Abstract
Contamination by heavy metals (HM) is a global concern due to their impact on terrestrial and aquatic environments. This question has great relevance in agricultural areas due to excessive chemical fertilization. In this sense, Cd is a toxic element that can reach agricultural soils through chemical fertilization or sewage sludges. Tobacco plants (Nicotiana tabacum L.) can uptake and accumulate Cd in their tissues, and therefore, an increased risk for human health due to tobacco consumption. This study was performed to evaluate the response of tobacco plants to a single and combined amendment of Cd and Zn on agricultural soil with a pot experiment. A factorial experiment was performed with four Cd levels (0, 25, 50 and 100 mg kg-1) and three Zn levels (0, 15 and 25 mg kg-1). Growth, Cd and Zn bioaccumulation and nutrient uptake parameters were assessed. The results revealed that during the tobacco growth, Cd was bioaccumulated on roots (translocation factor <1), while Zn was bioaccumulated on the aerial part (TF>1). Besides, the Zn amendment significantly decreased the Cd uptake and accumulation, especially under intermediate doses (15 mg kg-1 Zn). Zinc amendments could be helpful as a mitigation measure for Cd uptake in tobacco plants and, therefore, for health risk reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agricultural soils are essential for humans due to their role in food and fiber production. However, the intensive agricultural practices, including malpractices, such as excessive application of low-quality fertilizers, intensive use of saline waters, sewage sludges or pesticides, made those agricultural soils sometimes highly degraded at the physicochemical and biological levels (Fässler et al. 2010; Moghtaderi et al. 2020; Rodrigo-Comino et al. 2020). Besides, agricultural soils can also be a sink of contaminants [potentially toxic elements (PTEs), pesticides, antibiotics, microplastics, etc.], which could also risk food production and human health (Rodrigo-Comino et al. 2020). In this sense, Cd, Cu, Mn, Pb and Zn are the most critical PTEs in agricultural soils due to widespread and uncontrolled fertilization and pesticide application (Zoffoli et al. 2013; Tkalec et al. 2014; Tóth et al. 2016; Moghtaderi et al. 2020). As a result, mitigation measures, such as (bio-)remediation techniques, are needed to prevent soil contaminants from the food web and improve soil quality (Rodrigo-Comino et al. 2020; Rodríguez-Seijo et al. 2022).
In this sense, phytoremediation techniques (e.g., phytoextraction, phytostabilization, rhyzostabilization) are management practices widely used in the restoration processes of contaminated soils. Despite they are a slow process to remove contaminants from different environmental matrices (soils, waters, etc.), they are widely used due to their low economic and technological requirements and their ability to remove from the environment or facilitate the transformation to less bioavailable/toxic chemical form of contaminants (Rodríguez-Seijo et al. 2016; Daghan 2019; Rodrigo-Comino et al. 2020). In this sense, more than 700 plant species can (hyper)accumulate PTEs. Still, hyperaccumulator plants usually have several problems, such as slow growth, reduced biomass, or specifical conditions to grow (climate, geographic or soil conditions) (Liang et al. 2009; Liu et al. 2016; Reeves et al. 2018).
Tobacco (Nicotiana tabacum L.) is one of the idyllic plant species for phytoremediation techniques and metal (hyper)accumulation. Tobacco plants have some advantages over traditional species used for phytoremediation purposes, such as fast growth and high biomass production, wide climate adaptation range, can be genetically modified, and they have an adequate accumulation of different PTEs in all plant tissues, particularly Cd, Ni, Pb, and Zn, (Kirkham 2006; da Silva et al. 2016; Daghan 2019; Mei et al. 2022). Besides, tobacco is one of the most important non-edible agricultural products worldwide, and it significantly impacts several countries’ economies, such as Iran (Mirkarimi et al. 2021). However, this could also be a risk due to tobacco leaves being used for smoking, resulting in a potential soil-tobacco-human health risk (Zhang et al. 2018; Mei et al. 2022).
