1111/PCE.14532
Levitt J (1980) High-Temperature or Heat Stress. In: Levitt J (ed) Chilling, Freezing, and High Temperature Stresses. Academic Press, p 347-393. https://doi.org/10.1016/B978-0-12-445501-6.50016-6
Li T, Hu Y, Du X et al (2014) Salicylic Acid Alleviates the Adverse Effects of Salt Stress in Torreya grandis cv. Merrillii Seedlings by Activating Photosynthesis and Enhancing Antioxidant Systems. PLoS ONE 9:e109492. https://doi.org/10.1371/journal.pone.0109492
Li R, Liu C, Zhao R et al (2019) CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol 19:38. https://doi.org/10.1186/s12870-018-1627-4
Li X, Xu S, Fuhrmann-Aoyagi MB et al (2022c) CRISPR/Cas9 Technique for Temperature, Drought, and Salinity Stress Responses. Curr Issues Mol Biol 44:2664–2682. https://doi.org/10.3390/cimb44060182
Li F, Chen X, Yang R et al (2023a) Potato protein tyrosine phosphatase StPTP1a is activated by StMKK1 to negatively regulate plant immunity. Plant Biotechnol J 21:646–661. https://doi.org/10.1111/pbi.13979
Li W, He J, Wang X et al (2023b) PBS3: a versatile player in and beyond salicylic acid biosynthesis in Arabidopsis. New Phytol 237:414–422. https://doi.org/10.1111/nph.18558
Li X-C, Chang C, Pei Z-M (2023c) Reactive Oxygen Species in Drought-Induced Stomatal Closure: The Potential Roles of NPR1. Plants 12:3194. https://doi.org/10.3390/PLANTS12183194
Li P, Li Y-J, Zhang F-J et al (2017) The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J 89:85–103. https://doi.org/10.1111/tpj.13324
Li A, Sun X, Liu L (2022a) Action of Salicylic Acid on Plant Growth. Front Plant Sci 13:878076. https://doi.org/10.3389/fpls.2022.878076
Li T, Zhang H, Xu L et al (2022b) StMPK7 phosphorylates and stabilizes a potato RNA-binding protein StUBA2a/b to enhance plant defence responses. Hortic Res 9. https://doi.org/10.1093/hr/uhac177
Liao S, Qin X, Luo L et al (2019) CRISPR/Cas9-Induced Mutagenesis of Semi-Rolled Leaf1,2 Confers Curled Leaf Phenotype and Drought Tolerance by Influencing Protein Expression Patterns and ROS Scavenging in Rice (Oryza sativa L.). Agronomy 9:728. https://doi.org/10.3390/agronomy9110728
Lin L, Wu J, Jiang M et al (2021) Plant mitogen-activated protein kinase cascades in environmental stresses. Int J Mol Sci 22:1–24
Liu X, HUANG B (2005) Root physiological factors involved in cool-season grass response to high soil temperature. Environ Exp Bot 53:233–245. https://doi.org/10.1016/j.envexpbot.2004.03.016
Liu Y, Zhang J, Liu H et al (2008) Salicylic acid or heat acclimation pre-treatment enhances the plasma membrane-associated ATPase activities in young grape plants under heat shock. Sci Hortic 119:21–27. https://doi.org/10.1016/j.scienta.2008.06.027
Liu P, Xu Z-S, Lu P et al (2013) A wheat PI4K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J Exp Bot 64:2915–2927. https://doi.org/10.1093/jxb/ert133
Liu X, Wu D, Shan T et al (2020a) The trihelix transcription factor OsGTγ-2 is involved adaption to salt stress in rice. Plant Mol Biol 103:545–560. https://doi.org/10.1007/s11103-020-01010-1
Liu Y, Sun T, Sun Y et al (2020b) Diverse roles of the salicylic acid receptors NPR1 and NPR3/ NPR4 in plant immunity. Plant Cell 32:4002–4016. https://doi.org/10.1105/tpc.20.00499
Liu J, Qiu G, Liu C et al (2022) Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life-Basel 12:886. https://doi.org/10.3390/LIFE12060886
Liu X, Yu Y, Yao W et al (2023) CRISPR/Cas9-mediated simultaneous mutation of three salicylic acid 5-hydroxylase (OsS5H) genes confers broad-spectrum disease resistance in rice. Plant Biotechnol J 21:1873–1886. https://doi.org/10.1111/PBI.14099
Lone R, Shuab R, Kamili AN (eds) (2020) Plant Phenolics in Sustainable Agriculture: Volume 1. Springer Singapore. https://doi.org/10.1007/978-981-15-4890-1
Lou D, Wang H, Liang G et al (2017) OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00993
Ma J, Chen J, Wang M et al (2018) Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J Exp Bot 69:1051–1064. https://doi.org/10.1093/JXB/ERX458
Maier F, Zwicker S, Hückelhoven A et al (2011) Nonexpressor Of Pathogenesis-Related Proteins1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol Plant Pathol 12:73–91. https://doi.org/10.1111/j.1364-3703.2010.00653.x
