Abstract
Mycotoxin-producing molds which considered as common maize grains contaminants are the genera Fusarium, Aspergillus and Penicillium. There are natural and safe ways to protect grains from mold contamination as the use of essential oils and chemical treatments. A total number of 25 samples were used to study the natural frequency in five governorates in Egypt, Molecular identification indicated that the most frequent fungi were Fusarium verticillioides, Aspergillus niger, Talaromyces verruculosus, Aspergillus flavus and Aspergillus terreus. The in vitro studies have been done to determine mycelial growth and spore germination inhibition of the two A. flavus; isolated and reference isolates. Thyme and acetic acid were tested in direct contact assay to study their effects on mycelial growth. Treatments showed significant impact on mycelial growth and spore germination inhibition of both A. flavus isolates. In the postharvest application treatments: as vapour and carrier contact assay, Thyme and Acetic acid were tested to determine their influence on growth and aflatoxin production in A. flavus isolates by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS/MS). Results indicated that both treatments were effective in inhibition of aflatoxin production in both vapour and carrier assays as they succeeded in reducing AFB1 while they inhibited completely the production of AFB2. The extent of the inhibition of aflatoxin production was dependent on the concentration and storage duration of treatments applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi rank second among pathogens that cause diseases in maize as the reason for the loss in maize crop (Mohammadi et al. 2011). In order to increase maize production, it is crucial to prevent maize diseases (Lanubile et al. 2015). When conditions are ideal, fungi are responsible for 50 to 80 percent of the damage to stored maize grains.
Aspergillus spp., Fusarium spp., Penicillium spp., Alternaria spp., Pythium spp., Rhizoctonia spp., and Rhizopus spp., which are soil- and seed-borne fungal pathogens, are responsible for many of the common seed diseases in maize (Ashiq 2015; Benkerroum 2020; Rózewicz et al. 2021).
These fungi are able to produce mycotoxins, which are toxicologically dangerous to both human and animals' health. Aflatoxin, ochratoxin, patulin, trichothecenes, and fumonisin are the primary mycotoxins (Ashiq 2015; Mahato et al. 2019; Jallow et al. 2021).
The use of pesticides to control fungi has expanded as their infestation has increased (Christensen et al. 2014). The excessive use of chemicals had a negative impact on the environment and human health (Al-Ansary et al. 2022).
Mycotoxins, according to Leslie et al. (2008), affect the quality of harvested crops and have an adverse effect on the health of individuals as well as animals. Mycotoxins have a wide range of structural variations, which causes a wide range of effects (Ismaiel and Papenbrock 2015).
A number of mycotoxins produced by Aspergillus species, including aflatoxins, were described by Hussain et al. (2013). Mycotoxins known as aflatoxins, which are hazardous to people and animals, are produced by A. flavus and A. parasiticus (Somda et al. 2008).
The prevention of mold contamination and growth and the detoxification of contaminated items are two types of strategies that can be used to manage aflatoxins (Lavkor and Var 2017; Benkerroum 2020). Similarly, pre- and post-harvest controls are two general types of techniques that can be used to prevent aflatoxins (AFS) contamination (Kabak et al. 2006; Lavkor and Var 2017). AF-resistant crop varieties, use of fungal and bacterial inhibitors, application of natural and chemical agents and appropriate field management techniques (such as crop rotation, irrigation, and soil cultivation) are the main control strategies used prior to harvest. Following harvest, control tactics focus on enhancing storage and drying conditions using biological, chemical, and natural irradiation (Varga et al. 2010; Agriopoulou et al. 2020).
The current study aimed to develop strategies for management of fungal seed-borne pathogens and reduce their aflatoxin production depending on the alternatives of conventional fungicides or fumigants as post-harvest treatments.
Materials and methods
Plant material
Twenty five samples (1 kg each) of white maize grains were collected randomly from different locations in Cairo, Giza, Qalyubia, Fayoum and Sharqia governorates, Egypt. Each sample was placed in dry sterile containers. Collected samples were labeled and kept separately in sealed paper bags and transported to the laboratory of biopesticides production, Plant Pathology Department, Research of Agricultural and Biological Institute, National Research Center, where they were stored at 4 °C refrigerator for further analysis.
Isolation of seed-borne fungi associated with maize grains
One hundred maize grains were taken from each of the 25 samples, surface sterilized by dipping in 1 % aqueous sodium hypochloride solution for 3 min, followed by three successive rinses in sterile distilled water Then grains were dried with sterilized filter papers in a laminar flow hood. From each sample, 100 grains were selected then plated on each of Potato Dextrose Agar (PDA) and Malt salted agar (MSA) media, 50 grains/medium in 5 replicates for 7 day at 25±2 °C (Dhingra and Sinclair 1985). The isolated fungi were single-spored and the cultures were transferred onto PDA slants at 4 °C for further studies (Cumagun 2012).
Frequency of fungal isolates associated with grains
The frequency of occurrence of each fungus species isolated from maize grains was calculated by the following formula (Tsedaley and Adugna (2016)):
Fungal isolate Frequency (%) = Number of occurrence of fungus species/total number of isolated fungi × 100.
Biological identification of more frequent maize associated seed-borne fungi
The biological identification of the fungal isolates had been done and published in previous work (Al-Ansary et al. 2022).
Molecular identification of more frequent maize associated seed-borne fungi
The molecular identification of the fungal isolates had been done by Sigma Scientific Services co.
Extraction of fungal DNA
The fungal genomic DNA was extracted from single spored cultures of five fungal isolates recovered from maize grains collected from different governments in Egypt and grown on PD broth medium with the Quick-DNA Fungal Kit (Zymo Research, USA) according to the manufacturer's instructions.
Determination of the genomic DNA quantity and purity
The extracted DNA was estimated according to Sambrook et al. (1989) through reading the UV-absorbance at 260 and 280 nm using spectrophotometer to estimate the DNA quantity and purity.
