Abstract
The aim of this study was to understand how the application of decomposed stubble return (DSR), a type of bio-organic fertilization, affects soil microbial communities under crop rotation. The changes in microbial composition and diversity related to DSR were investigated based on metagenomic sequencing and comparative analysis of two groups of soil samples after a 3-year tomato-pepper-papaya rotation: the DSR and no-DSR (i.e., without DSR) groups, with the soils before crop rotation as the control group. Inter-group comparisons of the crop performance (growth and yield) and physicochemical soil properties (pH value, nutrient elements, and heavy metals) were also conducted to reveal the effects of DSR application on the soil. The relative abundance of bacteria was higher than 90% in all soil samples. Proteobacteria and Actinobacteria in the DSR group and Proteobacteria and Firmicutes in the no-DSR group, whereas Acidobacteria and Proteobacteria in the control, were the two most abundant phyla. The abundance of Proteobacteria decreased, whereas that of Actinobacteria increased, in the DSR-amended soil compared to the no-DSR soil. At genus level, Acidobacterium dominated in the control and genera Pseudomonas, Burkholderia, and Bacillus in the no-DSR group, while Burkholderia, Pseudomonas, and Bacillus in the DSR-amended soil comprised the majority of their microbiomes. The DSR soil had higher microbial diversity and relative abundance of Ascomycota fungi than the no-DSR group after the crop rotation. Along with higher diversity of microbial community, more favorable soil pH, better crop growth, higher crop yields, higher abundance of soil nutrient elements, and lower accumulation of heavy metals in the soil were found in the DSR group compared to the no-DSR one. Furthermore, the DSR soil had more similarities with the control than with the No-DSR soil, in aspects of microbial composition and microbe-derived potential gene functions. It was indicated that decomposed stubble return may improve soil conditions or prevent them from degradation incurred by long-term crop cultivation. It was suggested that the application of the compost derived from fermented post-harvest plant residue may be a general strategy for developing more sustainable agricultural systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chemical fertilizers are indispensable for modern agriculture to obtain higher crop yields that guarantee food security. However, excessive application of chemical fertilizers today has increasingly imposed a prominent threat on agricultural environment and ecosystem by causing direct pollution, soil degradation (acidification, salinization, hardening, infertility, loss of microbial diversity, etc.), and damage of ecological balance (Belay et al. 2002; Zang et al. 2016; Zheng et al. 2017); this affects the sustainable productivity of agricultural land, especially in those countries with a large population but relatively limited cultivated area, such as China, where soil improvement for long-term utilization of agricultural land is of great importance.
Soil microorganisms play crucial roles in an agricultural system (Panke-Buisse et al. 2015; van der Heijden et al. 2008). In agricultural system, they can establish symbiotic relationships with plants and contribute to higher plant productivity through driving nutrient cycling, promoting organic material transformation, enhancing plant productivity, and mediating host defense against soil-borne diseases and tolerance to abiotic stresses (Bakker et al. 2018; Barrios 2007; Berendsen et al. 2012; Cha et al. 2016; Liu et al. 2021; Mendes et al. 2011; Pieterse et al. 2016). Consequently, application of organic fertilizers amended with beneficial microorganisms (for instance, arbuscular mycorrhiza fungi, P-solubilizing bacteria, etc.) is considered an ecology-friendly, promising practice in agricultural production (Atieno et al. 2020). To date, returning decomposed crop straw to soil has emerged as a feasible approach to improve soil conditions and promote crop growth (Crecchio et al. 2007; Janvier et al. 2007; Lenka and Lal 2013; Said-Pullicino et al. 2014), which involves beneficial modifications of soil microbial community by bio-organic fertilizer (Su et al. 2020). In such regime, crop straw serves an ample source of organic matters for the soil and the post-harvest plant residue can be properly disposed. Most of the previous studies focused on the effects of cereal (rice, maize and wheat) straw return on crop performance and/or soil microbiota (Gaind and Nain 2007; Su et al. 2020; Yang et al. 2016). However, whether returning decomposed stubble of other types of crops like vegetables and fruits may take similar effects still needs estimation, and little is known about how it would impact the soil microbial communities.