Malhi GS, Kaur M, Kaushik P (2021) Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 13:1–21. https://doi.org/10.3390/su13031318
Manabe S (2019) Role of greenhouse gas in climate change**. Tellus Ser A-Dyn Meteorol Oceanogr 71:1–13. https://doi.org/10.1080/16000870.2019.1620078
Mansoor S, Wani OA, Lone JK et al (2022) Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 11:225. https://doi.org/10.3390/antiox11020225
Martínez C, Pons E, Prats G et al (2003) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37:209–217. https://doi.org/10.1046/j.1365-313x.2003.01954.x
Mehana M, Abdelrahman M, Emadeldin Y et al (2021) Impact of genetic improvements of rice on its water use and effects of climate variability in egypt. Agriculture-Basel 11. https://doi.org/10.3390/agriculture11090865
Mejía-Teniente L, de Dalia Duran-Flores F, Chapa-Oliver AM et al (2013) Oxidative and molecular responses in Capsicum Annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int J Mol Sci 14:10178–10196. https://doi.org/10.3390/ijms140510178
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145. https://doi.org/10.1242/dev.164376
Mikhaylov A, Moiseev N, Aleshin K et al (2020) Global climate change and greenhouse effect. Entrepreneurship Sustain 7:2897–2913. https://doi.org/10.9770/jesi.2020.7.4(21)
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489. https://doi.org/10.1111/j.1399-3054.2008.01090.x
Mishra AK, Baek KH (2021) Salicylic acid biosynthesis and metabolism: A divergent pathway for plants and bacteria. Biomolecules 11:705. https://doi.org/10.3390/biom11050705
Misra N, Misra R (2012) Salicylic Acid Changes Plant Growth Parameters and Proline Metabolism in Rauwolfia serpentina Leaves Grown under Salinity Stress. J Agric Environ Sci 12:1601–1609. https://doi.org/10.5829/idosi.aejaes.2012.12.12.1919
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177:181–189. https://doi.org/10.1016/j.plantsci.2009.05.007
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125. https://doi.org/10.1016/j.tibs.2011.11.007
Miura K, Lee J, Jin JB et al (2009) Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc Natl Acad Sci U S A 106:5418–5423. https://doi.org/10.1073/pnas.0811088106
Miura K, Okamoto H, Okuma E et al (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73:91–104. https://doi.org/10.1111/tpj.12014
Mo W, Tang W, Du Y et al (2020) PHYTOCHROME-INTERACTING FACTOR-LIKE14 and SLENDER RICE1 Interaction Controls Seedling Growth under Salt Stress1. Plant Physiol 184:506–517. https://doi.org/10.1104/PP.20.00024
Mohammed AR, Tarpley L (2013) Effects of Enhanced Ultraviolet-B (UV-B) Radiation and Antioxidative-type Plant Growth Regulators on Rice (Oryza sativa L.) Leaf Photosynthetic Rate, Photochemistry and Physiology. J Agric Sci 5. https://doi.org/10.5539/jas.v5n5p115
Molina A, Bueno P, Marín MC et al (2002) Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol 156:409–415. https://doi.org/10.1046/J.1469-8137.2002.00527.X
Mori IC, Pinontoan R, Kawano T et al (2001) Involvement of Superoxide Generation in Salicylic Acid-Induced Stomatal Closure in Vicia faba. Plant Cell Physiol 42:1383–1388. https://doi.org/10.1093/pcp/pce176
Mostofa MG, Saegusa D, Fujita M et al (2015) Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.01055
Mukherjee A, Gaurav AK, Singh S et al (2022) The bioactive potential of phytohormones: A review. Biotechnol Rep 35:e00748. https://doi.org/10.1016/j.btre.2022.e00748
Müller-Xing R, Xing Q, Goodrich J (2014) Footprints of the sun: memory of UV and light stress in plants. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00474
Murphy AM, Zhou T, Carr JP (2020) An update on salicylic acid biosynthesis, its induction and potential exploitation by plant viruses. Curr Opin Virol 42:8–17. https://doi.org/10.1016/j.coviro.2020.02.008
Nadarajah KK (2020) Ros homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 21:1–29. https://doi.org/10.3390/ijms21155208
Nandy S, Pathak B, Zhao S et al (2019) Heat-shock-inducible CRISPR/Cas9 system generates heritable mutations in rice. Plant Direct 3:e00145. https://doi.org/10.1002/pld3.145