PCR partial amplification and sequencing of internal transcribed spacer (ITS)
The fungal isolates were identified using molecular genetic analysis and internal transcribed spacer (ITS). A technique based on Boekhout et al. (1994) was used to get partial sequences of the isolate 18S rDNA. The gene’s divergent domain was amplified using two separate primers: the first primer (ITS1) sequence is 5' TCCGTAGGTGAACCTGCGG-3', while the second primer (ITS4) sequence is 5' TCCTCCGCTTATTGATATGC-3'. Utilized primer (12 ng) and 40 ng of the purified DNA sample were added to each polymerase chain reaction (PCR) bead. The amplified DNA products were electrophoresed on 1.0% agarose gel and 1X TBE (Tris-borate-EDTA) buffer at a constant 100 V for about 2 hours. The different band sizes were determined against Gene Ruler 100 bp DNA Ladder (Thermo SM0243) and the separated bands were stained with 0.5 µg/ml ethidium bromide and photographed using the Gel Documentation System with UV Transilluminator.
Fungal DNA purification
The PCR products were cleaned up using GeneJET™ PCR Purification Kit (Thermo K0701).
Isolate identification
The DNA sequencing of the purified PCR products were done with ABI 3730xl DNA sequencer (GATC Company, Germany) by using forward primer.
Evolutionary relationships of taxa
The DNA sequences of the fungal isolates were compared with the sequences available by the Basic Local Alignment Search Tool (BLAST) in the National Center for Biotechnology Information (NCBI), GenBank database (http://www.ncbi.nlm.nih.gov). The sequences were aligned together with those of reference taxa retrieved from public databases and the evolutionary distance was generated based on NCBI Neighbor Joining (Saitou and Nei 1987).
Source of standard Fungal isolate
An isolate of A. flavus isolates was kindly provided by Toxins and Food Pollutants Department, Food Industries and Nutrition Research Division, National Research Center. This isolate was known to induce aflatoxin and used as a standard isolate.
Essential oil
The ready-to-use Thyme essential oil was obtained from the Pressing and Extraction of Natural Oils Unit, National Research Center.
Direct contact assay
Antifungal activity was studied by using an in vitro direct contact assay. (Cakir et al. 2004; Gong et al. 2009).
Mycelial growth inhibition
Mycelial radial growth inhibition assay was conducted according to (Quiroga et al. 2001; Cakir et al. 2004; Song et al. 2004; Gong et al. 2009).
The percentage (%) of mycelial growth inhibition was determined as [(Mc – Mt)/Mc] ×100, where Mc average of five replicates of fungal mycelial growth measured on control agar medium, and Mt is an average of five replicates of fungal mycelial growth measured on treated agar medium with the treatments.
Inhibitory activity on spore germination
Spore germination inhibition assay was conducted according to (Liu et al. 2009; Wang et al. 2010).
The percentage (%) of the spore germination inhibition was determined as [(Gc – Gt)/Gc] ×100, where Gc is an average of five replicates of germinated spore in the control, and Gt is an average of five replicates of germinated spores in the treated ones.
Application methods
Two application methods i.e., Volatile and Carrier contact assays were tested with the acetic acid and thyme at different concentrations and evaluated for their capability to suppress aflatoxin production from A. flavus isolates; the one isolated throughout this study and the standard isolate, on maize grains during storage.
Volatile contact assay
The method of Paster et al. (1995), Abdallah (2005) and Bill et al., (2015) was modified as follow: The effect of volatile components on the treated maize grains with different concentrations of acetic acid and essential oils was tested in plastic containers. Maize grains treated without acetic acid or oils was used as the control. Three replicates were used for each treatment.
Carrier contact assay
Method by Wang et al. (2019) was modified as follow: Wheat bran as carrier was autoclaved at 120 °C for 60 min. Each of the tested acetic acid or oil added individually to the sterilized wheat bran with different concentrations, and then mixed thoroughly to ensure equal distribution of mixed carriers. The prepared mixture was added to sterilized maize grains. Three replicates were used for each treatment.
Aflatoxin production
Detection and analysis of aflatoxin was performed using liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) with an ExionLC AC system for separation and SCIEX Triple Quad 5500+ MS/MS system equipped with an electrospray ionization (ESI) for detection. The instrument data were collected and processed using the SCIEX OS 1.6.10.40973 software (Hu et al. 2017).
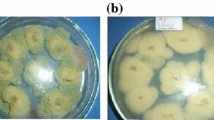
To determine the in vitro antifungal efficacy of three different concentrations (1/2 EC90, EC90 and 2EC90) of acetic acid and thyme essential oil, maize grains were divided into two methods, i.e.,, Volatile assay and Carrier contact assay, 1.0 Kg for each concentration of each treatment in both methods. Maize grains were primarily sterilized in Erlenmeyer flask for 20 min at 121 °C using autoclave.
Maize grains in both volatile and carrier contact methods were divided into two groups, each group inoculated with 20 mL/kg (106 spores/ml) of conidial suspension of one of the two A. flavus fungal isolates, then treatments with different concentrations were added and then maize was stored for 30 days. Untreated and inoculated maize grains were used as control treatments. Samples were taken from each method after 15 and 30 days of storage and AFB1 was extracted from maize grains.
Statistical analysis
The median effective concentrations (EC50 and EC90) against tested fungi were calculated using coefficient equation between probit of means of inhibition (%) and logarithm of concentrations used according to (Ramadan et al. 2007).
Results
Isolation of seed-borne fungi associated with maize grains
From the twenty five samples of maize grain collected from different governorates, the results showed that the most dominant genera isolated on PDA were Aspergillus (three species) followed by Penicillium sp. and Fusarium spp with average frequency of 62.5, 22.5 and 13.3%, respectively, while on MSA average frequency was 58.9, 29.4 and 8.3%, respectively. The other fungal genera such as Alternaria sp. (1.1–0.0 %) and other (0.9 & 0.5%) were also isolated with low frequency of occurrence (Fig.1).
