Abstract
Selecting appropriate candidates for genetic rescue mostly relies on previous genetic research and monitoring, while ecological and behavioural traits of the remnant and source populations are rarely considered for such conservation measures. Because of their slow recovery, Eurasian lynx Lynx lynx populations in Central and Western Europe have been a repeated target of genetic reinforcements and reintroductions in the past 50 years. Once inhabiting much of south-eastern Europe, the Balkan lynx L. l. balcanicus is now critically endangered and confined to a small population. Long-term isolation has caused loss of genetic diversity and has possibly led to inbreeding depression. Immediate actions need to consider genetic reinforcement to increase the genetic diversity and secure population viability. Here, we compared the Balkan lynx with two neighbouring populations: Dinaric population originating from the Carpathian subspecies (L.l. carpathicus) and Anatolian population of Caucasian subspecies (L.l. dinniki) to determine is better suited source from an ecological standpoint. Main findings suggest that the L.l. carpathicus is ecologically more similar to the L.l. balcanicus and therefore likely better suited for the environment of south-western Balkans on the basis of prey preference (roe deer being the main prey), local prey availability (lower lagomorph and higher ungulate availability) and habitat use (predominant use of the mixed and broadleaved forests). We discuss the contrasting results of genetic and ecological analyses from both the evolutionary and conservation perspective and provide potential solutions that would take into account both aspects to pave the road towards potential genetic rescue of the Balkan lynx.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations in need of reinforcement (genetic rescue) are usually affected by intrinsic factors that threaten their very existence (Whiteley et al. 2014). Selecting appropriate candidates for such a management measure can have implications for adaptation to local environmental condition and ecosystem function, but also on the way individuals interact with other species. It is thus important to match the ecological needs (local adaptation for instance) of the remnant and source populations in order for this process to be successful and to ensure the viability and health of the ecosystem (Frankham et al. 2011).
Eurasian lynx (Lynx lynx) reintroductions and reinforcements have been an integral part of wildlife management and conservation in the last 50 years in Europe (e.g., Vandel et al. 2006; Krofel et al. 2021). In light of the recent history of the Balkan lynx (Lynx lynx balcanicus Bureš 1941) and the constant shift of its status (Mirić 1981; Breitenmoser-Würsten & Breitenmoser 2001; Melovski et al. 2015), it is becoming increasingly urgent to focus on immediate actions that can save this population from extinction. In the past 100 years, the Balkan lynx has suffered harsh persecution that brought down the population to a mere 15–20 individuals in the 1930s (Mirić 1981). Species legal and site protection from the 1950s until the 1990s ensured an increase in the population, which faced extinction once again due to the political turmoil in the range countries and the missing on-the-ground protection for the lynx and its prey (Melovski et al. 2013). From 2006 onwards, the Balkan Lynx Recovery Programme solidified the conservation actions (Breitenmoser et al. 2008), but the programme has no warranty against stochastic events or potential inbreeding depression that can wipe out small populations. The ten-year (2008–2018) camera-trapping in the most promising Balkan lynx area in western North Macedonia evidenced stabilization and even slight increase of the population (Melovski et al. 2009; Stojanov 2020), but the 2021 camera-trapping in the same area yielded an all-time low with only five different individuals detected (Gonev et al. 2021). Further cause for concern is the fact that only one of the four collared females gave birth in two consecutive years (2020–2021), which may be the reason why only one new individual was identified during the latest camera-trapping session (Gonev et al. 2021).
The evolutionary history of the Balkan lynx lineage stretches for more than 50 kya years (Mengüllüoğlu et al. 2021a; Bazzicalupo et al. 2022) and, during this time, its population has always been relatively small compared to other populations in Asia, or Europe (Bazzicalupo et al. 2022). Given the long-term process of inbreeding, it is likely that reproductive success is already affected by inbreeding depression. Similar issues affected the reintroduced Dinaric lynx population, but negative trends were reversed with the start of the translocation of Eurasian lynx from the Carpathians to Slovenia and Croatia (Krofel et al. 2021). The intense genetic drift in the bottlenecked Balkan lynx contributes to further genetic erosion, whereas observed low genetic diversity and extremely small effective population size call for immediate genetic rescue actions (Bazzicalupo et al. 2022). Provided that the closest neighbours of the Balkan lynx population are Carpathian L. l. carpathicus (which were also reintroduced to the Dinaric Mountains) and Caucasian lynx L. l. dinniki, it is logical to seek potential alternatives for genetic rescue from these two subspecies (Mengüllüoğlu et al. 2021a). However, lynx populations may have been adapting to local conditions, including potentially developing different behaviour in terms of habitat use or prey selection. Therefore, in addition to information on genetic relatedness, comparison of behavioural ecology between the Balkan lynx and the neighbouring populations could provide important insights that should be considered when making decisions about the most suitable source population for reinforcement.