Nazar R, Iqbal N, Syeed S et al (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 168:807–815. https://doi.org/10.1016/j.jplph.2010.11.001
Nazar R, Iqbal N, Umar S (2017) Heat stress tolerance in plants: Action of salicylic acid. In: Nazar R, Iqbal N, Khan N (eds) Salicylic Acid: A Multifaceted Hormone. Springer, Singapore, p 145–161. https://doi.org/10.1007/978-981-10-6068-7_8/TABLES/1
Niu Y, Ye L, Wang Y et al (2023) Transcriptome analysis reveals salicylic acid treatment mitigates chilling injury in kiwifruit by enhancing phenolic synthesis and regulating phytohormone signaling pathways. Postharvest Biol Technol 205:112483. https://doi.org/10.1016/J.POSTHARVBIO.2023.112483
Njålsson R, Norgren S (2005) Physiological and pathological aspects of GSH metabolism. Acta Paediatr 94:132–137. https://doi.org/10.1111/j.1651-2227.2005.tb01878.x
Ogata T, Ishizaki T, Fujita M (2020) CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 15:e0243376. https://doi.org/10.1371/journal.pone.0243376
Omidi M, Khandan-Mirkohi A, Kafi M et al (2022) Biochemical and molecular responses of Rosa damascena mill. cv. Kashan to salicylic acid under salinity stress. BMC Plant Biol 22:1–20. https://doi.org/10.1186/S12870-022-03754-Y/FIGURES/1
Osakabe Y, Osakabe K, Shinozaki K et al (2014) Response of plants to water stress. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00086
Pál M, Szalai G, Kovács V et al (2013) Salicylic Acid-Mediated Abiotic Stress Tolerance. In: Hayat S, Ahmad A, Alyemeni M (eds) SALICYLIC ACID. Springer, Dordrecht, p 183–247. https://doi.org/10.1007/978-94-007-6428-6_10
Pan Q, Zhan J, Liu H et al (2006) Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci 171:226–233. https://doi.org/10.1016/j.plantsci.2006.03.012
Panchal S, Chitrakar R, Thompson BK et al (2016) Regulation of Stomatal Defense by Air Relative Humidity. Plant Physiol 172:2021–2032. https://doi.org/10.1104/pp.16.00696
Park J-J, Dempewolf E, Zhang W et al (2017) RNA-guided transcriptional activation via CRISPR/dCas9 mimics overexpression phenotypes in Arabidopsis. PLoS ONE 12:e0179410. https://doi.org/10.1371/journal.pone.0179410
Peng Y, Yang J, Li X et al (2021) Salicylic Acid: Biosynthesis and Signaling. Annu Rev Plant Biol 72:761–791. https://doi.org/10.1146/annurev-arplant-081320-092855
Pokotylo I, Kravets V, Ruelland E (2019) Salicylic acid binding proteins (SABPs): The hidden forefront of salicylic acid signalling. Int J Mol Sci 20:4377. https://doi.org/10.3390/ijms20184377
Prodhan MY, Munemasa S, Nahar MNEN et al (2018) Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol 178:441–450. https://doi.org/10.1104/pp.18.00321
Qiu T, Qi M, Ding X et al (2020) The SAUR41 subfamily of SMALL AUXIN up RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann Bot 125:805–819. https://doi.org/10.1093/aob/mcz160
Quilis J, Peñas G, Messeguer J et al (2008) The Arabidopsis AtNPR1 Inversely Modulates Defense Responses Against Fungal, Bacterial, or Viral Pathogens While Conferring Hypersensitivity to Abiotic Stresses in Transgenic Rice. Mol Plant Microbe Interact 21:1215–1231. https://doi.org/10.1094/MPMI-21-9-1215
Rahimi S, Devi BSR, Khorolragchaa A et al (2014) Effect of salicylic acid and yeast extract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ J Plant Physiol 61:811–817. https://doi.org/10.1134/S1021443714060156/METRICS
Rai KK, Pandey N, Rai SP (2020) Salicylic acid and nitric oxide signaling in plant heat stress. Physiol Plant 168:241–255. https://doi.org/10.1111/ppl.12958
Raja V, Majeed U, Kang H et al (2017) Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157. https://doi.org/10.1016/j.envexpbot.2017.02.010
Ramya A, Dhevagi P, Poornima R et al (2023) Effect of ozone stress on crop productivity: A threat to food security. Environ Res 236:116816. https://doi.org/10.1016/J.ENVRES.2023.116816
Rančelienė V, Vyšniauskienė R (2012) Modification of UV-B radiation effect on Crepis capillaris by antioxidant and environmental conditions. Emir J Food Agric 24. https://doi.org/10.9755/ejfa.v24i6.14680