A. flavus was the most frequent species among the Aspergilli group -in both PDA and MSA media- with frequency of occurrence 30.8 and 35.6 %, respectively, followed by A. niger with frequency of occurrence 26.0 and 19.9 %, respectively, while A. terrus was the least frequent isolate among the Aspergilli with frequency of occurrence 5.7 and 3.5 %, respectively.
Molecular Identification of more frequent maize associated seed-borne fungi
Following DNA extraction from pure fungal strains and concentration measurement by spectrophotometer, the ITS1 and ITS4 primers were used to amplify the area of the rDNA repeat unit that includes the ITS from the fungal strain's genomic DNA. (Fig. 2) demonstrate that after amplification, roughly 632 bp were produced.
After the DNA sequencing of the purified PCR products with ABI 3730xl DNA sequencer (GATC Company, Germany), the obtained DNA sequence with the identified fungal strains from NA01 to NA05 (Table 1) were conserved in the GenBank.
Evolutionary relationships of the identified strains
See Fig. 3
Determination of antimycotoxigenic activity
The antifungal effect of thyme and acetic acid was examined in vitro by the food poison (bi culture) technique. The EC50 and EC90 values of the thyme and acetic acid against mycelial growth and spore germination of isolated A. flavus and reference isolate of A. flavus are shown in Table 2.
Both thyme and acetic acid had an obvious significant inhibitory activity against isolated A. flavus mycelial growth, where there EC50 is 0.8704, 0.0016 mg/mL, respectively and EC90 is 1.6074, 0.0075 mg/mL, respectively with regression equations y = 3.7538x−6.0351 and y =1.4905x + 4.6916, respectively and coefficient of determination R2 = 0.872, R2 = 0.9701, respectively according to Table 2 and Fig. 4a. While, mycelial growth of reference isolate of A. flavus was significantly affected by thyme and acetic acid, as their EC50 is 0.8054, 0.0018 mg/mL, respectively and EC90 is 2.0808, 0.0088 mg/mL, respectively with regression equations y = 2.425x−2.0467 and y =1.4522x + 4.6249, respectively and coefficient of determination R2 = 0.9288, R2 = 0.9954, respectively according to Table 2 Fig. 4b.
Both thyme and acetic acid had an obvious significant inhibitory activity of spore germination against isolated A. flavus, where there EC50 is 0.7309, 0.0036 mg/mL, respectively and EC90 is 1.2616, 0.0129 mg/mL, respectively with regression equations y = 4.2188x−7.0821 and y = 1.8219x + 3.9773, respectively and coefficient of determination R2 = 0.75, R2 = 0.9757, respectively according to Table 2 Fig. 4c. While spore germination of reference isolate of A. flavus was significantly affected by thyme and acetic acid, as there EC50 is 1.1303, 0.0042 mg/mL, respectively and EC90 is 1.8781, 0.0215 mg/mL, respectively with regression equations y = 4.5344x−8.8444 and y =1.4129x + 4.116, respectively and coefficient of determination R2 = 0.9547, R2 = 0.9394, respectively according to Table 2 Fig. 4d.
Detection and determination of aflatoxin production
The effect of thyme essential oil and acetic acid on two types of aflatoxins, i.e., AFB1 and AFB2 production by two A. flavus isolates (isolated and reference) in sterilized maize grains, at two different assays (volatile and carrier) and two incubation periods was studied after 15 and 30 days using LC-ESI-MS/MS. Both of them had a significant impact on AFB1 and AFB2 accumulation.
In the untreated controls, the major levels of only AFB1 and AFB2 were observed after 15 days of storage in both volatile and carrier contact assays then they began to decrease till it reached the lowest level after 30 days of storage.
After 15 days of storage
Determination of aflatoxin B1
Volatile contact assay indicated that thyme and acetic acid treatments after 15 days of incubation were able to reveal important reduction of AFB1 accumulation produced from isolated A. flavus, where there EC50 is 1.0304, 0.1008 mg/mL, respectively and EC90 is 1.8415, 1.6853 mg/mL, respectively with regression equations Y = 5.0763x−10.295 and Y =1.0464x + 4.9036, respectively and coefficient of determination R2 = 0.8977, R2 = 0.9067, respectively (Table 3 and Fig. 5a). In carrier contact assay, the thyme and acetic acid treatments after 15 days of storage also had an obvious significant inhibitory activity against AFB1, where there EC50 is 5.9729, 0.1588 mg/mL, respectively and EC90 is 16.6064, 0.2724 mg/mL, respectively with regression equations Y = 2.8823x−5.8841 and Y = 5.4646x−7.0274, respectively and coefficient of determination R2 = 0.7905, R2 = 0.9454, respectively according to Table 3 Fig. 5b.
Correlation coefficient between the logarithm of the thyme and acetic acid concentrations and the probit of means of AFB1 content produced by a isolated A. flavus and b reference A. flavus under volatile contact assay and AFB1 content produced by c isolated A. flavus and d reference A. flavus under carrier contact assay
Also, volatile contact assay showed that thyme and acetic acid treatments after 15 days of incubation had significant inhibitory effect on AFB1 levels produced from reference A. flavus, where there EC50 is 0.0045, 0.0013 mg/mL, respectively and EC90 is 0.0744, 0.0134 mg/mL, respectively with regression equations Y = 1.0542x + 4.3072 and Y =1.2623x + 4.8554, respectively and coefficient of determination R2 = 0.9976, R2 = 0.9918, respectively (Table 3 Fig. 5c). In carrier contact assay, the thyme and acetic acid treatments after 15 days of storage also were effective in the inhibition of AFB1 accumulation, where there EC50 is 4.3877, 0.002 mg/mL, respectively and EC90 is 45.7908, 4.3841 mg/mL, respectively with regression equations Y = 1.2567x + 0.4228 and Y = 0.382x + 4.8888, respectively and coefficient of determination R2 = 0.9142, R2 = 0.9845, respectively (Table 3 Fig. 5d).