While microbes may help improve or maintain cultivation-favorable soil conditions (Arias et al. 2005; Schmidt et al. 2017), on the other hand, agronomic operations like the use of fertilizers and crop rotation can also alter the composition and structure of the microbial communities living in soils along with various changes of the soil properties (Ai et al. 2018; Guo et al. 2010; Liu et al. 2016; Peng et al. 2017). The agriculture based on intensive use of chemical fertilizers, in general, may cause harm to soil microbial communities (Banerjee et al. 2019; Hartmann et al. 2015), while bio-organic farming exerts beneficial effects on them on aspects of their abundance and diversity upon most occasions. Although it should be a common phenomenon, the relevant complex soil microbe-bio-organic fertilizer interactions and multifaceted mechanism deserve more in-depth research.
In this study, to understand the impact of decomposed vegetable/fruit stubble return on the soil microbial community under perennial rotation of the stubble-generating crops, we comparatively analyzed the structure of microbiome between the soils with and without decomposed stubble return (DSR), after a 3-year rotation. Our findings will provide insight into developing sustainable strategies for agricultural production.
2 Materials and Methods
2.1 Preparation of Stubble Decomposition Inoculant
Twenty-five kilograms of the post-harvest stubble of crops, tomato, pepper, and papaya (Carica papaya), was crushed into pieces and well-mixed in 20 kg water with 2.5 kg RW decomposition facilitator reagent (Hebi City Renyuan Bio-Technology Development Ltd. Co., China) containing several kinds of stubble-decomposing fungi, bacteria, and yeasts. The mixture was kept in a 50-L cylinder bucket sealed tight to allow fermentation for 3 weeks at room temperature, and then, the obtained compost was sterilized in an autoclave (121 ℃ for 20 min) before used to fertilize the soil matrix in the following experiments.
2.2 Crop Rotation, Comparison of Yields, and Collection of Soil Samples
The field experiment was conducted at the Nansha base of Guangzhou Academy of Agricultural Sciences in Nansha district, Guangzhou city, Guangdong province, China (22.71°N, 113.57°E). The site has a subtropical maritime monsoon climate. Crop seeds were sown in 5×5 cm plates to germinate and grow into 4-leaf seedlings, which were then transplanted to 80×40×50 cm rectangle flower pots filled with gardening soil matrix purchased from Guangzhou Shengsheng Agriculture Ltd. Co. (China). The basic chemical properties of the soil matrix were PH 5.5–6.5, EC value = 0.3 dS·m−1, total porosity >60%, organic content >45%, water content = 30%, and total nutrient content = 4%. Two groups of pot cultivation (10 pots × 3 replicates for each group) underwent a 3-year rotation for tomato, pepper, and papaya in sequence (1 year for each crop). One group of soil substrates (group B3, with three replicates B31, B32, and B33) got fertilized via DSR once per month by adding 1-L compost to the soil in each pot, while the other group (B2, with three replicates B21, B22, and B23) did not get this fertilization, during the 3-year rotation.
In each year, the obtained fruits of the respective crop from all the 10 plants in each of the three replicates for both DSR and no-DSR groups were collected and weighed, and their yields per square meter (total yield divided by 3.2 m2, the total area of ten pots) were calculated. Comparisons of the three crops’ yields were conducted between the two groups, using the t-test to reveal significance of differences at p = 0.05 level.
After the rotation finished, the 20-cm depth soil samples from the ten pots were collected according to a 9-point sampling method for each pot, and equally well-mixed into one combined sample for each group. The two mixed samples, along with the unused (i.e., just purchased) fresh soil substrate as a control (group B1, with three replicates B11, B12, and B13), were stored at −80 ℃ for the uses of physicochemical property assays and soil microbe investigation.