Rasmussen S, Dixon RA (1999) Transgene-Mediated and Elicitor-Induced Perturbation of Metabolic Channeling at the Entry Point into the Phenylpropanoid Pathway. Plant Cell 11:1537-1552. https://doi.org/10.1105/tpc.11.8.1537
Raza A, Charagh S, Abbas S et al (2023) Assessment of proline function in higher plants under extreme temperatures. Plant Biol 25:379–395. https://doi.org/10.1111/PLB.13510
Raza A, Razzaq A, Mehmood SS et al (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 8:34. https://doi.org/10.3390/plants8020034
Rekhter D, Lüdke D, Ding Y et al (2019) Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365:498–502. https://doi.org/10.1126/SCIENCE.AAW1720
Richmond TA, Bleecker AB (1999) A Defect in β-Oxidation Causes Abnormal Inflorescence Development in Arabidopsis. Plant Cell 11:1911–1923. https://doi.org/10.1105/tpc.11.10.1911
Roca Paixão JF, Gillet F-X, Ribeiro TP et al (2019) Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a Histone AcetylTransferase. Sci Rep 9. https://doi.org/10.1038/s41598-019-44571-y
Rodas-Junco BA, Cab-Guillén Y, Muñoz-Sánchez JA et al (2013) Salicylic acid induces vanillin synthesis through the phospholipid signaling pathway in Capsicum chinense cell cultures. Plant Signal. Behav 8:e26752. https://doi.org/10.4161/PSB.26752
Rossi CAM, Marchetta EJR, Kim JH et al (2023) Molecular regulation of the salicylic acid hormone pathway in plants under changing environmental conditions. Trends Biochem Sci 48:699–712. https://doi.org/10.1016/J.TIBS.2023.05.004
Sabagh EL, A, Islam MS, Hossain A et al (2022) Phytohormones as Growth Regulators During Abiotic Stress Tolerance in Plants. Front Agron 4:765068. https://doi.org/10.3389/FAGRO.2022.765068/BIBTEX
Sachdev S, Ansari SA, Ansari MI et al (2021) Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 10:1–37. https://doi.org/10.3390/antiox10020277
Sakuma Y, Maruyama K, Osakabe Y et al (2006) Functional Analysis of an Arabidopsis Transcription Factor, DREB2A, Involved in Drought-Responsive Gene Expression. Plant Cell 18:1292–1309. https://doi.org/10.1105/tpc.105.035881
Saleem M, Fariduddin Q, Janda T (2020) Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J Plant Growth Regul 40:464–485. https://doi.org/10.1007/S00344-020-10152-X
Saleem M, Fariduddin Q, Castroverde CDM (2021) Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol Biochem168:381–397. https://doi.org/10.1016/j.plaphy.2021.10.011
Sangwan S, Shameem N, Yashveer S et al (2022) Role of Salicylic Acid in Combating Heat Stress in Plants: Insights into Modulation of Vital Processes. Front Biosci 27:310. https://doi.org/10.31083/j.fbl2711310
Santisree P, Jalli LCL, Bhatnagar-Mathur P et al (2020) Emerging Roles of Salicylic Acid and Jasmonates in Plant Abiotic Stress Responses. In: Protective Chemical Agents in the Amelioration of Plant Abiotic Stress. p 342–373. https://doi.org/10.1002/9781119552154.ch17
Sarowar S, Kim YJ, Kim EN et al (2005) Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep 24:216–224. https://doi.org/10.1007/s00299-005-0928-x
Saruhan N, Saglam A, Kadioglu A (2011) Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol Plant 34:97–106. https://doi.org/10.1007/s11738-011-0808-7
Sarwar M, Saleem MF, Ullah N et al (2018) Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci Rep 8. https://doi.org/10.1038/s41598-018-35420-5
Saunders JA, Olechno JD (1988) Radioisotope detectors for investigations on phenolic biosynthesis. In: Progress in HPLC. p 167–189
Sawada H, Shim IS, Usui K (2006) Induction of benzoic acid 2-hydroxylase and salicylic acid biosynthesis-Modulation by salt stress in rice seedlings. Plant Sci 171:263–270. https://doi.org/10.1016/j.plantsci.2006.03.020
Shah T, Wahid S, Ilyas M et al (2019) Nitric Oxide and Phytohormones Cross-Talk During Abiotic Stresses Responses in Plants. In: Hasanuzzaman M et al (eds) Reactive Oxygen, Nitrogen and Sulfur Species in Plants. p 533–554. https://doi.org/10.1002/9781119468677.ch21
Shah Jahan M, Wang Y, Shu S et al (2019) Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hortic 247:421–429. https://doi.org/10.1016/J.SCIENTA.2018.12.047