Determination of aflatoxin B2
In volatile contact assay, acetic acid had the ability to completely inhibit the accumulation of AFB2 produced by isolated A. flavus after 15 days of storage in compare to the control. However thyme was able to reduce AFB2 accumulation produced from isolated A. flavus, where there EC50 is 0.8482 mg/mL and EC90 is 1.4336 mg/mL with regression equation Y = 55.6165x−11.448 and coefficient of determination R2 = 0.7527 (Table 4 and Fig. 6a). In carrier contact assay, the thyme and acetic acid treatments after 15 days of storage also had an obvious significant inhibitory activity against AFB2, where there EC50 is 7.007, 0.0894 mg/mL, respectively and EC90 is 11.5019, 0.1431 mg/mL, respectively with regression equations Y = 5.9469x−17.869 and Y = 6.2618x−7.2177, respectively and coefficient of determination R2 = 0.7872, R2 = 0.75, respectively (Table 4 and Fig. 6b).
Correlation coefficient between the logarithm of the thyme concentrations and the probit of means a Effect of different concentrations of thyme on AFB2 content produced by isolated A. flavus under volatile contact assay. Correlation coefficient between the logarithm of the thyme and acetic acid concentrations and the probit of means of AFB2 content produced by b isolated A. flavus and c reference A. flavus under carrier contact assay
In volatile contact assay, both thyme and acetic acid had the ability to completely inhibit AFB2 accumulation produced by reference A. flavus after 15 days of storage in compare to the control. On the other hand, in carrier contact assay, the thyme and acetic acid treatments after 15 days of storage also were effective in the inhibition of AFB2 accumulation, where there EC50 is 1.6428, 0.0024 mg/mL, respectively and EC90 is 4.6534, 0.0166 mg/mL, respectively with regression equations Y = 2.8306x−4.102 and Y = 1.4949x + 4.4433, respectively and coefficient of determination R2 = 00.9627, R2 = 0.75, respectively (Table 4 and Fig. 5c).
After 30 days of storage
After 30 days of incubation, thyme and acetic acid treatments had the ability to reduce AFB1 content produced by isolated A. flavus, where there EC50 is 0.5163, 0.0096 mg/mL, respectively and EC90 is 20.2622, 1.1595 mg/mL, respectively with regression equations Y = 0.8031x + 2.8213 and Y =0.6146x + 4.3967, respectively and coefficient of determination R2 = 0.9902, R2 = 0.9882, respectively under the condition of volatile contact assay (Table 5 and Fig. 7a). On the other hand and under carrier contact assay, thyme and acetic acid treatments also had an obvious significant inhibitory activity against AFB1, where there EC50 is 10.0956 0.0759 mg/mL, respectively and EC90 is 44.5858, 7.1775 mg/mL, respectively with regression equations Y = 1.9843x−2.9454 and Y = 0.6478x + 3.7821 (Table 5 and Fig. 7b).
Correlation coefficient between the logarithm of the thyme and acetic acid concentrations and the probit of means of AFB1 content produced by a isolated A. flavus under volatile contact assay and b reference A. flavus under carrier contact assay c isolated A. flavus under volatile contact assay and d reference A. flavus under carrier contact assay
While in volatile contact assay, both treatments of thyme and acetic acid possessed significant inhibitory effect on AFB1 levels produced from reference A. flavus, where there EC50 is 0.0159, 0.0209 mg/mL, respectively and EC90 is 0.1617, 0.0551 mg/mL, respectively with regression equations Y = 1.2707x + 3.4733 and Y =1.2623x + 4.8554, respectively and coefficient of determination R2 = 0. 9137, R2 = 0.9239, respectively (Table 5 and Fig. 7c). Although, treatments with thyme and acetic acid also had great effect in AFB1 accumulation inhibition under carrier contact assay, where there EC50 is 0.2608, 0.002 mg/mL respectively and EC90 is 5.992, 4.3841 mg/mL, respectively with regression equations Y = 0.3808x + 4.0799 and Y = 0.382x + 4.8888, respectively and coefficient of determination R2 = 0. 9051, R2 = 0.9845, respectively (Table 5 and Fig. 7d).
Determination of aflatoxin B2
Both thyme and acetic acid treatments using volatile and carrier contact assay had the ability to completely inhibit AFB2 accumulation produced by both isolated and reference A. flavus isolates after 30 days of storage in compare to the control.
Discussion
Grains are considered as one of the most economic important crops worldwide as well as in Egypt (Al-Ansary 2023). It’s found to be suitable for infection with a number of fungi, i.e.,, Aspergillus spp., Penicillium spp. and Fusarium spp. (Mansfield et al. 2008; Hussain et al. 2013).
In this study, corn grain samples were collected from different governorates in Egypt for detection of the presence of fungi-producing aflatoxin.
The experimental results showed that three genera of fungi (Aspergillus spp., Penicillium sp. and Fusarium spp.) were noted to be the most frequently fungi isolated from the corn grains. Out of them, three species of Aspergillus (A. flavus, A. niger and A. terreus) were isolated. The findings of this study could be suppoted by the work of Amadi and Adeniyi (2009); Goko et al. (2010); Krinjaja et al. (2013); Baka et al. (2014); Madbouly et al. (2014); Tsedaley and Adugna (2016); Shabana et al. (2022); Oldenburg et al. (2017) and Gromadzka et al. (2019) who also found that Aspergelli, Fusaria, and Penicilli were the three most prevalent post-harvest pathogenic fungi.
Results of isolation frequency in this study mentioned that Aspergillus spp. had the frequent presence and high level genera. These findings agree with the literature as Goko et al. (2010). On the other hand, Elwakil et al. (2020) stated that the most prevalent species were F. verticillioides (100%), Penicillium spp. (96.7%), A. flavus (80%), and A. niger (83.3%).