Linked to these questions, Cd and Zn are common elements in agricultural soils due to natural soil characteristics or human activities (e.g., excessive phosphate fertilizer application). While Zn is an essential micronutrient for organisms, Cd is a very toxic PTEs and non-essential for plants that, in combination with Zn, could interact and reduce the uptake, transport and biological functions of some essential nutrients in plants (Tkalec et al. 2014; Moghtaderi et al. 2020; Rassaei et al. 2020). In this sense, different studies (Vasiliadou and Dordas 2009; Fontes et al. 2014; Tkalec et al. 2014; Dong et al. 2019) showed that the Cd-Zn interactions reveal antagonistic interactions on plant functions. Moreover, different Zn levels can reduce the Cd accumulation and, therefore, the toxicity for plants, although sometimes contradictory effects were also reported since Cd controls the antagonistic effect: Zn ratio of Cd added to soil system (Vasiliadou and Dordas 2009; Fontes et al. 2014). Both elements have been widely reported in higher levels for agricultural soils, with moderate bioavailability and potential risk for plant uptake (e.g., Cheragi et al. 2012; Rezapour et al. 2019; Moghtaderi et al. 2020; Rassaei et al. 2020; Jalali et al. 2021; Rastegari Mehr et al. 2021; Aminiyan et al. 2022). Tavakkoli and Khanjani (2016) reviewed a high risk for Cd in Iranian smokers, while Ghoochani et al. (2018) reviewed that higher amounts of Cd appear in several kinds of crops, such as rice and lettuce, with high amounts of Cd in blood samples for Iranian population. Therefore, both elements represent an issue for crop production and food security, especially Cd.
According to the previous reports (Vasiliadou and Dordas 2009; Fontes et al. 2014; Tkalec et al. 2014; Dong et al. 2019), Zn amendments to agricultural soil can decrease the uptake and accumulation of Cd. Therefore, it is necessary to find an optimum concentration of Zn that can reduce Cd uptake. In this way, this study aims to go one step forward by evaluating the effects of different concentrations of Cd and Zn on tobacco plants' growth, development, and metal uptake. To achieve this, single and combined concentrations (11 treatments) will be assessed in a pot experiment under controlled conditions. The potential impact of Cd and Zn on plant development was studied through biometric parameters (root and shoot length) and metal accumulation on the roots and shoots of tobacco plants. The obtained data could be useful for further amelioration measures on contaminated soils and for the improvement of agriculture in potentially contaminated areas.
2 Material and methods
2.1 Soil characterization of agricultural soil
A topsoil sample (0-20 cm sampling depth) was collected from an agricultural area in the Kushkak Fars research station (Iran). Several soil samples were collected from this agricultural area to get a compound sample stored in polyethene bags. After the soil sampling and once in the laboratory, soil samples were air-dried, passed through a 2-mm sieve, and homogenized before analyses. Three subsamples were taken to carry out the physicochemical characterization: particle-size analysis carried out according to the hydrometer method (Bouyoucos 1962), organic matter content and soil carbon (Walkley and Black 1934), phosphorous content (Olsen and Sommers 1982), and soil pH and EC (electrical conductivity) were determined with a pH electrode in 1:2.5 water or KCl to soil extracts. Besides, the DTPA (diethylenetriaminepentaacetic acid) extraction method (0.005 M DTPA + 0.1 M triethanolamine + 0.01 M CaCl2 at pH 7.3) was used to assess the potential soil availability of Cd, Cu, Fe, Mn and Zn (1/10 w/v soil to extractant ratio, 2h shaking, 120 rpm) (Lindsay and Norvell 1978), and measured by atomic absorption spectroscopy (AAS).
Soil properties of used soil were silty clay loam (USDA classification), neutral soil pH (7.3) and non-saline (1.15 dS m-1), very low organic carbon content (1.35 %) and low available phosphorous (8.8 mg kg-1). Finally, the phytoavailable contents of the studied elements were 2, 49.16, 25.85 and 0.7 mg kg-1 for Cd, Fe, Mn and Zn, respectively. Available Cu was not detected.