Shahid M, Rahman KU, Haider S et al (2021) Quantitative assessment of regional land use and climate change impact on runoff across Gilgit watershed. Environ Earth Sci 80. https://doi.org/10.1007/s12665-021-10032-x
Sharma A, Sidhu GPS, Araniti F et al (2020) The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 25:540. https://doi.org/10.3390/MOLECULES25030540
Sharma A, Shahzad B, Kumar V et al (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285. https://doi.org/10.3390/biom9070285
Shemi R, Wang R, Gheith E-SMS etal (2021) Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci Rep 11:3195. https://doi.org/10.1038/s41598-021-82264-7
Shi Q, Bao Z, Zhu Z et al (2006) Effects of Different Treatments of Salicylic Acid on Heat Tolerance, Chlorophyll Fluorescence, and Antioxidant Enzyme Activity in Seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135. https://doi.org/10.1007/s10725-005-5482-6
Shi J, Gao H, Wang H et al (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15:207–216. https://doi.org/10.1111/pbi.12603
Shohani F, Hosseinin Sarghein S, Fazeli A (2023) Simultaneous application of salicylic acid and silicon in aerial parts of Scrophularia striata L. in response to drought stress. Plant Physiol Biochem 202:107936. https://doi.org/10.1016/J.PLAPHY.2023.107936
Singh PK, Gautam S (2013) Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol Plant 35:2345–2353. https://doi.org/10.1007/S11738-013-1279-9/FIGURES/1
Singh P, Choudhary KK, Chaudhary N et al (2022a) Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front Plant Sci 13:1006617. https://doi.org/10.3389/FPLS.2022.1006617/BIBTEX
Singh VP, Dwivedi P, Kashyap S (2022b) Effect of exogenous application of salicylic acid and sodium nitroprusside in wheat (Triticum aestivum L.) cultivars subjected to heat stress under early and late sown conditions. Pharma Innov J 11: 151–156
Skendžić S, Zovko M, Živković IP et al (2021) The impact of climate change on agricultural insect pests. Insects 12:440. https://doi.org/10.3390/insects12050440
Song W, Zhang P, Zhang H et al (2022) A low concentration of exogenous salicylic acid enhances cold tolerance in Hami melons (Cucumis melo var. saccharinus) by modulating salicylic acid-response CmGST genes. Postharvest Biol Technol 193:112034. https://doi.org/10.1016/J.POSTHARVBIO.2022.112034
Souri MK, Tohidloo G (2019) Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem Biol Technol Agric 6:1–7. https://doi.org/10.1186/S40538-019-0169-9/FIGURES/2
Srinivasan T, Kumar KRR, Meur G et al (2009) Heterologous expression of Arabidopsis NPR1 (AtNPR1) enhances oxidative stress tolerance in transgenic tobacco plants. Biotechnol Lett 31:1343–1351. https://doi.org/10.1007/s10529-009-0022-5
Staswick PE, Raskin I, Arteca RN (1995) Jasmonates, Salicylic acid and Brassinosteroids. In: Davies PJ (ed) Plant Hormones: Physiology, Biochemistry and Molecular Biology. Springer, Netherlands, Dordrecht, p 179–213
Steinwand MA (2017) Biochemical and Genetic Mechanisms Underlying Basal Salicylic Acid Synthesis In Arabidopsis thaliana. Dissertation, UC Berkeley
Surplus SL, Jordan BR, Murphy AM et al (1998) Ultraviolet-B-induced responses in Arabidopsis thaliana: role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant Cell Environ 21:685–694. https://doi.org/10.1046/j.1365-3040.1998.00325.x
Suzuki N, Bajad S, Shuman J et al (2008) The Transcriptional Co-activator MBF1c Is a Key Regulator of Thermotolerance in Arabidopsis thaliana. J Biol Chem 283:9269–9275. https://doi.org/10.1074/jbc.m709187200
Suzuki N, Miller G, Morales J et al (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699. https://doi.org/10.1016/j.pbi.2011.07.014
Swanson S, Gilroy S (2010) ROS in plant development. Physiol Plant 138:384–392. https://doi.org/10.1111/j.1399-3054.2009.01313.x
Szegediensis AB, Szepesi Á, Csiszár J et al (2005) Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol Szegediensis 49:123-125
Szepesi Á, Csiszár J, Gémes K et al (2009) Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J Plant Physiol 166:914–925. https://doi.org/10.1016/J.JPLPH.2008.11.012