2.3 Measurement of Physicochemical Properties of Soil Samples
Physicochemical properties of the soil samples were assayed by Guangzhou Zhonghe Bio-Tech Ltd. Co. (Guangzhou, China), including pH and the contents of nine nutrients and eight heavy metal elements using conventional and standard methods. For contents of the seventeen elements, inter-group comparisons were conducted via one-way ANOVA tests, and significance of differences between the groups was revealed by Tukey’s HSD tests (p = 0.05). These statistical analyses were performed using an SPSS software.
2.4 Metagenomic Sequencing and Analysis of Soil Microbial Population
The soil samples were subject to DNA extraction using PureLink™ Microbial DNA Purification Kit (Thermo Scientific, China) according to the manufacturer’s protocol. DNA quality verification and DNA library construction were performed using standard procedures, and metagenomic sequencing for the soil microbiomes was conducted using a paired-end 150-bp (PE150) sequencing strategy on an Illumina Novaseq platform. These operations were carried out by Microeco Sci-Technology Ltd. Co. (Shenzhen, China). After completion of high-throughput sequencing, the obtained sequence data (deposited in the NCBI SRA with an accession number as SRP385945) were subject to quality validation by FastQC and a KneadData software with Trimmomatic tool (Bolger et al. 2014), and the sequences of non-microbial hosts, adapters, and the fragments shorter than 50bp were removed using the bowtie2 program (Langmead and Salzberg 2012). To identify the microbial taxa contained in the soil samples, the clean data were annotated using the Kraken2 taxonomic sequence classification system (http://ccb.jhu.edu/software/kraken2/index.shtml?t=manual) with bacteria, fungus, archaebacteria, and virus databases. The relative abundance of the identified taxa at the genus and species levels was estimated using a Bracken software (Lu et al. 2017).
For analysis of gene functions, the clean reads from each sample were annotated in the UniRef90 database using an HUMAnN2 software (based on DIAMOND) with default parameters (translated_query_coverage_threshold = 90.0, prescreen_threshold = 0.01, evalue_threshold = 1.0, translated_subject_coverage_threshold = 50.0), and relative abundances (indicated as reads per kilobase per million, RPKM) of the proteins/genes they represented were calculated. Annotation of these genes in KEGG databases was conducted to further elucidate their functions. A principal coordinate analysis (PCoA) based on Bray-Curtis distance matrix was used to compare the genus-level structures of microbial communities between different groups.
3 Results
3.1 The Performance of Potted Crops and Physicochemical Properties of Soil Samples
During the 3-year rotation, all three crops were observed to have better performances in both vegetative and reproductive stages when grown in the DSR soil (group B3) than when in the unfertilized soil (group B2) (Supplemental Fig. S1). In the first year of rotation, tomato yields of 43.44 and 53.65 kg per m2 averagely from the no-DSR and DSR soils were obtained respectively (t=8.55, p<0.01). In the second year, pepper were harvested at 14.38 and 20.50 kg·m−2 averagely from the no-DSR and DSR pots respectively (t=14.14, p<0.01). In the last year of rotation, an average papaya yield of 22.66 kg·m−2 came from the no-DSR group while 107.92 kg·m−2 from the DSR group. Meanwhile, obvious soil compaction was found in the B2 but not in the B3 group.
The fresh soil matrix was pH 6.2. After the rotation, the soil pH of DSR group increased to 6.8, falling within the pH range favorable for growing of most crops, whereas that of no-DSR group increased to 7.8, an alkalescent condition less favorable for crop growth. The contents of eight nutrient elements (N, P, K, Ca, Fe, B, Mn, and Mo) were significantly higher (1.68~3.86 times) in DSR soils than in no-DSR soils, and N, P, Ca, B, Mn, and Mo were significantly more abundant in DSR soils then in the control; but the Mg content in the DSR group was only 36% and 22% of that in no-DSR samples and the control, respectively, and elements P and Mo were more abundant in the B2 group than in the control (Table 1), suggesting that the crops’ growth might be associated with accumulation of these two elements. As to heavy metals, seven elements (Pb, Cd, Cr, Ni, Hg, Cu, and Zn) were significantly lower in the DSR group than in the no-DSR group, whereas no significance of difference in element As content was found among the three groups (Table 1). These results indicated that bio-organic fertilization via DSR was conducive to retain soil nutrients, promoting crop growth, and could effectively prevent accumulation of heavy metals and soil degradation.