2.2 Pot experiment
To evaluate the response of tobacco plants to residual and interaction effects of Cd and Zn in agricultural soil, the study soil was spiked with a suspension of Cd and Zn as CdCl2 and ZnSO4, respectively, at levels of 0 (no addition treatment), 25, 50 and 100 mg kg -1 for Cd, and 0 (no addition), 15 and 25 mg kg-1 for Zn, with individual and combined contamination, obtaining twelve treatments and with three replicates by each treatment (Table 1).
Seeds of Nicotiana tabacum L. cv Bamiri, a local variety in the Parsian County (South of Iran), obtained from a local supplier, were planted in three-kilogram pots. First, the seeds were planted in a nursery, and after about six weeks, when the seedlings became four to six leaves, plants were transferred from the nursery to the pots (± 1.5 L) containing soil previously contaminated with Cd and Zn. The experiment was performed on pot experiments under open-air conditions in a completely randomized design, as previously described. The pots were rinsed with a four-day periodic irrigation regime for 10 weeks. After establishing the plants, the number of plants decreased to three to have enough biomass; during the growing period, the lighting hours were twelve hours a day, and the air temperature was about 20°C. After 10 weeks, the plants were collected from the soil; the plants were cut, and the roots and the aerial parts (shoots) were differentiated. In the laboratory, the specimens were washed with tap and distilled water to remove the remaining soil particles that adhered to their surface. After weighing the roots and shoots (f.w. fresh weight), they were placed in an oven at 60°C until reaching constant weight. Afterwards, these roots and shoots were weighed (d.w. dry weight), crushed and stored in hermetically sealed polythene bags, ready for use. The total fraction of Cd and Zn in the plant (in the shoot and root) was extracted from 0.5g of sample and was digested with HCl 2M in hot plate digestion (550°C, 7h). The digests were diluted to 100 mL with distilled water and filtered with Whatman 42 filter paper. In all of the extracts, the concentration of Cd and Zn were determined by AAS. In addition, Cu, Fe and Mn levels were also determined for shoots.
2.3 Statistical analysis
All data were analyzed statistically with IBM SPSS Statistics 29 software using the analysis of the variance (ANOVA) test. Differences were considered statistically significant according to Duncan’s Multiple Range at p < 0.05 (Sharma et al. 2007; Gao et al. 2022). Pearson’s correlation analysis was conducted to determine the main associations between Cd and Zn and their accumulation on roots and shoots.
The translocation factor (TF) was estimated as the ratio between the trace metal content (mg kg-1) in shoots (Cs) and that in the roots (Cr) (TF=Cs / Cr). Plants with TF > 1 indicate that the plant translocates metals effectively from the root to the shoot, showing potential as phytoextractors, while TF < 1 indicates their phytostabilizing property (Baker and Brooks 1989; Kumar et al. 2022). The bioconcentration factor (BCF) in the roots and shoots was determined by calculating the ratio of the metal concentration in the plant (Cp) to that in the soil (Cso) (BCF=Cp / Cso), where Cp and Cso are the metal concentrations in each plant tissue (root (BCFr) or shoot (BCFs)) to that bioavailable metal concentration in the soil medium (Cso), respectively. BCF values above 1 indicate that they are hyperaccumulator plants and sometimes range from 50 to 100, although BCF values can differ for each plant species (Rodríguez-Seijo et al. 2016; Mishra and Pandey 2019; Usman et al. 2019).