Tajik S, Zarinkamar F, Soltani BM et al (2019) Induction of phenolic and flavonoid compounds in leaves of saffron (Crocus sativus L.) by salicylic acid. Sci Hortic 257:108751. https://doi.org/10.1016/J.SCIENTA.2019.108751
Takagi H, Tamiru M, Abe A et al (2015) MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat Biotechnol 33:445–449. https://doi.org/10.1038/nbt.3188
Tang Y, Abdelrahman M, Li J et al (2021) CRISPR/Cas9 induces exon skipping that facilitates development of fragrant rice. Plant Biotechnol J 19:642–644. https://doi.org/10.1111/pbi.13514
Tezuka D, Matsuura H, Saburi W et al (2021) A ubiquitously expressed UDP-glucosyltransferase, uGT74J1, controls basal salicylic acid levels in rice. Plants 10:1875. https://doi.org/10.3390/PLANTS10091875/S1
Tonnessen BW, Manosalva P, Lang JM et al (2015) Rice phenylalanine ammonia-lyase gene OsPAL4 is associated with broad spectrum disease resistance. Plant Mol Biol 87:273–286. https://doi.org/10.1007/S11103-014-0275-9/TABLES/2
Torrens-Spence MP, Bobokalonova A, Carballo V et al (2019) PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol Plant 12:1577–1586. https://doi.org/10.1016/j.molp.2019.11.005
Torun H (2019) Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol Plant 165:169–182. https://doi.org/10.1111/ppl.12798
Torun H, Novák O, Mikulík J et al (2020) Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.). Sci Rep 10:13886. https://doi.org/10.1038/s41598-020-70807-3
Tran MT, Son GH, Song YJ et al (2023) CRISPR-Cas9-based precise engineering of SlHyPRP1 protein towards multi-stress tolerance in tomato. Front Plant Sci 14:1186932. https://doi.org/10.3389/fpls.2023.1186932
Tyagi P, Singh A, Gupta A et al (2022) Chapter 4 - Mechanism and function of salicylate in plant toward biotic stress tolerance. In: Aftab T, Naeem M (eds) Emerging Plant Growth Regulators in Agriculture. Academic Press, p 131–164
Usman B, Nawaz G, Zhao N et al (2020) Precise Editing of the OsPYL9 Gene by RNA-Guided Cas9 Nuclease Confers Enhanced Drought Tolerance and Grain Yield in Rice (Oryza sativa L.) by Regulating Circadian Rhythm and Abiotic Stress Responsive Proteins. Int J Mol Sci 21:7854. https://doi.org/10.3390/ijms21217854
van Verk MC, Bol JF, Linthorst HJM (2011) WRKY Transcription Factors Involved in Activation of SA Biosynthesis Genes. BMC Plant Biol 11. https://doi.org/10.1186/1471-2229-11-89
Verma N, Parihar P, Singh R et al (2022) Salicylic acid induced signaling against nickel-induced toxicity: Responses of growth, exopolysaccharides, phostosystem II photochemistry, nitrogen metabolism status and antioxidant system of Anabaena sp. PCC 7120. S Afr J Bot 151:185–199. https://doi.org/10.1016/j.sajb.2022.09.026
Wang LJ, Li SH (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170:685–694. https://doi.org/10.1016/J.PLANTSCI.2005.09.005
Wang L, Fan L, Loescher W et al (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34. https://doi.org/10.1186/1471-2229-10-34
Wang L, Chen L, Li R et al (2017) Reduced Drought Tolerance by CRISPR/Cas9-Mediated SlMAPK3 Mutagenesis in Tomato Plants. J Agric Food Chem 65:8674–8682. https://doi.org/10.1021/acs.jafc.7b02745
Wang W, Wang X, Huang M et al (2018b) Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front Plant Sci 9:397026. https://doi.org/10.3389/FPLS.2018.01137/BIBTEX
Wang Y, Mopper S, Hasenstein KH (2001) EFFECTS OF SALINITY ON ENDOGENOUS ABA, IAA, JA, and SA IN Iris hexagona. J Chem Ecol 27:327-342. https://doi.org/10.1023/A:1005632506230
Wang N, Xiao B, Xiong L (2011) Identification of a cluster of PR4-like genes involved in stress responses in rice. J Plant Physiol 168:2212–2224. https://doi.org/10.1016/j.jplph.2011.07.013
Wang B, Song N, Zhang Q et al (2018a) TaMAPK4 acts as a positive regulator in defense of wheat stripe-rust infection. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.00152
Wani AB, Chadar H, Wani AH et al (2017) Salicylic acid to decrease plant stress. Environ Chem Lett 15:101–123. https://doi.org/10.1007/s10311-016-0584-0
Wassie M, Zhang W, Zhang Q et al (2020) Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotox Environ Safe 191:110206. https://doi.org/10.1016/J.ECOENV.2020.110206