The foraging ecology of the Eurasian lynx has been well studied in the past, with studies from the European part (e.g., Okarma et al. 1997; Jobin et al. 2000; Krofel et al. 2014; Mattisson et al. 2013; Melovski et al. 2020) and, to a lesser extent, from the Asian part (Sedalischev et al. 2014; Weidong 2010). As a strictly carnivorous mammal, the Eurasian lynx diet consists of more than 30 species (Odden et al. 2006), although only few compose its principal prey (Krofel et al. 2011; Jobin et al. 2000; Melovski et al. 2020). This specialization comes with different prey availability that mostly depends on the region (Soyumert et al. 2019). The Eurasian lynx is one of the most widely distributed felid species, which can be found as far west as the Jura Mountains between Switzerland and France, and as far east as the Kamchatka region in Russia (von Arx et al. 2004). Living in a variety of habitats, ecosystems, climatic zones and demographic conditions, the different meta-populations of Eurasian lynx adopted a wide variety of foraging habits. The change is mostly evident in the Anatolia and Caucasus, where lynx differ considerably in body size and foraging behaviour from the European populations (Mengüllüoğlu et al. 2018). This variety of foraging habits may be a product of their evolutionary history, especially conditions in the glacial refugia during the last glacial maximum (LGM) (Mengüllüoğlu et al. 2021a). For example, forest refugia in the central and northern parts of the Palearctic hosted several prey species, including ungulates, while more southern regions were less affected by the last ice age (Hemmer 1993). The primary prey of the Eurasian lynx ranges from ungulates, such as roe deer (Capreolus capreolus), chamois (Rupicapra rupicapra), red deer (Cervus elaphus), reindeer (Rangifer tarandus), ibex (Capra aegerus) and wild sheep (Oivs orientalis) (Okarma et al. 1997; Jobin et al. 2000; Matyushkin and Vaisfeld 2003; Mattisson et al. 2013; Belotti et al. 2015, Vogt et al. 2019), to lagomorphs (brown hare Lepus europaeus, mountain hare Lepus timidus) and larger birds (Zhelukhin 1986; Matyushkin and Vaisfeld 2003; Sedalischev et al. 2014; Mengüllüoğlu et al. 2018). In some areas, also larger rodents (Krofel et al. 2011) and meso-predators (fox, marten) can represent an important secondary source of food (Odden et al. 2006; Ivanov et al. 2018). Furthermore, lynx diet can shift seasonally as the abundance or vulnerability of the main prey species changes, where some become more vulnerable during feeding or ruminating (Molinari-Jobin et al. 2007). Possible reasons for the dietary shift could vary and range from environmental to biogeographic to evolutionary (Mengüllüoğlu et al. 2018). Additionally, the abundance of different species, including alternative prey, can play a key role in prey selection (Krofel et al. 2011). Jedrzejewski et al. (1993), for example, argue that dense tree cover is consequential to the lagomorph percentage in lynx diet, being a prerequisite for high roe deer and low brown hare density. Accordingly, the proportion of brown hare in lynx diet is higher in exploited forests than in the pristine Białowieża forest in Poland. Diet-shift from one preferred, primary species to another is also seen in other members of the cat family, and more specifically in the Lynx genus. In southern Florida, for instance, bobcats (Lynx rufus) most often prey on rodents and lagomorphs (Labisky and Boulay 1998), although white-tailed deer (Odocoileus virginanus) is typically preferred in the northern areas of the United States (Svoboda et al. 2013).
This study investigates prey availability, prey selection and habitat preference of three Eurasian lynx populations/subspecies: the Balkan population and two neighbouring populations, the Dinaric (belonging to the Carpathian subspecies) and the Anatolian (belonging to the Caucasian subspecies), to explore prospects for genetic rescue of the Balkan lynx. We hypothesize that prey preference changes from the continental forests in Central Europe to the Mediterranean forests, and scrub in Anatolia, is due to environmental factors (climate, habitat), prey availability and the evolutionary history of the Eurasian lynx. The Balkan population is ecologically intermediate between the Dinaric and Anatolian population, and therefore, we expect a transitional pattern of prey shift. We also discuss the taxonomic implications of potential admixture of the Balkan lynx with either Carpathian or Caucasian conspecifics in regard to further conservation efforts and the ecosystem role of the Balkan lynx population.
Methods
Study area
Dinaric study area
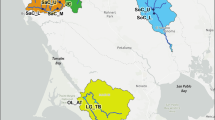
The Dinaric study area is located in the Northern Dinaric Mountain Range in Slovenia (Fig. 1) in mixed temperate forests dominated by fir and beech (Omphalodo-Fagetum s. lat.). The altitudes range from 200 m to 1796 m. The climate is a mix of influences from the Alps, the Mediterranean Sea and the Pannonian Basin with annual temperature averaging 5–8 °C, ranging from average maximum of 32 °C to a minimum of − 20 °C, and average annual precipitation of 1400–3500 mm. In the Dinaric Mountains, lynx were completely exterminated by the beginning of the 20th century, but so far, it remains unclear to which subspecies the extinct lynx population in this region belonged to, given its geographical position relative to other current or extinct native populations. These include the Carpathian subspecies, the Balkan subspecies, the extinct Alpine form identified genetically from museum specimens (Gugolz et al. 2008), or it could represent a mix of several of them, given that species distribution range is historically believed to have been continuous in this part of Europe (Kratochvil 1968). The current Dinaric lynx population was formed after a successful reintroduction initiated in 1973 when six Carpathian lynx from Slovakia were released in Slovenia (Čop 1990). While the population afterwards expanded to several neighbouring countries, it has so far remained isolated. The Dinaric lynx population is currently one of the most endangered lynx populations in Europe, threatened primarily by the high levels of inbreeding (Sindičić et al. 2013). To counteract this, additional Carpathian lynx are currently being translocated to Slovenia and Croatia, as part of the on-going reinforcement project (Krofel et al. 2021). The Slovenian part of the population currently consists of at least 20 adult individuals (Krofel et al. 2021). Prey species inhabiting the area include wild ungulates, such as roe deer, red deer and the Alpine chamois (R. r. rupicapra), as well as smaller mammals, such as brown hare and edible dormouse (Glis glis). Main competitors for the lynx in the area include brown bear (Ursus arctos), grey wolf (Canis lupus), and golden jackal (Canis aureus), among which the most important interaction is kleptoparasitism by scavenging bears (Krofel 2012; Krofel et al. 2012).
Balkan study area
The isolated population of the Balkan lynx is distributed in the south-western Balkan Peninsula, mainly in the western part of North Macedonia and the northern and eastern parts of Albania, western Kosovo, with possibility of occurrence in Montenegro and northern Greece (Fig. 1). The core reproductive area of the population is Mavrovo National Park (730 km2) in N. Macedonia. The current study focuses on this core reproductive area and its surrounding mountains. Around 45% of the park area is forested, of which over 70% are broadleaved forests (predominantly European beech, Fagus sylvatica and several oak species (Quercus spp.), mixed forests comprise more than 18% (mainly beech–fir mixed forests), nearly 10% are shrublands and around 1% are coniferous trees (Macedonian fir, Abies borisii-regis and European spruce, Picea abies) (Ivanov et al. 2018). The area has a mountain continental climate with Mediterranean influences (Filipovski et al. 1996). Prey species inhabiting the area include wild ungulates, such as roe deer and the Balkan chamois (R. rupicapra balcanica), and lagomorphs such as brown hare. Competition includes the large carnivores present in the area, such as grey wolf, golden jackal and brown bear.