3 Results
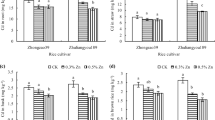
After ten weeks of the pot experiment, plants were harvested, and plant production parameters were measured (Fig. 1). Although a root biomass reduction was observed for the highest Cd with Zn, both at 15 and 25 mg kg-1 (Figs. 1a and 1c), no significative differences were observed for root fresh (p = 0.110) and dry biomass (p = 0.242). However, significant differences were detected for fresh (p = 0.002) and dry weight (p = 0.018) for shoots, especially for treatments with Cd without Zn addition (Figs. 1b and 1d). Interestingly, treatments with Cd 50 and 100 mg kg-1 mixed with Zn 15 and 25 mg kg-1 showed the highest reduction, except for the highest combined concentration of Cd and Zn, where the highest aerial part mass biomass was recorded.
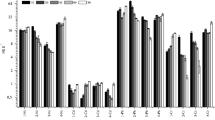
Regarding the metal bioaccumulation, significant differences were recorded for Cd accumulation in roots (p < 0.001) (Fig. 2a) and shoots (p < 0.001) (Fig. 3a) and Zn accumulation in roots (p < 0.01) (Fig. 2b) and shoots (p = 0.027) (Fig. 3e). Significant differences were also observed for nutrients in the aerial part, as shown for Cu (p < 0.001) (Fig. 3b), Fe (p < 0.001) (Fig. 3c) and Mn (p < 0.001) (Fig. 3d). In this sense, Cd was mainly accumulated on roots, while Zn was accumulated on shoots, as verified by translocation factors values for Cd (TF < 1) and Zn (TF > 1) (Fig. 2c and d). Significant differences were observed for both translocation factors (p < 0.01).
Cd (a) and Zn (b) accumulation in roots of N. tabacum and translocation factors for Cd (c) and Zn (d) when plants were exposed to different concentrations of Cd and Zn. Data are shown as means ± standard deviation of three replicates. Bars marked by different letters indicate significant differences according to Duncan’s test (p < 0.05)
Similar patterns were also for the bioaccumulation factors for roots (Cd and Zn, p < 0.001) (Fig. 4a, b) and shoots (Cd, Fe and Mn p < 0.001; Zn p = 0.027) (Fig. 4c, d, e and f). According to the obtained data, tobacco showed BCF values over > 50 for Zn, while BCF values for Cd were below 20 and 8 for Fe and Mn, respectively. These data suggest that N. tabacum can act as a hyperaccumulator for Zn since BCF and TF values are > 1, while Cd can act as a phytostabilizer (BCF >1, TF <1) (Rodríguez-Seijo et al. 2016).
Bioaccumulation factor (BCF) of roots and shoots for Cd (a and c, respectively), in shots for Fe and Mn (d and e) and Zn (b and f, respectively) for tobacco plants exposed to different concentrations of Cd and Zn. Data are shown as means ± standard deviation of three replicates. Bars marked by different letters indicate significant differences according to Duncan’s test (p < 0.05)
A correlation analysis was carried out between metal dose application, growth parameters and plant metal bioaccumulation (Table 2). The Cd contents of tobacco from the aerial parts (r = 0.92, p < 0.01) and roots (r = 0.76, p < 0.01; respectively) were positively and highly correlated with the concentration of Cd in soil. Similar correlations were observed for BCF values in roots (BCFr) and shoots (BCFs) (Table 2), being these findings consistent with earlier reports (Liu et al. 2016). Besides, a significative correlation between the different Cd application doses and Zn accumulation on roots (r= - 0.63, p< 0.05).
Interestingly, a similar effect was observed for Cd accumulated on shoots but with less Cd accumulation when Zn was added at 25 mg kg-1 (Cd reduction between 14 to 48%). An inverse behaviour was observed for Zn accumulation, with most Zn accumulated when Cd was added under 15 Zn mg kg-1 treatment. At the same time, on the aerial part, the higher Zn contents were observed under 25 Zn mg kg-1 treatment, even at larger doses of Cd (50 to 100 mg kg-1) (an increment from 20-65%), with the maximum accumulation under 50 and 25 mg kg-1 of Cd and Zn, respectively.