Wassmann R, Jagadish SVK, Sumfleth K et al (2009) Chapter 3 Regional Vulnerability of Climate Change Impacts on Asian Rice Production and Scope for Adaptation. Adv Agron 102:91–133. https://doi.org/10.1016/S0065-2113(09)01003-7
Wei Z, Abdelrahman M, Gao Y et al (2021) Engineering broad-spectrum resistance to bacterial blight by CRISPR-Cas9-mediated precise homology directed repair in rice. Mol Plant 14:1215–1218. https://doi.org/10.1016/j.molp.2021.05.012
Wen PF, Chen JY, Wan SB et al (2008) Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul 55:1–10. https://doi.org/10.1007/S10725-007-9250-7/FIGURES/5
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: A new player in plant signalling and defence responses. Curr Opin Plant Biol 7:449–455
Wildermuth MC, Dewdney J, Wu G et al (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414:562–565. https://doi.org/10.1038/35107108
Wu Y, Zhang D, Chu JY et al (2012) The Arabidopsis NPR1 Protein Is a Receptor for the Plant Defense Hormone Salicylic Acid. Cell Rep 1:639–647. https://doi.org/10.1016/j.celrep.2012.05.008
Wu J, Zhu W, Zhao Q (2023) Salicylic acid biosynthesis is not from phenylalanine in Arabidopsis. J Integr Plant Biol 65:881–887. https://doi.org/10.1111/jipb.13410
Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54:440–451. https://doi.org/10.1111/J.1365-313X.2008.03433.X
Xu E, Vaahtera L, Brosché M (2015) Roles of Defense Hormones in the Regulation of Ozone-Induced Changes in Gene Expression and Cell Death. Mol Plant 8:1776–1794. https://doi.org/10.1016/j.molp.2015.08.008
Xu C, Fu X, Liu R et al (2017a) PtoMYB170 positively regulates lignin deposition during wood formation in poplar and confers drought tolerance in transgenic Arabidopsis. Tree Physiol 37:1713–1726. https://doi.org/10.1093/treephys/tpx093
Xu L, Zhao H, Ruan W et al (2017b) ABNORMAL INFLORESCENCE MERISTEM1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell 29:560–574. https://doi.org/10.1105/tpc.16.00665
Xu L, Zhao H, Wang J et al (2023) AIM1-dependent high basal salicylic acid accumulation modulates stomatal aperture in rice. New Phytol 238:1420–1430. https://doi.org/10.1111/NPH.18842
Yang L, Li B, Zheng X et al (2015) Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat Commun 6:7309. https://doi.org/10.1038/ncomms8309
Yang A, Akhtar SS, Li L et al (2020) Biochar mitigates combined effects of drought and salinity stress in Quinoa. Agronomy 10. https://doi.org/10.3390/agronomy10060912
Yang W, Zhou Z, Chu Z (2023) Emerging Roles of Salicylic Acid in Plant Saline Stress Tolerance. Int J Mol Sci 24:3388. https://doi.org/10.3390/ijms24043388
Yasuda M, Ishikawa A, Jikumaru Y et al (2008) Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20:1678–1692. https://doi.org/10.1105/tpc.107.054296
Yokoo S, Inoue S, Suzuki N et al (2018) Comparative analysis of plant isochorismate synthases reveals structural mechanisms underlying their distinct biochemical properties. Biosci Rep 38:20171457. https://doi.org/10.1042/BSR20171457/57492
Yuan Y, Chung J-D, Fu X et al (2009) Alternative splicing and gene duplication differentially shaped the regulation of isochorismate synthase in Populus and Arabidopsis. Proc Natl Acad Sci U S A 106:22020–22025. https://doi.org/10.1073/pnas.0906869106
Yuan P, Tanaka K, Poovaiah BW (2021) Calmodulin-binding transcription activator AtSR1/CAMTA3 fine-tunes plant immune response by transcriptional regulation of the salicylate receptor NPR1. Plant Cell Environ 44:3140–3154. https://doi.org/10.1111/pce.14123
Zafar Z, Rasheed F, Mushtaq NU et al (2023) Exogenous Application of Salicylic Acid Improves Physiological and Biochemical Attributes of Morus alba Saplings under Soil Water Deficit. Forests 14:236. https://doi.org/10.3390/f14020236
Zeier J (2021) Metabolic regulation of systemic acquired resistance. Curr Opin Plant Biol 62:102050. https://doi.org/10.1016/j.pbi.2021.102050
Zeng W, He SY (2010) A Prominent Role of the Flagellin Receptor FLAGELLIN-SENSING2 in Mediating Stomatal Response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153:1188–1198. https://doi.org/10.1104/pp.110.157016