Anatolian study area
The study area in Anatolia covers around 400 km2 in the Nallıhan Mountains (NM, Fig. 1) and represents a small part of the distribution range of the subspecies belonging to the forest–steppe ecosystem (Mengüllüoğlu et al. 2018). The elevation varies between 500 m and 1550 m, and the study area is located in the transition zone between the dry western Black Sea (xeric), central Anatolian (Iran-Turan) and Mediterranean floristic zones (Aksoy 2009). Vegetation composition and structure depends on altitude and historical human use. The lower areas (500–1000 m) are covered by Turkish pine (Pinus brutia). Above this belt, temperate coniferous forest reaches up to 1500 m and is composed of black pine (Pinus nigra), junipers (Juniperus excelsa and J. oxycedrus) with an understory of oak-dominated scrub (Quercus pubescens, Pyrus elaeagnifolia, Crataegus spp.—Aksoy 2009). The study area does not hold any form of protection status, and is part of the state forests management system. The human population in and around NM is low (12 villages, Ntotal = 1780; Turkstat 2017) and more than half of the registered population (mostly young people) resides in Nallıhan district centre or close by big cities, such as Ankara, Eskişehir and Bolu (Mengüllüoğlu, pers. comm. 2014–2016, with village heads). Potential lynx prey in the area is roe deer and brown hare, while other large carnivores, including brown bear, grey wolf and golden jackal (Mengüllüoğlu 2010, Mengüllüoğlu et al. 2019).
Prey preference and sample collection
Lynx scats in the Dinaric Mts. were collected during snow-tracking and at telemetry locations of radio-collared lynx in the period from 2016 to 2021 (Fig. 1). If more than one scat was found during the same snow-tracking session, only one (the first) was used for analysis to avoid pseudo-replication.
Lynx scats in the Balkan study area were collected either by chance, line transects, or near the feeding sites of the radio-collared lynx (Ivanov et al. 2018; Melovski et al. 2020) (Fig. 1). The scats were collected in the period from 2010 to 2020 and in 2017 the search was aided by trained dogs.
Scats in Anatolia were collected with a scat detection dog, by walking on dirt roads, game trails or ridgeline, and on predefined transects below the tree line (Fig. 1). The sampling took place between 2013 and 2015 (see more details in Mengüllüoğlu et al. 2018), as well as 2015 to 2017. Scats samples originated from at least 14 lynx individuals (Mengüllüoğlu et al. 2019).
The scat analysis included macroscopic (general form, size, colour, rigidness, shape of the apex) and microscopic (structure and pattern of cuticular scales, shape of the cross section, shape and size of the medullar space) characteristics of hairs. Microstructure of the cuticle was inspected using cuticular imprints made on celluloid plates with the use of acetone (Wachter et al. 2012). Species determination was performed according to Teerink (1991) and with the help of our own reference material. Due to the difficulties of separating between the roe and red deer hair in the Dinaric Mountains, we limited our classification to the family level (Cervidae) and inferred species use based on the ratio between the roe and red deer among prey remains found at lynx kill sites (for details, see Krofel et al. 2011).
We calculated Frequency of Occurrence (FO%) as percentage of scats containing different food types relative to the total number of analysed samples; and Relative Frequency of Occurrence (RFO%)—the frequency of occurrence in relation to the total occurrence of the food type (Ivanov et al. 2018). Since our study is focused on the relative proportion of ungulate versus lagomorph prey, we distinguished between ungulates and the brown hare, while pooling the remaining prey items into one category. These items featured small carnivores, such as foxes, martens, domestic animals, rodents, shrews and birds. We used Pearson's chi-squared to compare lynx diet according to three prey categories (ungulates, hares and other) among the three study areas.
Prey availability
With prey species where individuals cannot be distinguished in camera-trap photographs, density estimation in forest habitats is difficult and generally gives biased results, as the actual population numbers are underestimated (Jobin et al. 2000). We therefore used the Random Encounter Model (REM; Rowcliffe et al. 2008) to estimate the density D of lagomorphs, mid-sized and large herbivore prey as \(D=\frac{y}{t}\times \frac{\pi }{Vr\left(2+\theta \right)}\), where y is the number of independent photographic events, t is camera-trap days (ctd), V is average speed of animal movement, and r and θ are the camera-trap detection distance (in kilometre) and angle (in radian). Animal movement speeds were taken from the published literature with GPS fix frequencies of 15 min for red deer (Pépin et al. 2004) and wild boar (Spitz and Janeau 1990), 2 h for chamois (Krofel et al. 2013a, b), and 1 h for brown hare (Schai-Braun et al. 2012). Speed for roe deer was calculated by Marcon et al. (2019) using the model of day range by Carbone et al. (2005). Camera-trap detection distance and angles were obtained from Meek et al. (2012). While we estimated absolute values for each prey species, we were primarily interested in the relative differences in prey availability among species, to compare it with proportion of prey use in lynx diet among the tree study areas.
We used the following six types of camera traps (Cuddeback X-Change™ Color Model 1279 with white or IR flash, Cuddeback X-Change™ Color Model 1213 with black IR and white flash, Cuddeback Ambush with white flash, Cuddeback Attack and StealthCam STC-G42NG with black IR). Traps were installed on forest trails, dirt roads, game trails, mountain trails and along mountain ridges or cliffs.
The design for camera-trapping lynx in the Dinaric study area used 100 3 × 3 km2 grid cell for a total sampling area of 900 km2. For each camera-trap, we noted the location details (GPS coordinates, the name and the district of the hunting ground) (Fležar et al. 2019).
The camera-trapping for the Balkan population was conducted in Mavrovo National Park, N. Macedonia, on three consecutive sessions in 2013, 2015 and 2018, each starting from March until May and lasting for 60 days (Fig. 1). In each session, a total of 60 camera traps were installed in 30 different sites (each site had two opposing cameras and every year the same sites were selected). The sampling design of the cameras accommodated the monitoring of the Balkan lynx and covered a referenced area of around 450 km2 using 2.5 × 2.5 km grid cells, with one out of the two cells sampled (Stojanov 2020).
Camera-trapping data for the study site in Anatolia were gathered from a 684 effective trapping nights survey in the spring of 2014 (Fig. 1). In this survey, 12 camera-trapping stations were installed covering a minimum convex polygon (MCP) of 148 km2. While the monitoring area in this region was smaller compared to the other two, lynx population here occurred at a very high density (Mengüllüoğlu et al. 2021b), thus camera-trapping survey nevertheless included several individuals (i.e. at least part of the home ranges of 7 female and 4 male lynx individuals; Mengüllüoğlu et al. 2019). We set a minimum interval of 30 min to assign two pictures of the same species as independent captures.
As study area sizes and camera-trapping efforts were variable depending on the study area, we calculated relative trapping effort by dividing the camera-trapping day by study area size (ctd/km2).