Regarding the Cu, Fe and Mn uptake, Zn seems to play a crucial role in their uptake, as shown for Cd. In the case of Fe, more accumulation was observed under 25 Zn mg kg-1 (r=0.42, p < 0.05), while Mn uptake was reduced when Zn was added, both at 15 and 25 mg kg-1, which seems more marked under 15 mg kg-1. In this case, a negative correlation between Zn amendment and Mn bioaccumulated on shoots was also observed for shoots and the bioaccumulated contents in shoots and BCF values (r= - 0.69, p< 0.05) (Table 2). Regarding Cu, their uptake was reduced under the different Zn applications, but their accumulation was correlated when Cd was also uptakes on shoots (r = 0.63, p< 0.05) (Table 2).
4 Discussion
The observed results have shown that Cd and Zn both tend to accumulate in tobacco shoots, especially in the case of Zn, which is in accordance with the data reported for N. tabacum (Tkalec et al. 2014), although some studies also indicate that tobacco plants can translocate Cd and Zn very efficiently from roots to shoots (Liang et al. 2009; da Silva et al. 2016; Kozak and Antosiewicz 2023). However, Huang et al. (2021) indicated that tobacco plants' ability to accumulate Cd in the aerial part is highly dependent on tobacco cultivars, while Mei et al. (2022) also highlighted that Cd translocation could be dependent on involved genes for Cd uptake.
Despite the different availability and mobility of Cd and Zn in soil solution and their potential uptake by plants, Cd was higher in roots than in shoots due to plants trying to reduce their translocation to the aerial part to prevent metal-induced damage in the shoots (Tkalec et al. 2014; Huang et al. 2021; Kozak and Antosiewicz 2023). The observed results suggest that Zn addition has a protective effect over Cd bioaccumulation on leaves and, therefore, their potential risk for human health due to Cd intake by smokers (Kozak and Antosiewicz 2023). Although Cd can be efficiently uptake by tobacco plants under some conditions (i.e., Cd background and fertilization input, growth conditions or tobacco cultivars), the results suggest that Zn addition has a potential protective effect over a genotoxic element such as Cd (Gichner et al. 2006; Huang et al. 2021; Sadiku and Rodríguez-Seijo 2022) and their bioaccumulation on plants (Tkalec et al. 2014), especially under intermediate zinc doses (e.g., 15 mg kg-1 Zn).
Different authors observed this pattern with tobacco plants, where treatment of 25-50 μM Zn, especially under intermediate concentrations (25 μM Zn), significantly reduced the plant's oxidative stress and the Cd bioaccumulation (Tkalec et al. 2014). Although Zn had antagonistic effects on Cd uptake, it also slightly impacted the Cu, Fe and Mn uptake, which plants need as nutrients. Erdem et al. (2017) also reported similar results with the application of Zn-rich biochar on different doses (0, 1, 2 and 3%) over Cd contamination (0, 10 and 20 mg kg-1) and their uptake by tobacco plants. In their case, biochar application reduced Cd bioaccumulated on shoots, whereas other nutrients such as Fe, Mn and Cu were also reduced under the higher biochar dose. Similar results also were reported by Liu et al. (2019a) with Zn and K foliar application (0.5%) combined with biochar to alleviate Cd stress, where Zn and K foliar application can increase the antioxidant abilities (not measured in this work) and promote the resistance to Cd and growth of crops (Adrees et al. 2021; Szerement et al. 2022). Following this question, Zhou et al. (2020) also observed a similar behaviour for wheat, with foliar and soil Zn application, that can effectively decrease grain Cd and increase food safety. However, tobacco cultivars can also play a role in the Zn alleviation of toxic effects of Cd over tobacco (Liu et al. 2019b; Kinay et al. 2021).