3.2 Identification of Microbial Taxa in the Soil Samples
The high-throughput sequencing generated 39.2, 28.4, and 29.8 Mbp nucleotides, in average, from the samples in groups B1, B2, and B3 respectively. After removal of low-quality and adaptor sequences, 37.5, 26.6, and 28.1 Mbp of clean reads were obtained for the three groups respectively. The clean data were well annotated by Kraken2 at the kingdom, phylum, class, order, family, genus, and species levels. For each group, less than 0.3% of the obtained sequences remain unannotated at genus level because they were not found homologous to any reported sequences in the public sequence databases. In total, 23 phyla, 50 classes, 92 orders, 158 families, 334 genera, and 728 species of microorganisms were identified from the nine samples in this study (Table S1). The annotation indicated that 98.2%, 98.2%, and 93.4% of these data were bacterial sequences, whereas 1.84%, 1.81%, and 6.45% were derived from fungal genomes (almost all from phylum Ascomycota), for groups B1, B2, and B3, respectively. The B3 samples contained only 0.12%, while B1 and B2 groups had as less as 0.004% and 0.001% of the reads derived from Archaea (phylum Euryarchaeota).
As indicated in Table 2, more than one-half of the sequences came from phylum Acidobacteria, followed by the second most abundant phylum Proteobacteria, in the control group (B1), whereas in the unfertilized soil (B2), the microbiome largely consisted of the bacteria in phylum Proteobacteria, which also dominated but were less abundant in the DSR-treated samples (B3). Furthermore, abundance of the bacteria in phylum Actinobacteria apparently elevated in the DSR group, as compared to the other two groups (Table 2).
In fresh soil, Acidobacterium was the dominating genus (54.05% of the clean reads) and the second most abundant one was Hydrogenophaga (11.49%). In the unfertilized soil, Pseudomonas (22.59%), Burkholderia (15.07%), and Bacillus (10.46%) represented the majority of the microbiome, and these three genera were relatively less abundant (Burkholderia, 14.62%; Pseudomonas, 10.63%; Bacillus, 9.58%) in the DSR-amended soil with the fourth abundant genus Mycobacterium (9.00%). The twenty most abundant generic taxa and their relative abundances in the three groups were charted by Fig. 1.
3.3 Inter-group Comparison of the Microbial Compositions
At all the six levels (from phylum to species), the B3 (DSR group) contained the largest quantity of identified taxa, and it was significantly more abundant in identified orders, families, genera, and species than the B2 (no-DSR) group (Table S1), which suggested the highest diversity of microbial community in DSR soil samples. For instance, groups B1, B2, and B3 comprised 187, 128, and 269 identified genera, whereas 325, 202, and 565 identified species respectively (Fig. 2a). Averagely, the three groups had 5.0, 4.8, and 9.6 genera and 8.7, 7.6, and 20.1 species per Mb clean reads, respectively, with the reads from group B3 having the highest “taxon density.” Each group contained a number of specific taxa who had higher abundance as compared to the other two groups, as shown in Fig. 2b, wherein the LDA score indicates the extent of difference in abundance. The three groups shared 86 common species and contained 111, 46, and 293 group-specific species, respectively; the fresh soil control and the no-DSR group shared 92 common species, the two rotation groups shared 150, whereas the fresh and the DSR-amended soils shared as many as 208 common species (Fig. 2a). These findings suggested that perennial crop plantation reduced microbiological diversity while the application of DSR could prevent the reduction and prominently elevate the species density in the soil, suggesting that the application of the DSR fertilization plays roles to maintain both the microbiological diversity and a favorable soil conditions to facilitate crop production.