Habitat preference
To assess the habitat preference where the three populations are found, we analysed the relation of a habitat’s use to its overall availability in the respective home range of the individuals (Fig. 1). We used CORINE Land cover vector data (v. 2018, ESRI geodatabase format, available at https://land.copernicus.eu/pan-european/corine-land-cover/clc2018?tab=download) for representation of the habitats that were available within the 100% MCP of the lynx (third order of selection, Johnson 1980). We arrived at seven different categories that belong to two general Corine groups: agricultural areas and forests and semi natural areas. Few relocations found in the categories artificial surfaces or water bodies were excluded from calculations due to the unrepresentative sample or GPS inaccuracy (Table S1 and S2). We calculated the Resource Selection Index (RSI) by dividing the relocations found in a certain habitat by the expected number of relocations in the same habitat (calculated with the area representation of this habitat within the total MCP of each lynx). Results were then normalized in a range from 0 to 100 for all study areas.
In total, 19 Eurasian lynx were captured and fitted with GPS/GSM collars in the period from 2006 to 2020 (Table 1).
Euclidian distance analysis
Aiming to determine the optimal population for genetic rescue of the Balkan lynx, we performed a multivariate ecological distance analysis among the three populations. We did the cluster analysis (nearest neighbour method, Euclidian distance) in Statgraphics Centurion 16 (StatPoint Technologies, USA 2009) using the frequency of occurrence of prey species (Table 2) and Resource Selection Index (Table S1&S2) as input data.
Results
Prey preference
Across the three study areas, we collected a total of 315 scats (Fig. 1; Table 2) in which we detected 350 prey items (Table 2). The preference for brown hare showed a clear north–south gradient, ranging from almost no significance for the lynx in Dinaric Mts., to an almost complete dependence for lynx in Anatolia. The same is true but in reverse order for the ungulates, especially cervids, which is avoided by the lynx in Anatolia, while the lynx in the Dinaric range are preferentially selecting these species (Table 2). The Balkan lynx, with relative frequency of occurrence for ungulates (roe deer, chamois and wild boar) of 76% as opposed to 12% for brown hare, shows a transitional pattern between the two, but is much closer to the Dinaric population (χ2 = 2.9, P = 0.24, df = 2, nDinaric = 42, nBalkan = 61) than Anatolian population (χ2 = 190, df = 2, p < 0.00001, nAnatolian = 247; Table 2).
Prey availability
The highest relative trapping effort was 7.9 ctd/km2 for Dinaric Mt., while the values were somewhat lower and very similar for the Balkan and Anatolian study areas at 4.5 and 4.6 ctd/km2, respectively. The prey availability was calculated with the help of the Random Encounter Model (Table 3). These results are consistent with prey preferences (Table 2) since there is a strong correlation between the availability of the prey and the preferred prey in each of the three populations.
Habitat preference
The Resource Selection Index (RSI) shows the importance of certain habitat based on the lynx GPS relocations and its overall availability in each home-range of the lynx. The index shows that forests make up more than 60% of habitats used by lynx in Slovenia, 48% by lynx found in N. Macedonia and only 34% in Turkey (Fig. 2, Table S1 and S2). Lynx in N. Macedonia and Turkey selected transitional woodland-shrub and sparsely vegetated areas. Agricultural areas and natural grasslands are somewhat evenly represented in all three countries, whereas sparsely vegetated areas are preferred in Anatolia (Fig. 2). In total, 83% of the RSI is shared among the three forested habitats and transitional woodland-shrub in Slovenia, 80% in N. Macedonia and 64% in Anatolia.
Euclidian distance analysis
Based on prey and habitat preference, the Balkan population clearly clusters with the Dinaric population (Fig. 3).
Discussion
In this study, we assessed which of the two genetically and geographically closest lynx subspecies to the critically endangered Balkan lynx (Mengüllüoglu et al. 2021a; Bazzicalupo et al. 2022) is more suitable for population rescue from the ecological standpoint. We analysed prey preference, prey availability and habitat preference to infer whether the Carpathian or the Caucasian subspecies better suits the environment in south-western Balkans. Our main findings suggest that the Balkan population is ecologically closer to the Dinaric population, i.e. the Carpathian subspecies. As a result, we recommend that any future reinforcements to the Balkan lynx population should consider individuals from the Carpathian subspecies.
However, this recommendation is limited to the behavioural ecology in current environmental conditions and does not appear to correspond with the genetic relationship among the subspecies. Mitochondrial DNA has been widely used to assess the genetic relatedness among the populations from a phylogenetic perspective (Moritz 1994). Recent studies have suggested that Balkan lynx is mitogenomically closest to the lynx populations in Anatolia (see Clade B, Lineage B2 in Mengüllüoglu et al. 2021a), but closest to Carpathian lynx populations regarding autosomal genomes, likely due to male-biased dispersal and introgression (Bazzicalupo et al. 2022). Given the distinct haplotypes found in the Balkan population (Gugolz et al. 2008; Lucena-Perez et al. 2020), it is important to consider the genetic uniqueness of the mitogenomes in this population and the implications of potential translocations from other populations, given that the Balkan lynx is recognized as both an evolutionary significant unit (Melovski et al. 2013) and its own subspecies (Kitchener et al. 2017). Similarly, the Florida panther (Puma concolor) experienced a variety of genetic maladies due to depleted genetic diversity that were all overcome after the reinforcement with female individuals from Texas (Johnson et al. 2010). This conservation measure risked compromising the genetic uniqueness of the Florida panther, but ultimately provided reproductive success, survival and fitness, and doubled the genetic heterozygosity, while the level of inbreeding declined (Johnson et al. 2010).