Different mechanisms have been proposed to explain this behaviour. First, the metal uptake and transport through the cells are controlled by several mechanisms, but a group of proteins from the NRAMP family (Natural Resistance Associated Macrophage Proteins), that have a crucial role in the transport of Cd, Zn, Fe, Mn and Ni, with groups of proteins that can regulate different metal uptakes, such as NtNRAMP5 for Cd and Mn uptake (Kozak and Antosiewicz 2023; Mei et al. 2022). In this sense, the fact that Cd was mainly accumulated in roots than shoots suggest that HMA4, Nramp5 and Nramp1 genes were overexpressed under Cd stress, as highlighted in our results by reduction on Cd translocation from root to shoot, higher biomass and Fe accumulation in shoots (Figs. 1, 3, 4 and 5) (Mei et al. 2022). Furthermore, Zn can also reduce Cd uptake and their translocation because Zn is involved in the Rubisco activase (RCA) and the activity of chloroplast Zn-SOD, which could effectively reduce the toxicity induced by Cd (Liu et al. 2019a; Zhou et al. 2020). Similar results were reported for other species such as wheat (Oliver et al. 1994; Adiloglu 2002; Zhou et al. 2020; Gu et al. 2022), garden cosmos (Du et al. 2020), tomato (Ammar et al. 2015); corn (Adiloglu 2002; Adıloglu and Adıloglu 2009), barley (Adiloglu 2002), pepper (Wang et al. 2013), rice (Hassan et al. 2005; Slamet-Loedin et al. 2015; Wang et al. 2018; Zhen et al. 2021) or oat (Adiloglu 2002; Wu and Zhang 2002; Akay and Koleli 2008) where Zn application reduce Cd uptake and translocation, and alleviates Cd-induced oxidative stress by improving the antioxidant system (especially superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and lipid peroxidation), or even improve the photosynthesis (Wu and Zhang 2002; Rizwan et al. 2019; Zhou et al. 2020). However, in this study, oxidative stress parameters or overexpression genes were not measured, and further studies should be focused on the effect of oxidative parameters and the antioxidant response of exposed tobacco plants to soil contamination (Mei et al. 2022).
Besides, Zn application can increase help to increase the plant biomass and reduce the Cd bioaccumulation through a dilution effect of Cd level in the plants (Rehman et al. 2019; Zhou et al. 2020), as suggested for the shoot’s biomass increment under 25 mg kg-1 Zn (Fig. 1b and d). Finally, soil Zn application can reduce metal uptake by plants due to can block the element migration to the root through deposition on their root surface (Zhou et al. 2020), which seems more probable in our case as shown by element contents and translocation factor values. Besides, other researchers showed that Zn amendment could enhance Cd chelation on cell walls of leaves (Zhou et al. 2020), but this question was not studied.
In any case, optimum Zn applications should be determined considering that an excessive Zn amount can produce no significative effects as indicated for higher Zn doses or increase the Cd uptake (Rojas-Cifuentes et al. 2012; Akoumianaki-Ioannidou et al. 2015; Sozubek et al. 2015), and due to higher Zn concentrations also can represent a risk for smokers (Zhang et al. 2018). Still, these results could be helpful for other species where Cd can be a risk due to soil transfer.
5 Conclusions
Zinc had a protective role over Cd uptake, preventing their translocation to the aerial part and favouring their bioaccumulation on roots, especially under intermediate Zn levels. However, nutrient uptake was also slightly impacted, and higher Zn bioaccumulation was also observed, which could also be a risk for smokers under some concentrations. This study offers valuable information on the Zn effect on Cd uptake and smokers' risk reduction for Cd intake.