The five fungal isolates were biologically and molecularly identified using ITS region among using two primers flanking a PCR product of 632 nts. Results of molecular identification confirmed the biological identification as they were documented in GenBank under the accession numbers of OQ135182.1, OQ135183.1, OQ135184.1, OQ135185.1 and OQ135186.1 representing the strains of A. flavus, A. niger, A. terreus, F. verticillioides and T. verruculosus respectively. Results of molecular identification agreed with some investigations (Galletti et al. 2019; Gaige et al. 2020; Rahm et al. 2020; Tran et al. 2021) that revealed that maize grains were accompanied by several fungal pathogens, including A. flavus, A. niger, Penicillium spp., F. verticillioides and others, which causes various diseases in the majority of the world maize-growing areas.
At the level of inducing aflatoxin, the strain of A. flavus (OQ135182.1) was tested for aflatoxin production in fungal-infected grains compared to the standard strain.
Management of aflatoxigenic fungi was conducted using the selected strain as well as the standard. This was done by using two materials (thyme essential oil and acetic acid).
Tzortzakis and Economakis (2007); Martínez (2012) and Daniel et al. (2015) mentioned that essential oils can comprise more than 60 individual components in which up to 85% of the EO can be mainly composed of major components, while the remaining 15% is simply a trace amount (Senatore 1996). Several active compounds, including: aldehydes, phenols, and alcohols show significant bioactivities against fungi development (Jerković et al. 2019). Also acetic acid and other weak organic treatments can be successfully replace chemical treatments as they have an important role such as: preservatives in the food sector (Kang et al. 2003), reduce the development of fungi (Hassan et al. 2015), and prevent them from producing mycotoxin (Guimaries et al., 2018). These qualities inspired scientists to employ it for seed treatment as well as plant protection (Szopińska 2013; El-Saidy and El-Hai 2016; Rioux et al. 2016; Dorna et al. 2018; Escamilla et al. 2019). It was shown that acetic acid vapors at different concentrations inhibited the growth of Alternaria sp., Aspergillus spp., F. moniliforne, and Penicillium spp. as well as their ability to produce spores (Abdalla 2005; Morsy et al. 2000a, b; Luz et al. 2021).
The ability of thyme essential oil and acetic acid to inhibit the aflatoxin was detected within detection of their effect on mycelial growth, spore germination and aflatoxin production among two methods (volatile and carrier contact assay).
Acetic acid and EOs application techniques range from fumigation to combining seeds, to soaking seeds in the treatment solutions, to loading treatments on carrier in order to suppress seed-borne diseases. EOs and acetic acid should be applied practically (volatile and carrier contact applications) as inhibitors of mold growth because they are generally regarded as safe. Acetic acid and EOs have strong bioactive effect in the vapour phase, which makes them attractive as fumigants for stored product protection (Čvek et al. 2010).
Results revealed that the two materials were significantly effective on mycelial growth and spore germination inhibition. The findings of thyme essential oil effect on the toxgenic fungi could be supported by the work of Sinha et al. (1993) and Thanaboripat et al. (2004) who demonstrated that A. flavus growth in maize grain was inhibited with citronella EO. Simillarly, EOs were reported to affect the growth of F. culmorum (Sahab et al. 2014; Perczak et al. 2019) and Aspergillus flavus, A. parasiticus, A. ochraceus, and F. verticilloides (Soliman and Badeaa 2002). On the other hand, Nesci et al. (2011) observed that thyme oil couldn’t reduce the counts of Aspergillus section Flavi in stored peanut. Results presented by El-Aziz et al. (2015) indicated that the tested toxigenic fungi were sensitive to the essential oils particularly to thyme and cinnamon. These results were confirmed by many researchers, as the mycelium treated with thyme oil showed alteration in the morphology of the hyphae, which appeared collapsed and caused a reduction in mycelial growth. (Rad et al. 2011; Eweis et al. 2012). While the experimental result of acetic acid treatment was in harmony with earlier results which showed that acetic acid as post-harvest treatments directly inhibit pathogen growth, spore germination and aflatoxin production by affecting the active sites of enzymes and cellular metabolism (Arrebola et al. 2010; Bozik et al. 2017). Organic acids according to Kang et al. (2003) and Wang et al. (2021), lower the pH of the fungal cell, which requires a lot of energy to maintain intracellular pH equilibrium. Fungal growth will be constrained as a result of the significant energy consumption required for this task. Kang et al. (2003) found that when applying acetic acid, fungal respiration was inhibited and suggested that the action of acetic acid in its dissociated form would be more potentiated and exerted within the cell.
Results of aflatoxin reduction with thyme EO and acetic acid proved their significant inhibitory activity against AFB1 and AFB2 produced by both isolated A. flavus and standard A. flavus strains under volatile and carrier assays after 15 and 30 days of storage. Similarly, Abd El-Aziz et al. (2015) proved that A. flavus and A. parasiticus produced less aflatoxins (B) when exposed to studied essential oils compared to the control especially when thyme and cinnamon applied. Numerous researchers have backed up this finding (Rad et al. 2011; Eweis et al. 2012). Additionally, it has been demonstrated that the thyme oil can effectively stop fungi growth and the production of its toxins (Rasooli and Owlia 2005; Kumar et al. 2008; Ismaiel and Papenbrock 2015).
Chang et al. (2022) stated that who stated that volatile contact assay had stronger antifungal activity as a post-harvest treatment than carrier contact assay and this work agrees with our proved results.
As a conclusion, the natural products as thyme and acetic acid can be utilized to suppress fungal seed-borne pathogens and their aflatoxin production in stored maize grains.
Data availability
Data presented in this study are available in this article.
References
Abd El-Aziz ARM, Mahmoud MA, Al-Othman MR, Al-Gahtani MF (2015) Use of selected essential oils to control aflatoxin contaminated stored cashew and detection of aflatoxin biosynthesis gene. Sci World J 15:1–13. https://doi.org/10.1155/2015/958192
Abdallah MA (2005) Effect of acetic acid fumigation on common storage fungi of some medicinal and aromatic seeds. Egypt J Phytopathol 33:77–86
Agriopoulou S, Stamatelopoulou E, Varzakas Th (2020) Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 9:137–184. https://doi.org/10.3390/foods9020137
Al-Ansary NA, Haggag WM, Ali MK (2022) Evaluation of antifungal activity of some natural essential oils against fungal pathogens associated with maize grains. IJAT 18:1897–1916
Al-Ansary NAM., (2023) Seed-borne fungi of stored corn grains and their effect on the contamination with mycotoxins. Doctoral dissertation, Ain Shams University.