Zhang S, Klessig DF (1998) The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc Natl Acad Sci U S A 95:7225–7230. https://doi.org/10.1073/pnas.95.12.7225
Zhang Y, Zhou Y, Zhang D et al (2020) PtrWRKY75 overexpression reduces stomatal aperture and improves drought tolerance by salicylic acid-induced reactive oxygen species accumulation in poplar. Environ Exp Bot 176:104117. https://doi.org/10.1016/J.ENVEXPBOT.2020.104117
Zhang D, Zhang Z, Unver T et al (2021a) CRISPR/Cas: A powerful tool for gene function study and crop improvement. J Adv Res 29:207–221. https://doi.org/10.1016/J.JARE.2020.10.003
Zhang H, Huang Q, Yi L et al (2021b) PAL-mediated SA biosynthesis pathway contributes to nematode resistance in wheat. Plant J 107:698–712. https://doi.org/10.1111/tpj.15316
Zhang Z, Zhang Q, Wu J et al (2013) Gene Knockout Study Reveals That Cytosolic Ascorbate Peroxidase 2(OsAPX2) Plays a Critical Role in Growth and Reproduction in Rice under Drought, Salt and Cold Stresses. PLoS One 8:e57472. https://doi.org/10.1371/journal.pone.0057472
Zhang A, Liu Y, Wang F et al (2019) Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol Breed 39:47. https://doi.org/10.1007/s11032-019-0954-y
Zhang B, Huang S, Guo Z et al (2023) Salicylic acid accelerates carbon starvation-induced leaf senescence in Arabidopsis thaliana by inhibiting autophagy through Nonexpressor of pathogenesis-related genes 1. Plant Sci 336:111859. https://doi.org/10.1016/j.plantsci.2023.111859
Zhao Y, Zhang C, Liu W et al (2016) An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci Rep 6:23890. https://doi.org/10.1038/srep23890
Zhao J, Lu Z, Wang L et al (2020) Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int J Mol Sci 22:117. https://doi.org/10.3390/ijms22010117
Zhao B, Liu Q, Wang B, Yuan F (2021a) Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J Agric Food Chem 69:3566–3584. https://doi.org/10.1021/acs.jafc.0c07908
Zhao Y, Song C, Brummell DA et al (2021b) Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem 358:129867. https://doi.org/10.1016/J.FOODCHEM.2021.129867
Zheng Z, Qualley A, Fan B et al (2009) An important role of a BAHD acyl transferase-like protein in plant innate immunity. Plant J 57:1040–1053. https://doi.org/10.1111/J.1365-313X.2008.03747.X
Zheng M, Lin J, Liu X et al (2021) Histone acetyltransferase TaHAG1 acts as a crucial regulator to strengthen salt tolerance of hexaploid wheat. Plant Physiol 186:1951–1969. https://doi.org/10.1093/plphys/kiab187
Zhong Q, Hu H, Fan B et al (2021) Molecular Sciences Biosynthesis and Roles of Salicylic Acid in Balancing Stress Response and Growth in Plants. Int J Mol Sci 22:11672. https://doi.org/10.3390/ijms
Zhou H, Ren S, Han Y et al (2017) Identification and Analysis of Mitogen-Activated Protein Kinase (MAPK) Cascades in Fragaria vesca. Int J Mol Sci 18:1766. https://doi.org/10.3390/IJMS18081766
Zhou JM, Zhang Y (2020) Plant Immunity: Danger Perception and Signaling. Cell 181:978–989. https://doi.org/10.1016/j.cell.2020.04.028
Acknowledgements
We thank the entire team of school of biotechnology at Nile University, Egypt for their support during the development of this manuscript. The authors would like to thank Ms. Yasmin Elghanamy, Scenography Department- Faculty of Fine Arts- Helwan University- Egypt for her support in drawing and finetuning the figures.
Funding
This research didn’t receive funding.
Author information
Authors and Affiliations
Contributions
ME, MELSH( Moaz ELSheikh), NS, MAz and MAb designed the content. MAb, KA, FI, JC discussed the content. ME, MELSH( Moaz ELSheikh), NS and Maz wrote the initial draft. JC and MAb advanced the writing. FI, JC, and MAb critically reviewed the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Dr. Huan Chen.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsisi, M., Elshiekh, M., Sabry, N. et al. The genetic orchestra of salicylic acid in plant resilience to climate change induced abiotic stress: critical review. Stress Biology 4, 31 (2024). https://doi.org/10.1007/s44154-024-00160-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-024-00160-2