Similarity and specialty in microbiomic composition of the fresh (B1), unfertilized (B2), and decomposed stubble return-fertilized (B3) soils. A Venn diagram indicating the numbers of common and specific species for the three groups of soil samples; B the characteristic microbial taxa in the three groups. The taxon names are given on the left of the histogram (p_=phylum, c_=class, o_=order, f_=family, g_=genus). Each horizontal column represents a taxon that is significantly more abundant in one group than in others. The LDA scores (length of column) indicate the extent of difference in abundance
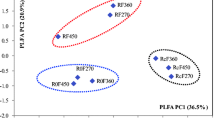
A cluster analysis based on abundances of the 20 most abundant genera indicated that the samples of each group formed a cluster; more similar microbiomic structures were observed between the DSR group and the control than between the DSR and no-DSR groups (Fig. 3a). However, the clustering of the 20 most abundant species showed that the two rotation groups had more similar microbial compositions than each of them and the control did (Fig. 3b). PCoA analysis also revealed the relatively higher structural similarity between the DSR group and the control (Fig. 4). No phylogenetically close taxa clustered together in the abundance-based analysis (data not shown), suggesting that genetically diverse microbial groups are more likely to coexist in the same ecological niche.
Heatmap generated using the relative abundance of the top 20 abundant genera in the nine soil samples. The x-axis represents the genus-level taxonomy, and y-axis represents the different samples. The heatmap scale displays the value of log2(x+1), where x represents the relative abundance of a microbial taxon. Ad, Acidobacterium; At, Actinospica; Ba, Bacillus; Bu, Burkholderia; Ch, Chelatococcus; Cr, Crenotalea; D, Dyella; E, Enhydrobacter; Ha, Halomonas; Hy, Hydrogenophaga; M, Mycobacterium; Pb, Paraburkholderia; Pd, Pandoraea; Po, Porphyrobacter; Ps, Pseudomonas; R, Ralstonia; S, Streptomyces; Tl, Thielavia; Tm, Thermomonas; X, Xanthobacter
3.4 Inter-group Comparison Based on KEGG Annotation
To investigate the differences between the microbiomes from the three types of soil on the aspects of gene function and/or biological processes, the obtained sequences in this study were subject to annotation in the KEGG database. Based on the annotation result, comparison analysis with Duncan test revealed significant differences in the abundance of the genes involved in two level 1 KEGG pathways, “cellular processes” and “Human Diseases,” between the control and the unfertilized group (both p = 0.011; three replicates per group for statistical inter-group comparisons, and the same hereinafter) and in that of the genes involved in “genetic information processing” and “organismal systems” pathways between the unfertilized and DSR-fertilized groups (both p = 0.038), whereas no difference for any level 1 KEGG pathways was found between the fresh and DSR soil samples. Fourteen level 2 KEGG pathways (“cell growth and death,” “digestive system,” “energy metabolism,” “glycan biosynthesis and metabolism,” “metabolism of cofactors and vitamins,” “metabolism of other amino acids,” “translation,” “chemical structure transformation maps,” “infectious diseases: parasitic,” “transcription,” “transport and catabolism,” “cell motility,” “environmental adaptation,” and “neurodegenerative diseases”) involving genes of different abundances between B1 and B2 groups (p = 0.011~0.038) and six pathways (“metabolism of terpenoids and polyketides,” “amino acid metabolism,” “carbohydrate metabolism,” “cellular community—prokaryotes,” “drug resistance: antineoplastic,” and “nucleotide metabolism”) between B2 and B3 samples (p = 0.011~0.038), but only two pathways (“excretory system” and “Xenobiotics biodegradation and metabolism,” both p = 0.011) between the fresh and the DSR-amended substrates, were identified. Similar patterns of gene quantity distribution were observed when annotation for the KEGG pathways in GO, metabolism, antibiotic resistance, and enzyme databases was performed (Table 3, Supplemental Table S2). These results indicated the obvious difference between the fresh and the perennially used soils, but high similarity between the fresh and the DSR-amended soils, on the aspect of the microbial genes and their cellular, biological, and/or genetic functions.