Amadi JE, Adeniyi DO (2009) Mycotoxin production by fungi isolated from stored grains. AJB 49:1235–1245
Arrebola F, Pérez-Marín CC, Santiago-Moreno J (2010) Limitation of seasonality in reproductive parameters of Mediterranean bucks using photoperiod treatment. Small Rumin Res 89:31–35. https://doi.org/10.1016/j.smallrumres.2009.11.016
Ashiq S (2015) Natural occurrence of mycotoxins in food and feed: Pakistan perspective. CRFSFS 14:159–175. https://doi.org/10.1111/1541-4337.12122
Baka ZAM, Serag MS, Kardosha TA (2014) Evaluation of some plant extracts for controlling mycoflora causing spoilage of stored cereals and legumes. SJDFS 3:53–61. https://doi.org/10.21608/sjdfs.2014.194312
Benkerroum N (2020) Chronic and acute toxicities of aflatoxins: mechanisms of action. Int J Environ Res Public Health 17:423–450
Bill M, Sivakumar D, van Rooyan Z, Mavuso ZS (2015) New methods of postharvest disease control: using thyme oil fumigation. South Afr Avocado Growe Assoc Year Book 38:98–104
Boekhout T, Kurtzman CP, O’Donnell K, Smith MT (1994) Phylogeny of the yeast genera Hanseniaspora (anamorph Kloeckera), Dekkera (anamorph Brettanomyces), and Eeniella as inferred from partial 26s ribosomal DNA nucleotide sequences. Int J Syst Bacteriol 44:781–786. https://doi.org/10.1099/00207713-44-4-781
Bozik M, Císarová M, Tancinová D, Kourimská L, Hleba L, Kloucek P (2017) Selected essential oil vapors inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind Crops Prod 98:146–152. https://doi.org/10.1016/j.indcrop.2016.11.044
Cakir A, Kordali S, Zengin H, Izumi S, Hirata T (2004) Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. FFJ 19:62–68. https://doi.org/10.1016/j.indcrop.2016.11.044
Chang Y, Harmon PF, Treadwell DD, Carrillo D, Sarkhosh A, Brecht JK (2022) Biocontrol potential of essential oils in organic horticulture systems: from farm to fork. Front Nutr 8:1–26. https://doi.org/10.3389/fnut.2021.805138
Christensen SA, Nemchenko A, Park YS, Borrego E, Huang PC, Schmelz EA, Kunze S, Feussner I, Yalpani N, Meeley R (2014) The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. MPMI 27:1263–1276. https://doi.org/10.1094/MPMI-06-13-0184-R
Cumagun CJR (2012) Plant pathology. InTech, Croatia, p 362
Čvek D, Markov K, Frece J, Dragiˇcevi´c T, Majica M, Delas F (2010) Growth inhibition of Aspergillus ochraceus ZMPBF 318 and Penicillium expansum ZMPBF 565 by four essential oils. Arh Hig Rada Toksikol 61:191–196. https://doi.org/10.2478/10004-1254-61-2010-2009
Daniel CK, Lennox CL, Vries FA (2015) In-vitro effects of garlic extracts on pathogenic fungi Botrytis cinerea, Penicillium expansum and Neofabraea alba. South Afr J Sci 111:1–8. https://doi.org/10.17159/sajs.2015/20140240
Dhingra OD, Sinclair JB (1985) Culture of pathogens basic plant pathology methods. CRC Press, Boca Raton, pp 11–47
Dorna H, Qi Y, Szopińska D (2018) The effect of acetic acid, grapefruit extract and selected essential oils on germination, vigor and health of carrot (Daucus carota L.) seeds. Acta Sci Pol Hortorum Cultus 17:27–38. https://doi.org/10.24326/asphc.2018.2.3
El-Aziz ARM, Mahmoud MA, Al-Othman MR, Al-Gahtani MF (2015) Use of selected essential oils to control aflatoxin contaminated stored cashew and detection of aflatoxin biosynthesis gene. Sci World J. https://doi.org/10.1155/2015/958192
El-Saidy AEA, El-Hai AKM (2016) Effect of some evaporation matters on storability of sunflower (Helianthus annuus L.) seed. Pak J Biol Sci 19:239–249. https://doi.org/10.3923/pjbs.2016.239.249
Elwakil MA, Ghoneem KM, Rehan NA (2020) Prevalence and transmission of seed-borne fungi of maize and their control by phenolic antioxidants. Plant Pathol J 19:176–184. https://doi.org/10.3923/ppj.2020.176.184
Escamilla D, Rosso ML, Zhang B (2019) Identification of fungi associated with soybeans and effective seed disinfection treatments. Food Sci Nutr 7:3194–3205. https://doi.org/10.1002/fsn3.1166
Eweis M, Imhemmed AA, Gad AS (2012) Influence of Thymus serpyllum essential oil on Aspergillus parasiticus morphology and aflatoxins production. RJPBCS 3:322–332
Gaige AR, Todd T, Stack JP (2020) Interspecific competition for colonization of maize plants between Fusarium proliferatum and Fusarium verticillioides. Plant Dis 104:2102–2110. https://doi.org/10.1094/PDIS-09-19-1964-RE
Galletti S, Paris R, Cianchetta S (2019) Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol Res 233:1–36. https://doi.org/10.1016/j.micres.2019.126406