Our findings revealed that a variety of forests is preferred by lynx in the European part of this study compared to the coniferous forests and more open landscape selected by the Caucasian lynx in Anatolia. It furthermore showed that the importance of forested habitats decreases from north to south. This can be attributed to two reasons: lynx in Central Europe prefer more forested areas, and/or, the prey preference of the lynx in Anatolia require the selection of transitional woodland–shrub as a type of forested habitat and sparsely vegetated areas that support a high density of brown hares (Fig. 2). Compared to the Dinaric study area, the structure of the forest and the available prey densities are likely the main reasons for the slight prey shift in the Balkan population. Empirical data suggest that two of the 12 radio-tagged individuals (a male and a female) in the Balkan study area mainly preyed on brown hares due to the higher share of the natural grasslands and the transitional woodland–shrub found in their overlapping home ranges (MKD_m_07 Bistri & MKD_f_10 Atidze in Table S2), while the other lynx with home ranges in more forested habitats mainly relied on ungulates (Tab S1 and S2; Melovski et al. 2020). Moreover, brown hare density in Slovenia is by far the lowest compared to the other two study sites. The Random Encounter Model (REM) allows calculation of animal density from camera-trapping rates by modelling the underlying detection process without the need for individual recognition (Rowcliffe et al. 2008). Although the camera-trap surveys in all three study areas were primarily designed to photograph lynx, such camera deployment has been shown to also provide reliable information on other (non-target) mammals (Soofi et al. 2017; Hofmeester et al. 2021). Therefore, we assume that data can be used to provide information about relative abundances among the three ungulate species and the brown hare. However, the method can potentially bias the results through the estimation of animal movement speed and the activity level (proportion of the day spent active) and therefore the estimation of daily average distance covered by an animal (Halotel 2020). Since all camera-trapping sessions for our study were done with an initial design to estimate Eurasian lynx densities, we feel comfortable comparing the results among the three study areas. Our results for the roe deer density in N. Macedonia are also close to the estimate (3.35/km2) from a study designed to estimate roe deer density in the same region (Halotel 2020).
The Eurasian lynx has evolved in sympatry with larger felids in Central Asia (e.g., Panthera uncia and P. pardus), where it supposedly preyed on both lagomorphs and small ungulates to avoid competition with the ancestral L. issiodorensis while retaining a similar body size (Werdelin 1981; Mengüllüoglu et al. 2021b). In contrast, the body sizes of the Canada lynx (L. canadensis), the bobcat, and the Iberian lynx (L. pardinus), all of which evolved to prey almost exclusively on lagomorphs and other small prey, were considerably reduced (to approximately 10–12 kg on average) during their evolution (Werdelin 1981). Although less extreme than in these species, reduction in size was observed in the Caucasian subspecies of Eurasian lynx (average body weight of females and males 13.1 and 16.6 kg, respectively; Mengüllüoğlu et al. 2021b) and may suggest a similar evolutionary adaptation to the local conditions since the LGM of open and dry habitats with high biomass of lagomorphs, which also form its main prey. Compared to the Caucasian subspecies, the Carpathian and the Balkan lynx are similar in size: the average body mass of Carpathian lynx is 18.0 kg for females and 22.4 kg for males (Marti and Ryser-Degiorgis 2018); average body mass of Balkan lynx is 16.3 kg for females and 22.1 kg for males (Melovski et al. 2020; Melovski, unpublished data). Therefore, average body size, habitat preference, prey selection and current prey availability all suggest closer ecological and morphological similarity of Balkan lynx towards the Carpathian lynx, rather than the Caucasian subspecies, which contrasts with the phylogenetic relationships, at least at the mitogenomical level. The reason for the observed pattern might be connected with the separation that occurred during the LGM, causing the Balkan lynx to evolve during the last 20 kya years in isolation from the Caucasian lynx populations in the landscape of south-eastern Europe, adapting to the local habitats and prey availability, with likely gene flow through immigrating male Carpathian lynx. If the conservation measures (including current reinforcement of the Dinaric population; Krofel et al. 2021) will result in population recovery, we can eventually expect natural dispersal and gene flow between individuals from the Dinaric and Balkan populations on the south-eastern and north-western ends of their distribution, respectively. Another option is connection between the Balkan and Carpathian population via Serbia. So far, no natural dispersal among these populations has been recorded, but the distances among the current edges of these populations are within the reported dispersal ability of this species (Gajdárová et al. 2021) and therefore we recommend further monitoring for potential dispersers.
Moreover, an understudied Eurasian lynx population in south-western Turkey, which might represent another remnant population of the Balkan lynx subspecies (Cömert et al. 2018; Mengüllüoğlu et al. 2021a), could be another candidate source for population rescue and needs special attention from both the ecological and genetic point of view. Previous studies, however, showed that this lynx population occur in montane coniferous forests and at very high densities with brown hare as its principal prey (Avgan et al. 2014; Mengüllüoglu et al. 2018), suggesting it is ecologically more similar to other Caucasian lynx populations in Anatolia. On the other hand, the Caucasian lynx from the Greater Caucasus might well be another candidate for reinforcement given its genetic makeup and prey preference. Finally, our study did not take into account the possibility for translocation (exchange) of individuals between the two confirmed Balkan lynx reproductive nuclei in Mavrovo NP in N. Macedonia, and Munella Mt. in Albania (Melovski et al. 2018), which is a prospect worth considering in the coming years, especially if no natural gene flow exists between them.
The results presented in this paper give a roadmap for future research, with robust and targeted methodology to provide further insights into the ecological differences among the subspecies.
Conclusion
Due to its extremely small (effective) population size, the Balkan lynx is in immediate risk of extinction and will likely need genetic reinforcement in the imminent future. Our study stresses the need that not only genetic relatedness, but also ecological and behavioural characteristics of potential source populations need to be studied and compared with the targeted population to provide informed decision about the most suitable donor population. While the genetically closest populations are often assumed to be ecologically most similar, our study on three neighbouring populations belonging to three different Eurasian lynx subspecies revealed that the Balkan lynx, although more closely related to the Caucasian subspecies at the level of the mitogenome, is considerably more similar to the Carpathian lynx in its behavioural ecology. This creates a dilemma in respect to the most suitable source for genetic rescue of the Balkan lynx.
Based on available knowledge provided by this study and previous results of genetic analyses, we suggest reinforcement with the use of only (or predominantly) male Carpathian lynx, which would mimic the natural male-biased gene flow, as was apparently repeated through sex-biased dispersal from the Carpathian population in the past (Bazzicalupo et al. 2022). This would provide ecologically similar individuals to the existing Balkan population and at the same time enable it to retain the unique mitogenome of this population. Although population rescue with the use of only male lynx seems to be giving positive results in another population threatened by inbreeding depression (Krofel et al. 2021), the potential impact of altered sex structure of small remnant populations caused by sex-biased translocation needs to be carefully evaluated.
The future of the Balkan lynx may depend on the reinforcement actions from neighbouring population(s). However, we encourage that in-situ conservation activities already in place through the Balkan Lynx Recovery Programme continue as an integrative approach to recovery, species-wide management and conservation.