4 Discussion
In this study, we estimated the impacts of decomposed stubble return (DSR), as an organic fertilization approach, on physicochemical properties of soil and composition of the microbial communities resident in the soils that underwent perennial rotation involving three vegetable and fruit crops. Our results indicated that DSR application could enhance crop growth, boost crop production, maintain soil nutrients and favorable pH condition, prevent heavy metal accumulation in the soil, and increase the diversity of soil microbes and their functions. Notably, the DSR-treated soil shared more similar microbial characteristics with the fresh one rather than the no-DSR soil. These findings suggest that DSR could keep the soil fertile after nutrition consumption by the rotation crops. Our conclusions were, in general, in agreement with the previous studies, which demonstrated the improvement of soil fertility and health (Su et al. 2020; Turmel et al. 2015) and soil microbial structure (Tao et al. 2020). Besides the established cases of beneficial effects of decomposed cereal straw return (Gaind and Nain 2007; Su et al. 2020), to our knowledge, this is the first report of the modifications of microbial community by the application of a bio-organic fertilizer derived from decomposed stubble of vegetable and fruit crops. In our study, the stubble used for biological fermentation came from the same crops for rotation after their harvest. Our results provided an informative complement to the knowledge of beneficial influence of bio-organic fertilizers on soil health, and suggested a potentially general, cost-effective approach for developing sustainable agricultural production strategies, which involve “recycling” of the crops’ unharvestable parts, to address the issues of soil degradation caused by excessive use of chemical fertilizers or long-term monoculture (Huang et al. 2013; Liu et al. 2021) or rotation. Our ongoing research is examining the effects of DSR in more cases (i.e., with other crops), and further confirmation will be conducted based on the new experimental data.
Our study showed that the quantity of identified microbial species in DSR-amended soil was 2.8 and 1.7 times higher than that in the unfertilized and fresh (unused) soils, respectively. Still, there were near one-half of clean reads from unidentified species in group B3. These findings indicate that DSR allows a much more diverse microbiota to inhabit in the soil. Logically, such taxonomic diversity should result in versatility of biological and/or ecological functionality. In the DSR-amended soil, the relative abundance of phylum Actinobacteria was 1.6 and 2.7 times higher than that in the fresh and the no-DSR soils respectively. At the genus level, DSR significantly elevated the relative abundances of Mycobacterium and Streptomyces in Actinobacteria and Pandoraea and Paraburkholderia in Proteobacteria. Most actinomycetes have been found to be antagonistic against fungal pathogens (El-Tarabily and Sivasithamparam 2006; Zaitlin et al. 2004). Pandoraea species are believed to be associated with cystic fibrosis, a human disease (Coenye et al. 2000). Some bacteria in genera Paraburkholderia and Bacillus (which was a dominating genus in DSR-amended soil too) are probiotic that may enhance plant growth and significantly increase fruit yield (Rahman et al. 2018). Interestingly, although the microbial communities in all the nine samples contained a very little portion of fungal organisms (probably because the growth of these fungi had been inhibited by some bacteria in the soils during the whole rotation), there was a prominent increase in relative abundance of the dominating fungal phylum Ascomycota, the key decomposers of cellulose and lignocellulose that play important roles in plant residue degradation (Ma et al. 2013), in the DSR-amended group (6.4%) in comparison with the other two groups (1.7–1.8%). Similarly, Ascomycota was also found to dominate in the soil microbiota with cereal straw return in the previous studies, but unlike in this study, its relative abundance decreased in cereal straw return-amended soils (Ma et al. 2013; Su et al. 2020; Yang et al. 2016). Deeper research is needed to elucidate the exact roles played by different microbial components in improving crop growth and the soil micro-environment when responding to DSR, and that will provided insight into strategy design of sustainable agriculture via well-directed use of microbiological agents.