Further studies should combine potentially toxic elements with different chemical behaviour and soil properties, especially soil pH values and organic matter contents, and also the assessment of oxidative stress parameters on tobacco plants.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Adiloglu A (2002) The effect of zinc (Zn) application on uptake of cadmium (Cd) in some cereal species. Arch Agron Soil Sci 48:553–556. https://doi.org/10.1080/0365034021000071837
Adıloglu S, Adıloglu A (2009) Effect of nitrogen and zinc application on nickel, lead and cadmium contents of maize plant in typic xerochrept and calcixeroll soils. Asian J Chem 21:1475–1480. https://doi.org/10.3923/ajps.2006.504.507
Adrees M, Khan ZS, Hafeez M, Rizwan M, Hussain K, Asrar M, Alyemeni MN, Wijaya L, Ali S (2021) Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotox Environ Saf 208:111627. https://doi.org/10.1016/j.ecoenv.2020.111627
Akay A, Koleli N (2008) Interaction between cadmium and zinc in barley (Hordeum vulgare L.) grown under field conditions. Bangladesh J Bot 36:13–19. https://doi.org/10.3329/bjb.v36i1.1543
Akoumianaki-Ioannidou A, Papadimitriou K, Barouchas P, Moustakas N (2015) The effects of Cd and Zn interactions on the concentration of Cd and Zn in sweet bush basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.). Fresenius Environ Bull 24:77–83
Aminiyan MM, Rahman MM, Rodríguez-Seijo A, Hajiali Begloo R, Cheragi M, Aminiyan FM (2022) Elucidating of potentially toxic elements contamination in topsoils around a copper smelter: Spatial distribution, partitioning and risk estimation. Environ Geochem Health 44:1795–1811. https://doi.org/10.1007/s10653-021-01057-z
Ammar WB, Zarrouk M, Nouairi I (2015) Zinc alleviates cadmium effects on growth, membrane lipid biosynthesis and peroxidation in Solanum lycopersicum leaves. Biologia 70:198–207. https://doi.org/10.1515/biolog-2015-0026
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bouyoucos GJ (1962) Hydrometer Method Improved for Making Particle Size Analysis of Soils. Agron J 54:464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Cheraghi M, Lorestani B, Merrikhpour H (2012) Investigation of the Effects of Phosphate Fertilizer Application on the Heavy Metal Content in Agricultural Soils with Different Cultivation Patterns. Biol Trace Elem Res 145:87–92. https://doi.org/10.1007/s12011-011-9161-3
da Silva CP, de Almeida TE, Zittel R, de Oliveira Stremel TR, Domingues CE, Kordiak J, de Campos SX (2016) Translocation of metal ions from soil to tobacco roots and their concentration in the plant parts. Environ Monit Assess 188:663. https://doi.org/10.1007/s10661-016-5679-3
Daghan H (2019) Chapter 13 - Transgenic Tobacco for Phytoremediation of Metals and Metalloids. In: Prasad MNV (ed) Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids. Academic Press, Elsevier, pp 279–297. https://doi.org/10.1016/b978-0-12-814389-6.00013-4
Dong Q, Hu S, Fei L, Liu L, Wang Z (2019) Interaction between Cd and Zn on Metal Accumulation, Translocation and Mineral Nutrition in Tall Fescue (Festuca arundinacea). Int J Mol Sci 20:3332. https://doi.org/10.3390/ijms20133332
Du J, Zeng J, Ming X, He Q, Tao QN, Jiang M, Gao S, Li X, Lei T, Pan Y, Chen Q, Liu S, Yu X (2020) The presence of zinc reduced cadmium uptake and translocation in Cosmos bipinnatus seedlings under cadmium/zinc combined stress. Plant Physiol Biochem 151:223–232. https://doi.org/10.1016/j.plaphy.2020.03.019
Erdem H, Kinay A, Günal E, Yaban H, Tutus Y (2017) The effects of biochar application on cadmium uptake of tobacco. Carpathian J Earth Environ Sci 12:447–456