References
Aksoy N (2009) Flora of Karakiriş Mountain (Seben-Nallıhan). Düzce Univ J for 5:104–125 ([In Turkish])
Avgan B, Zimmermann F, Güntert M, Arıkan F, Breitenmoser U (2014) The first density estimation of an isolated Eurasian lynx population in southwest Asia. Wildlife Biol 20:217–221. https://doi.org/10.2981/wlb.00025
Bazzicalupo E, Lucena-Perez M, Kleinman-Ruiz D et al (2022) History, demography and genetic status of Balkan and Caucasian Lynx lynx (Linnaeus, 1758) populations revealed by genome-wide variation. Divers Distrib 28(1):65–82
Belotti E, Weder N, Bufka L, Kaldhusdal A, Küchenhoff H, Seibold H, Woelfing B, Heurich M (2015) Patterns of lynx predation at the interface between protected areas and multi-use landscapes in Central Europe. PLoS ONE 10(9):e0138139
Breitenmoser U, von Arx M, Bego F, et al (2008) Strategic planning for the conservation of the Balkan Lynx. In: Proceedings of the III Congress of Ecologists of the Republic of Macedonia with International Participation. Macedonian Ecological Society, Struga, FYR Macedonia, pp 242–248
Breitenmoser-Würsten CH, Breitenmoser U (2001) The Balkan lynx population—history, recent knowledge on its status and conservation needs. KORA Report Nr. 7
Carbone C, Cowlishaw G, Isaac NJB, Rowcliffe JM (2005) How far do animals go? Determinants of day range in mammals. Am Nat 165:290–297
Cömert N, Carlı O, Dinçtürk HB (2018) The missing lynx of Eurasia at its southern edge: a connection to the critically endangered Balk`an lynx. Mitochondrial DNA Part A DNA Mapping Seq Anal 29(8):1269–1275. https://doi.org/10.1080/24701394.2018.1445240
Čop J (1990) Review of the resettlement of lynx (Lynx lynx L.) in Slovenia (YU) 1973–1990
Filipovski GJ, Rizovski R, Ristevski P (1996) The characteristics of the climate-vegetation-soil zones (regions) in the Republic of Macedonia. Makedonska akademija na naukite i umetnostite, Skopje, 1996 [in Macedonian]
Fležar U, Pičulin A, Bartol M, Černe R, Stergar M, Krofel M (2019) Eurasian lynx (Lynx lynx) monitoring with camera traps in Slovenia in 2018–2019, Ljubljana
Frankham R, Ballou JD, Eldridge MDB, Lacy RC, Ralls K, Dudash MRR, Fenster CB (2011) Predicting the probability of outbreeding depression. Conserv Biol 25(3):465–475. https://doi.org/10.1111/j.1523-739.2011.01662.x
Gajdárová B, Belotti E, Bufka L, Duľa M, Kleven O, Kutal M, Ozoliņš J, Nowak C, Reiners TE, Tám B, Volfová J, Krojerová-Prokešová J (2021) Long-distance Eurasian lynx dispersal—a prospect for connecting native and reintroduced populations in Central Europe. Conserv Genet 22:799–809. https://doi.org/10.1007/s10592-021-01363-0
Ivanov Gj, Melovski D, Janevski R, Stojanov A, Avukatov V, Pavlov A (2018) Detecting alternative prey of the Balkan lynx using scat analysis. For Rev 49(1):06–13
Gonev A, Stojanov A, Pavlov A, Melovski D, Veapi E (2021) The 2021 Intensive camera-trapping session in Mavrovo National Park. MES report. Skopje
Gugolz D, Bernasconi MV, Breitenmoser-Würsten C, Wandeler P (2008) Historical DNA reveals the phylogenetic position of the extinct alpine lynx. J Zool 275(2):201–208. https://doi.org/10.1111/j.1469-7998.2008.00428.x
Halotel Y (2020) Estimation of roe deer density in the Mavrovo National Park under the Balkan lynx Recovery Programme. Master Ingénierie Écologique et Gestion de la Biodiversité, Montpellier, p 53
Hemmer H (1993) Felis (Lynx) lynx Linnaeus, 1758—Luchs, Nordluchs. In: Stubbe M, Krapp F (eds) Handbuch der Säugetiere Europas, Faubsäuger (Teil II). Aula-Verlag, Wiesbaden
Hofmeester TR, Thorsen NH, Cromsigt JPGM, Kindberg J, Andren H, Linnell JDC, Odden J (2021) Effects of camera-trap placement and number on detection of members of a mammalian assemblage. Ecosphere 12(7): https://doi.org/10.1002/ecs2.3662
Jedrzejewski W, Schmidt K, Milkowski L, Jedrzejewska B, Okarma H (1993) Foraging by lynx and its role in ungulate mortality: the local (Białowieza Forest) and the Palaearctic viewpoints. Acta Theriol 38:385–403
Jobin A, Molinari P, Breitenmoser U (2000) Prey spectrum, prey preference and consumption rates of Eurasian lynx in the Swiss Jura Mountains. Acta Theriol 45:243–252
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Johnson WE, Onorato DP, Roelke ME et al (2010) Genetic restoration of the Florida panther. Science (new York, n.y.) 329(5999):1641–1645. https://doi.org/10.1126/science.1192891
Kitchener AC, Breitenmoser-Würsten Ch, Eizirik E, et al (2017) A revised taxonomy of the Felidae. The final report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group. CatNews SI 11, 80 p
Kratochvil J (1968) Survey of the distribution of populations of the genus Lynx in Europe. Acta Scientiarum Naturalium Acadamiae Scientarum Bohemoslovacae - Brno 4:5–12
Krofel M, Huber DJ, Kos I (2011) Diet of Eurasian lynx Lynx lynx in the northern Dinaric Mountains (Slovenia and Croatia): importance of edible dormouse Glis glis as alternative prey. Acta Theriol 56:315–322. https://doi.org/10.1007/s13364-011-0032-2
Krofel M (2012) Predation-related interspecific interactions in Eurasian lynx (Lynx lynx) in northern Dinaric Mountains. Doctorate Thesis. University of Ljubljana
Krofel M, Kos I, Jerina K (2012) The noble cats and the big bad scavengers: effects of dominant scavengers on solitary predators. Behav Ecol Sociobiol 66:1297–1304
Krofel M, Luštrik R, Stergar M, Jerina K (2013a) Habitat use of Alpine chamois (Rupicapra rupicapra) in Triglav National Park
Krofel M, Skrbinšek T, Kos I (2013b) Use of GPS location clusters analysis to study predation, feeding, and maternal behavior of the Eurasian lynx. Ecol Res 28:103–116. https://doi.org/10.1007/s11284-012-1005-x
Krofel M, Jerina K, Kljun F et al (2014) Comparing patterns of human harvest and predation by Eurasian lynx Lynx lynx on European roe deer Capreolus capreolus in a temperate forest. Eur J Wildl Res 60:11–21