Goko S, Kimura A, Harada H, Oshima M, Ohta M et al (2010) Measurement of neutron capture cross section ratios of 244Cm resonances using NNRI. J Nucl Sci Technol 47:1097–1100. https://doi.org/10.1080/18811248.2010.9720976
Gong Y, Huang Y, Zhou L, Shi X, Guo Z, Wang M, Jiang W (2009) Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum Maxim. (Rutaceae) from China. J Essent Oil Res 21:174–178. https://doi.org/10.1080/10412905.2009.9700141
Gromadzka K, Chełkowski J, Basińska-Barczak A, LalakKańczugowska J (2019) Diversity and mycotoxin production by Fusarium temperatum and Fusarium subglutinans as causal agents of pre-harvest Fusarium maize ear rot in Poland. J Appl Genet 60:113–121. https://doi.org/10.1007/s13353-018-0478-x
Guimarães A, Venancio A, Abrunhosa L (2018) Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit Contam: Part A 35:1803–1818. https://doi.org/10.1080/19440049.2018.1500718
Hassan R, El-Kadi S, Sand M (2015) Effect of some organic acids on some fungal growth and their toxins production. Int J Adv Biol 2:1–11. https://doi.org/10.21608/jacb.2012.55011
Hu Y, Zhang J, Kong W, Zhao G, Yang M (2017) Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem 220:1–8. https://doi.org/10.1016/j.foodchem.2016.09.179
Hussain N, Hussain A, Ishtiaq M, Azam S, Hussain T (2013) Pathogenicity of two seed-borne fungi commonly involved in maize seeds of eight districts of Azad, Jammu and Kashmir, Pakistan. AJP 12:1360–1370. https://doi.org/10.5897/AJB12.454
Ismaiel AA, Papenbrock J (2015) Mycotoxins: producing fungi and mechanisms of phytotoxicity. J Agric Ext Rural Dev 5:492–537. https://doi.org/10.3390/agriculture5030492
Jallow A, Xie H, Tang X, Qi Z, Li P (2021) Worldwide aflatoxin contamination of agricultural products and foods: from occurrence to control. CRFSFS 20:2332–2381. https://doi.org/10.1111/1541-4337.12734
Jerković I, Kranjac M, Marijanović Z, Šarkanj B, Cikoš AM, Aladić K, Pedisić S, Jokić S (2019) Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids and insight into its antifungal activity. Molecules 24:842–858. https://doi.org/10.3390/molecules24050842
Kabak B, Dobson ADW, Var I (2006) Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit Rev Food Sci Nutr 46:593–619. https://doi.org/10.1080/10408390500436185
Krinjaja V, Levic J, Stankovic S, Petrović T, Tomić Z, Mandić V, Bijelić Z (2013) Molds and mycotoxins in stored maize grains. Biotechnol Anim Husb 29:527–536. https://doi.org/10.2298/BAH1303527K
Kumar A, Shukla R, Singh P, Prasad CS, Dubey NK (2008) Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post-harvest fungal infestation of food commodities. IFSET 9:575–580. https://doi.org/10.1016/j.ifset.2007.12.005
Lanubile A, Maschietto V, de Leonardis S, Battilani P, Paciolla C, Marocco A (2015) Defense responses to mycotoxinproducing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. MPMI 28:546–557. https://doi.org/10.1094/MPMI-09-14-0269-R
Lavkor I, Var I (2017) The new approach: The control of aflatoxin contamination at harvest, drying, pre-storage and storage periods in peanut. In: Aflatoxin- Control, Analysis, Detection and Health Risks, Abdulra’uf L.B. (Ed.). Croatia Tech Open Science. 290 p
Leslie JF, Bandyopadhay B, Visconti A (2008) Mycotoxins: detection methods, management, public health and agricultural trade, CABI. 422 p
Liu H, Wang J, Zhao J, Lu S, Wang J, Jiang W, Ma Z, Zhou L (2009) Isoquinoline alkaloids from Macleaya cordata active against plant microbial pathogens. Nat Prod Commun 4:1557–1560. https://doi.org/10.1177/1934578X0900401120
Luz C, Carbonell R, Quiles JM, Torrijos R, de Melo NT, Mecca G (2021) Antifungal activity of peracetic acid against toxigenic fungal contaminants of maize and barley at the postharvest stage LWT. Food Sci Technol 148:111754–111763. https://doi.org/10.1016/j.lwt.2021.111754
Madbouly AK, Ibrahim MIM, Abdel-Wahhab MA (2014) Efficacy of corn and rice seed-borne mycoflora in controlling aflatoxigenic Aspergillus flavus. Comun Sci 5:118–130. https://doi.org/10.14295/cs.v5i2.284
Mahato DK, Lee KE, Kamle M, Devi S, Dewangan KN, Kumar P, Kang SG (2019) Aflatoxins in food and feed: an overview on prevalence, detection and control strategies. Front Microbiol 10:2266. https://doi.org/10.3389/fmicb.2019.02266
Mansfield MA, Jones AD, Kuldau GA (2008) Contamination of fresh and ensiled Maize by multiple Penicillium Mycotoxins. Phytopathology 98:330–336. https://doi.org/10.1094/PHYTO-98-3-0330
Martínez JA (2012) Natural Fungicides Obtained From Plants. Chapter 1. In: Dhanasekaran D, Thajuddin N, Panneerselvam A (Eds). Fungicides for Plant and Animal Diseases. Europe: InTech, pp. 3–28.