A plenty of microbial genes involved in various metabolism pathways, antibiotic resistance, and enzymatic activities were found with differential abundances between the DSR and no-DSR groups, showing that there is an intricate physiological and biochemical network underlying the comprehensive DSR effects. Further studies should be conducted to identify the key agents responsible to these effects from the whole complex and uncover their relevant mechanisms.
5 Conclusions
It was indicated that decomposed stubble return may improve soil conditions or prevent them from degradation incurred by long-term crop cultivation. It was suggested that application of the compost derived from fermented post-harvest plant residue may be a general strategy for developing more sustainable agricultural systems.
Data Availability
The original sequence data relevant to this work are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Ai C, Zhang S, Zhang X et al (2018) Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geofis Int 319:156–166
Arias ME, Gonzalez-Perez JA, Gonzalez-Vila FJ et al (2005) Soil health – a new challenge for microbiologists and chemists. Int Microbiol 8:13–21
Atieno M, Herrmann L, Nguyen HT et al (2020) Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J Environ Manage 275:111300. https://doi.org/10.1016/j.jenvman.2020.111300
Bakker P, Pieterse CMJ, de Jonge R et al (2018) The soil-borne legacy. Cell 172:1178–1180. https://doi.org/10.1016/j.cell.2018.02.024
Banerjee S, Walder F, Büchi L et al (2019) Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. Isme J 13:1722–1736. https://doi.org/10.1038/s41396-019-0383-2
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285
Belay A, Claassens AS, Wehner FC (2002) Effect of direct nitrogen and potassium and residual phosphorus fertilizers on soil chemical properties, microbial components and maize yield under long-term crop rotation. Biol Fert Soils 35:420–427
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Cha JY, Han S, Hong HJ et al (2016) Microbial and biochemical basis of a fusarium wilt-suppressive soil. Isme J 10:119–129. https://doi.org/10.1038/ismej.2015.95
Coenye T, Falsen E, Hoste B et al (2000) Description of pandoraea gen nov with pandoraea apista sp nov, pandoraea pulmonicola sp nov, pandoraea pnomenusa sp nov, pandoraea sputorum sp nov and pandoraea norimbergensis comb nov. Int J Syst Evol Microbiol 50(Pt 2):887–899. https://doi.org/10.1099/00207713-50-2-887
Crecchio C, Curci M, Pellegrino A et al (2007) Soil microbial dynamics and genetic diversity in soil under monoculture wheat grown in different long-term management systems. Soil Biol Biochem 39:1391–1140
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520
Gaind S, Nain L (2007) Chemical and biological properties of wheat soil in response to paddy straw incorporation and its biodegradation by fungal inoculants. Biodegradation 18:495–503. https://doi.org/10.1007/s10532-006-9082-6
Guo JH, Liu XJ, Zhang Y et al (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010. https://doi.org/10.1126/science.1182570
Hartmann M, Frey B, Mayer J et al (2015) Distinct soil microbial diversity under long-term organic and conventional farming. Isme J 9:1177–1194. https://doi.org/10.1038/ismej.2014.210
Huang LF, Song LX, Xia XJ et al (2013) Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J Chem Ecol 39:232–242. https://doi.org/10.1007/s10886-013-0244-9
Janvier C, Villeneuve F, Alabouvette C et al (2007) Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol Biochem 39:1–23
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with bowtie 2. Nat Methods 9:357–359. https://doi.org/10.1038/nmeth.1923
Lenka NK, Lal R (2013) Soil aggregation and greenhouse gas flux after 15 years of wheat straw and fertilizer management in a no-till system. Soil Till Res 126:78–89