Krofel M, Fležar U, Hočevar L, et al (2021) Surveillance of the reinforcement process of the Dinaric—SE Alpine lynx population in the lynx-monitoring year 2019–2020. Technical Report. Ljubljana, January 2021
Labisky R, Boulay MC (1998) Behaviors of bobcats preying on white-tailed deer in the everglades. Am Midl Natural 139:275–281
Lucena-Perez M, Marmesat E, Kleinman-Ruiz D et al (2020) Genomic patterns in the widespread Eurasian lynx shaped by Late Quaternary climatic fluctuations and anthropogenic impacts. Molec Ecol 29(4):812–828. https://doi.org/10.1111/mec.15366
Marcon A, Battocchio D, Apollonio M, Grignolio S (2019) Assessing precision and requirements of three methods to estimate roe deer density. PLoS ONE 14(10):e0222349. https://doi.org/10.1371/journal.pone.0222349
Marti I, Ryser-Degiorgis M-P (2018) Morphometric characteristics of free-ranging Eurasian lynx Lynx lynx in Switzerland and their suitability for age estimation. Wildl Biol 2018:1–10. https://doi.org/10.2981/wlb.00432
Mattisson J, Arntsen GB, Nilsen EB, Loe LE, Linnell JDC, Odden J, Persson J, Andrén H (2013) Lynx predation on semi-domestic reindeer: do age and sex matter? J Zool 292:56–63
Matyushkin EN, Vaisfeld MA (eds) (2003) The lynx. Regional features of ecology, use and protection (in Russian with English summary). Nauka, Moscow
Meek PD, Ballard G, Fleming P (2012) An introduction to camera trapping surveys in Australia. Petsmart Toolkit Publication, Invasive Animals Cooperative Research Unit, Canberra, Australia
Melovski D, Ivanov G, Stojanov A et al (2020) First insight into the spatial and foraging ecology of the critically endangered Balkan lynx (Lynx lynx balcanicus, Buresh 1941). Hystrix, Ital J Mamm 31(1):26–34. https://doi.org/10.4404/hystrix-00254-2019
Melovski D, Ivanov GJ, Stojanov A, Trajçe A, Zimmermann F, von Arx M (2009) First camera-trap survey in the National Park Mavrovo, Macedonia. International Conference on Biological and Environmental Sciences, University of Tirana, Faculty of Natural Sciences, pp 312–315
Melovski D, Ivanov G, Stojanov A, et al. (2013) Distribution and conservation status of the Balkan lynx (Lynx lynx balcanicus Bureš, 1941). In Proceedings of the IV Congress of Ecologists of the Republic of Macedonia with international participation. Macedonian Ecological Society, Ohrid, Republic of Macedonia, pp 50–60
Melovski D, Breitenmoser U, von Arx M, Breitenmoser-Würsten C, Lanz T (2015) Lynx lynx ssp. balcanicus (errata version published in 2016). The IUCN Red List of Threatened Species 2015: Downloaded on 03 March 2021
Melovski D, Von Arx M, Avukatov V, Breitenmoser-Würsten C, Ðurović M, Elezi R, Gimenez O, Hoxha B, Hristovski S, Ivanov G, Karamanlidis AA, Lanz T, Mersini K, Perović A, Ramadani A, Sanaja B, Sanaja P, Schwaderer G, Spangenberg A, Stojanov A, Trajçe A, Breitenmoser U (2018) Using questionnaire surveys and occupancy modelling to identify conservation priorities for the Critically Endangered Balkan lynx Lynx lynx balcanicus. Oryx 54(5):706–714. https://doi.org/10.1017/S0030605318000492
Mengüllüoglu D, Ambarlı H, Berger A, Hofer H (2018) Foraging ecology of Eurasian lynx populations in southwest Asia: Conservation implications for a diet specialist. Ecol Evol 8(18):1–13
Mengüllüoğlu D, Fickel J, Hofer H, Förster DW (2019) Non-invasive faecal sampling reveals spatial organization and improves measures of genetic diversity for the conservation assessment of territorial species: Caucasian lynx as a case species. PLoS ONE. https://doi.org/10.1371/journal.pone.0216549
Mengüllüoğlu D, Ambarlı H, Barlow A et al (2021a) Mitogenome phylogeny including data from additional subspecies provides new insights into the historical biogeography of the Eurasian lynx Lynx lynx. Genes 12:16
Mengüllüoğlu D, Edwards S, Hofer H, Berger A (2021b) Female and male Eurasian lynx have distinct spatial tactics at different life-history stages in a high-density population. Ecol Evol 11:10432–10445. https://doi.org/10.1002/ece3.7846
Mengüllüoğlu D (2010) An inventory of medium and large mammal fauna in pine forests of Beypazarı through camera trapping. MSc Thesis, METU, Ankara, Turkey
Miric Ð (1981) The lynx populations of the Balkan Peninsula (Lynx lynx martinoi Miric, 1978). Pos. Izd. SANU 139 Odel Prir Mat Nauka 55:1–154
Molinari-Jobin A, Zimmermann F, Ryser A, Molinari P, Haller H, Breitenmoser-Wursten C, Capt S, Eyholzer R, Breitenmoser U (2007) Variation in diet, prey selectivity and home-range size of Eurasian lynx Lynx lynx in Switzerland. Wildl Biol 13:393–405
Moritz C (1994) Applications of mitochondrial-DNA analysis in conservation—a critical-review. Mol Ecol 3:401–411
Odden J, Linnell JDC, Andersen R (2006) Diet of Eurasian lynx, Lynx lynx, in the boreal forest of south-eastern Norway: the relative importance of livestock and hares at low roe deer density. Eur J Wildl Res 52:237–244
Okarma H, Jedrzejewski W, Schmidt K, Kowalczyk R, Jedrzejewska B (1997) Predation of Eurasian lynx on roe deer and red deer in Bialowieza Primeval Forest. Pol Acta Theriol 42:203–224
Pépin D, Adrados C, Mann C, Janeau G (2004) Assessing real daily distance travelled by ungulates using differential GPS locations. J Mamm 85:774–780. https://doi.org/10.1644/BER-022
Rowcliffe JM, Field J, Turvey ST, Carbone C (2008) Estimating animal density using camera-traps without the need for individual recognition. J Appl Ecol 45:1228–1236. https://doi.org/10.1111/j.1365-2664.2008.01473.x
Schai-Braun SC, Rödel HG, Hackländer K (2012) The influence of daylight regime on diurnal locomotor activity patterns of the European hare (Lepus europaeus) during summer. Mamm Biol 77(6):434–440. https://doi.org/10.1016/j.mambio.2012.07.004