Mohammadi M, Anoop V, Gleddie S, Harris LJ (2011) Proteomic profiling of two maize inbreds during early gibberella ear rot infection. Proteomics 11:3675–3684. https://doi.org/10.1002/pmic.201100177
Morsy AA, Abd-El-Kareem F, Abd-Alla MA (2000a) Effect of acetic acid fumigation on common storage fungi of some grains. Egypt J Phytopathol 28:95–106
Morsy AA, Abd-Alla MA, Abd-El-Kareem F (2000b) Effect of acetic acid fumigation of wheat grains on fungal infection during storage. Egypt J Phytopathol 28:107–117
Nesci A, Montemarani A, Passone MA, Etcheverry M (2011) Insecticidal activity of synthetic antioxidant, natural phytochemicals, and essential oils against an Aspergillus section Flavi vector (Oryzaephilus surinamensis L.) in microcosm. J Pest Sci 84:107–115. https://doi.org/10.1007/s10340-010-0333-2
Oldenburg E, Höppner F, Ellner F, Weinert J (2017) Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res 33:167–182. https://doi.org/10.1007/s12550-017-0277-y
Paster N, Menasherov M, Ravid U, Juven B (1995) Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grains. J Food Prot 58:81–85. https://doi.org/10.4315/0362-028X-58.1.81
Perczak A, Gwiazdowska D, Marchwińska K, Juś K, Gwiazdowski R, Waśkiewicz A (2019) Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch Microbiol 201:1085–1097. https://doi.org/10.1007/s00203-019-01673-5
Quiroga EN, Sampietro AR, Vattuone MA (2001) Screening antifungal activities of selected medicinal plants. J Ethnopharmacol 74:89–96. https://doi.org/10.1016/S0378-8741(00)00350-0
Rad S, Afshari H, Moghadam MM, Tahmasbi S, Ziaolhagh H, Ebadi A (2011) The study of antifungal effects of herbal essences on Aspergillus parasiticus, an aflatoxin producer in pistachio (Pistacia vera). J Med Plant Res 5:5155–5159. https://doi.org/10.5897/JMPR.9001237
Rahm MA, Gh KM, Reha NAE (2020) Prevalence and Transmission of Seed-borne Fungi of Maize and Their Control by Phenolic Antioxidants. Plant Pathol J 19:176–184. https://doi.org/10.3923/ppj.2020.176.184
Ramadan KM, Ali MK, El-Gobashy RE, Georghiou PE, Ali NA, Zaher EA (2007) Application of volatile fractions from Ageratum houstonianum and Tagetes erecta as safe management of some root phytopatogenic Fungi. Arab Univ J Agric Sci 15:185–193. https://doi.org/10.21608/ajs.2007.14798
Rasooli I, Owlia P (2005) Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochem 66:2851–2856. https://doi.org/10.1016/j.phytochem.2005.09.029
Rioux S, Pouleur S, Randall P, Vanasse A, Turkington TK, Dion Y, Belkacemi K (2016) Efficacy of acetic acid vapors and dry heat to control Fusarium graminearum and Bipolaris sorokiniana in barley and wheat seeds. Phytoprotection 96:1–11. https://doi.org/10.7202/1037531ar
Rózewicz M, Wyzinska M, Grabinski J (2021) The most important fungal diseases of cereals problems and possible solutions. Agron 11:714–726. https://doi.org/10.3390/agronomy11040714
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor LaboratoryPress, New York, p 1546
Senatore F (1996) Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L.) growing wild in Campania (Southern Italy). J Agric Food Chem 44:1327–1332. https://doi.org/10.1021/jf950508z
Shabana YM, Ghoneem KM, Rashad YM, Arafat NS, Fitt BDL, Richard B, Qi A (2022) Distribution and biodiversity of seed-borne pathogenic and toxigenic fungi of maize in Egypt and their correlations with weather variables. Plants 11:2347. https://doi.org/10.3390/plants11182347
Sinha KK, Sinha AK, Prasad G (1993) The effect of clove and cinnamon oils on growth of and aflatoxin production by Aspergillus flavus. Lett Appl Microbiol 16:114–117. https://doi.org/10.1111/j.1472-765X.1993.tb01373.x
Soliman KM, Badeaa RI (2002) Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol 40:1669–1675. https://doi.org/10.1016/S0278-6915(02)00120-5
Somda I, Sanou J, Sanop P (2008) Seed-borne infection of farmer saved Maize seeds by pathogenic Fungi and their transmission to seedlings. Plant Pathol J 7:98–103. https://doi.org/10.3923/ppj.2008.98.103
Song W, Zhou L, Yang C, Cao X, Zhang L, Liu X (2004) Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop Prot 23:243–247. https://doi.org/10.1016/j.cropro.2003.08.007
Szopińska D (2013) The effect of organic acids treatment on germination, vigor and health of zinnia (Zinnia elegans Jacq.) seeds. Acta Sci Pol Hortorum Cultus 12:17–29
Thanaboripat D, Mongkontanawur N, Suvathi Y, Ruangrattanamatee V (2004) Inhibition of aflatoxin production and growth of Aspergillus flavus by citronella oil. KMITL Sci 4:1–8
Tran TM, Ameye M, Landschoot S, Devlieghere F, De Saeger S, Eeckhout M, Audenaert K (2021) Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates. J Fungi 7:724–737. https://doi.org/10.3390/jof7090724
Tsedaley B, Adugna G (2016) Detection of fungi infecting maize (Zea mays L.) seeds in different storages around Jimma Southwestern Ethiopia. J Plant Pathol Microbiol 7:1–6. https://doi.org/10.4172/2157-7471.1000338
Tzortzakis NG, Economakis CD (2007) Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. IFSET 8:253–258. https://doi.org/10.1016/j.ifset.2007.01.002
Varga J, Kocsubé S, Péteri Z, Vágvölgyi C, Tóth B (2010) Chemical, physical and biological approaches to prevent ochratoxin induced toxicoses in humans and animals. Toxins 2:1718–1750
Wang J, Zhao J, Liu H, Zhou L, Liu Z, Wang J, Han J, Yu Z, Yang F (2010) Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 15:6411–6422. https://doi.org/10.3390/molecules15096411
Wang Y, Qiao Y, Zhang M, Ma Z, Xue Y, Mi Q, Wang A, Feng J (2021) Potential value of small-molecule organic acids for the control of postharvest gray mold caused by Botrytis cinerea. Pestic Biochem Physiol 177:1–18. https://doi.org/10.1016/j.pestbp.2021.104884
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Communicated by Maria Rosa Simon.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haggag, W.M., Diab, M.M., Al-Ansary, N.A. et al. Molecular identification and management of mycotoxigenic fungi in stored corn Grains. CEREAL RESEARCH COMMUNICATIONS (2024). https://doi.org/10.1007/s42976-024-00502-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-024-00502-w