Liu J, Wu N, Wang H et al (2016) Nitrogen addition affects chemical compositions of plant tissues, litter and soil organic matter. Ecology 97:1796–1806. https://doi.org/10.1890/15-1683.1
Liu Q, Wang S, Li K et al (2021) Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl Microbiol Biot 105:7035–7050. https://doi.org/10.1007/s00253-021-11542-1
Lu J, Breitwieser FP, Thielen P et al (2017) Bracken: estimating species abundance in metagenomics data. Peerj Comput Sci 3:e104
Ma A, Zhuang X, Wu J et al (2013) Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. Plos One 8:e66146. https://doi.org/10.1371/journal.pone.0066146
Mendes R, Kruijt M, de Bruijn I et al (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. https://doi.org/10.1126/science.1203980
Panke-Buisse K, Poole AC, Goodrich JK et al (2015) Selection on soil microbiomes reveals reproducible impacts on plant function. Isme J 9:980–989. https://doi.org/10.1038/ismej.2014.196
Peng Y, Guo D, Yang Y (2017) Global patterns of root dynamics under nitrogen enrichment. Global Ecol Biogeogr 26:102–114
Pieterse CMJ, de Jonge R, Berendsen RL (2016) The Soil-Borne Supremacy. Trends Plant Sci 21:171–173. https://doi.org/10.1016/j.tplants.2016.01.018
Rahman M, Sabir AA, Mukta JA et al (2018) Plant probiotic bacteria bacillus and paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci Rep 8:2504. https://doi.org/10.1038/s41598-018-20235-1
Said-Pullicino D, Cucu MA, Sodano M et al (2014) Nitrogen immobilization in paddy soils as affected by redox conditions and rice straw incorporation. Geoderma 228:44–53
Schmidt J, Fester T, Schulz E et al (2017) Effects of plant-symbiotic relationships on the living soil microbial community and microbial necromass in a long-term agro-ecosystem. Sci Total Environ 581–582:756–765. https://doi.org/10.1016/j.scitotenv.2017.01.005
Su Y, Lv JL, Yu M et al (2020) Long-term decomposed straw return positively affects the soil microbial community. J Appl Microbiol 128:138–150. https://doi.org/10.1111/jam.14435
Tao C, Li R, Xiong W et al (2020) Bio-organic fertilizers stimulate indigenous soil pseudomonas populations to enhance plant disease suppression. Microbiome 8:137. https://doi.org/10.1186/s40168-020-00892-z
Turmel MS, Speratti A, Baudron F et al (2015) Crop residue management and soil health: a systems analysis. Agr Syst 134:6–16
van der Heijden MG, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Yang H, Feng J, Zhai S et al (2016) Long-term ditch-buried straw return alters soil water potential, temperature, and microbial communities in a rice-wheat rotation system. Soil Till Res 163:21–31
Zaitlin B, Turkington K, Parkinson D et al (2004) Effects of tillage and inorganic fertilizers on culturable soil actinomycete communities and inhibion of fungi by specific actinomycetes. Appl Soil Ecol 26:53–62
Zang H, Wang J, Kuzyakov Y (2016) N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol 108:47–53
Zheng BX, Hao XL, Ding K et al (2017) Long-term nitrogen fertilization decreased the abundance of inorganic phosphate solubilizing bacteria in an alkaline soil. Sci Rep 7:42284. https://doi.org/10.1038/srep42284
Funding
This work was supported by Guangzhou Science and Technology Program Item (No. 2020020086) and Guangzhou Science and Technology Bureau Fund Item (No. 201909020001).
Author information
Authors and Affiliations
Contributions
YT and DG conceived and designed the research. YQ and DX conducted experiments. YQ, DX, and JP analyzed the data. DX wrote the manuscript. YQ and HL revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Yanchun Qiao and Donglin Xu contributed equally to this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiao, Y., Xu, D., Peng, J. et al. Influence of Decomposed Stubble Return on the Soil Microbial Community Under Perennial Crop Rotation. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-023-01472-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-023-01472-4