Sedalischev VT, Odnokurtsev VA, Ohlopkov IM (2014) The materials on ecology of the lynx (Lynx lynx, 1758) in Yakutia. News Samara Sci Center Russ Acad Sci 16:175–182 ([In Russian])
Sindičić M, Polanc P, Gomerčić T, Jelenčič M, Huber D, Trontelj P, Skrbinšek T (2013) Genetic data confirm critical status of the reintroduced Dinaric population of Eurasian lynx. Cons Gen 14:1009–1018
Soofi M, Ghoddousi A, Hamidi AKh, et al (2017) Precision and reliability of indirect population assessments for the Caspian red deer Cervus elaphus maral. Wildl Biol 2017:1–8 https://doi.org/10.2981/wlb.00230
Soyumert A, Erturk A, Tavşanoğlu Ç (2019) The importance of lagomorphs for the Eurasian lynx in Western Asia: Results from a large-scale camera-trapping survey in Turkey. Mamm Biol 95:18–25
Spitz F, Janeau G (1990) Spatial strategies: an attempt to classify daily movements of wild boar. Acta Theriol 35(1–2):129–149. https://doi.org/10.4098/0001-7051
Stojanov A (2020) The use of the camera-trapping method as a tool for determining the abundance and density of the Balkan lynx population (Lynx lynx balcanicus Bureš, 1941) in Mavrovo National Park. MSc thesis. St Ciryll and Methodius University, Skopje, N. Macedonia [in Macedonian]
Svoboda NJ, Belant JL, Beyer DE, Duquette JF, Martin JA (2013) Identifying bobcat Lynx rufus kill sites using a global positioning system. Wildl Biol 19(1):78–86. https://doi.org/10.2981/12-031
Teerink BJ (1991) Hairs of west European mammals. Cambridge University Press, Cambridge
Turkstat (2017) https://www.tuik.gov.tr/Home/Index. Accessed 13 Feb 2022
Vandel JM, Stahl P, Herrendschmidt V, Marboutin E (2006) Reintroduction of the lynx into the Vosges Mountain massif: From animal survival and movements to population development. Biol Cons 131:370–385
Vogt K, Signer S, Ryser A, Schaufelberger L, Nagl D, Breitenmoser U, Willisch C (2019) Einfluss von Luchsprädation und Jagd auf die Gämse—Teil 1 und 2. Bericht in Zusammenarbeit mit dem Jagdinspektorat des Kantons Bern. KORA Bericht Nr.84. KORA, Muri bei Bern, Schweiz
Von Arx M, Breitenmoser-Würsten C, Zimmermann F, Breitenmoser U (2004) Status and Conservation of the Eurasian Lynx (Lynx lynx) in Europe in 2001. KORA Report, Bern
Wachter B, Blanc A-S, Melzheimer J, Höner OP, Jago M, Hofer H (2012) An advanced method to assess the diet of free-ranging large carnivores based on scats. PLoS One 7(6):e38066. https://doi.org/10.1371/journal.pone.0038066
Weidong B (2010) Eurasian lynx in China—present status and conservation challenges. CatNews SI 5:22–26
Werdelin L (1981) The evolution of lynxes. Ann Zool Fenn 18:37–71
Whiteley AR, Fitzpatrick SW, Funk WC, Tallmon DA (2014) Genetic rescue to the rescue. Trends Ecol Evol 1:42–49. https://doi.org/10.1016/j.tree.2014.10.009
Acknowledgements
Much of the field work was aided by Panajot Chrovski, but also game-wardens from Mavrovo National Park, Jasen Protected Area, and Tajmishte Hunting Ground, as well as researchers and students of University of Ljubljana and Slovenia Forest Service.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Balkan lynx study was financially supported by the SCOPES programme (Scientific Cooperation between Eastern Europe and Switzerland) from 2010 until 2012, the People’s Trust for Endangered Species (2013–2014), the German Federal Environmental Foundation (DBU) for the period 2015–2018, as well as the MAVA foundation through the Balkan Lynx Recovery Programme in the period from 2006 until the present day. Part of this study was funded by the Slovenian Research Agency (Grants P4-0059 and N1-0163) and by the European Commission under the LIFE Programme (Project LIFE16 NAT/SI/000634) and INTERREG IIIA Neighbourhood Programme Slovenia/Hungary/Croatia 2004–2006 (Project DinaRis). Lynx study in Turkey was funded by Rufford Foundation (RSGF 11447-1), German Academic Exchange Service (DAAD), General Directorate of Nature Conservation and National Parks, Turkish Ministry of Agriculture and Forestry and Leibniz Gemeinschaft.
Author information
Authors and Affiliations
Contributions
Dime Melovski, Miha Krofel and Deniz Mengüllüoğlu contributed to the study conception and design. Material preparation and data collection were performed by all authors. The first draft of the manuscript was written by Dime Melovski and all authors made editorial comments on previous versions of the manuscript. Analyses were made by Deniz Mengüllüoğlu, Miha Krofel, Vasko Avukatov, Ursa Fležar, Lan Hočevar and Dime Melovski. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Ethical approval
Data collection and fieldwork were carried out after obtaining research permission from the Ministry of Environment and Physical Planning (MOEPP) of the Republic of North Macedonia (Permits number: 11-2186/2; 11-546/2; 11-1006/10, 11/1-1417/2019); Wildlife Department of the Turkish Ministry of Agriculture and Forestry (WDT) (Permit number 30057506-030-1867); and the Slovenian Environmental Agency (nos. 35601-45/2006-6 and 35601-76-2020-6).
Consent to participate
All the authors approved this manuscript and no data have been used for which copyright is needed.
Consent for publication
All the authors give consent to publish this manuscript for the publication and no data have been used for which copyright is needed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Stephanie Schai-Braun.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Melovski, D., Krofel, M., Avukatov, V. et al. Diverging ecological traits between the Balkan lynx and neighbouring populations as a basis for planning its genetic rescue. Mamm Biol 102, 1697–1708 (2022). https://doi.org/10.1007/s42991-022-00268-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00268-w