Fässler E, Robinson BH, Stauffer W, Gupta SK, Papritz A, Schulin R (2010) Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric Ecosyst Environ 136:49–58. https://doi.org/10.1016/j.agee.2009.11.007
Fontes RLF, Pereira JMN, Neves JCL (2014) Uptake and translocation of Cd and Zn in two lettuce cultivars. Anais Acad Brasil Ci 86:907–922. https://doi.org/10.1590/0001-37652014117912
Gao F, Zhang X, Zhang J, Li J, Niu T, Tang C, Wang C, Xie J (2022) Zinc oxide nanoparticles improve lettuce (Lactuca sativa L.) plant tolerance to cadmium by stimulating antioxidant defense, enhancing lignin content and reducing the metal accumulation and translocation. Front. Plant Sci 13:1015745. https://doi.org/10.3389/fpls.2022.1015745
Ghoochani M, Rastkari N, Yunesian M, Nabizadeh Nodehi R, Mesdaghinia A, Houshiarrad A, Shamsipour M, Dehghani MH (2018) What do we know about exposure of Iranians to cadmium? Findings from a systematic review. Environ Sci Pollut Res 25:1–11. https://doi.org/10.1007/s11356-017-0863-8
Gichner T, Patková Z, Száková J, Demnerová K (2006) Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotox Environ Saf 65:420–426. https://doi.org/10.1016/j.ecoenv.2005.08.006
Gu X, Wen X, Yi N, Liu Y, Wu J, Li H, Liu G (2022) Effect of foliar application of silicon, selenium and zinc on heavy metal accumulation in wheat grains in field studies. Environ Pollut Bioavailab 34:246–252. https://doi.org/10.1080/26395940.2022.2085630
Hassan MJ, Zhang G, Wu F, Wei K, Chen Z (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168:255–261. https://doi.org/10.1002/jpln.200420403
Huang W, Zhang D, Cao Y, Dang B, Jia W, Xu Z, Han D (2021) Differential cadmium translocation and accumulation between Nicotiana tabacum L. and Nicotiana rustica L. by transcriptome combined with chemical form analyses. Ecotox Environ Saf 208:111412. https://doi.org/10.1016/j.ecoenv.2020.111412
Jalali M, Antoniadis V, Najafi S (2021) Assessment of trace element pollution in northern and western Iranian agricultural soils: a review. Environ Monit Assess 193:823. https://doi.org/10.1007/s10661-021-09498-w
Kinay A, Erdem H, Karakoç E (2021) Chemical composition of tobacco genotypes in response to zinc application under cadmium toxicity. Rom Agric Res 38:301–310. https://www.incda-fundulea.ro/rar/nr38/rar38.32.pdf. Accessed 08.06.2023
Kirkham MB (2006) Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32. https://doi.org/10.1016/j.geoderma.2006.08.024
Kozak K, Antosiewicz DM (2023) Tobacco as an efficient metal accumulator. Biometals 36:351–370. https://doi.org/10.1007/s10534-022-00431-3
Kumar A, Tripti Raj D, Maiti SK, Maleva M, Borisova G (2022) Soil Pollution and Plant Efficiency Indices for Phytoremediation of Heavy Metal(loid)s: Two-Decade Study (2002–2021). Metals 12:1330. https://doi.org/10.3390/met12081330
Liang HM, Lin TH, Chiou JM, Yeh KC (2009) Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ Pollut 157:1945–1952. https://doi.org/10.1016/j.envpol.2008.11.052
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Liu H, Wang H, Ma Y, Wang H, Shi Y (2016) Role of transpiration and metabolism in translocation and accumulation of Cadmium in tobacco plants (Nicotiana tabacum L.). Chemosphere 144:1960–1965. https://doi.org/10.1016/j.chemosphere.2015.10.093
Liu H, Wang H, Zhang Y, Wang H, Yang J, Liu J, Shi Y (2019b) Comparison of heavy metal accumulation and cadmium phytoextraction rates among ten leading tobacco (Nicotiana tabacum L.) cultivars in China. Int J Phytoremediation 21:699–706. https://doi.org/10.1080/15226514.2018.1556589
Liu L, Yue F-x, Ji-W L, Li D, Wang Y-f (2019a) Interaction between biochar and Zn or K foliar fertilizer on the growth and Cd uptake of tobacco under cadmium stress. J Plant Nutr 25:982–990. https://doi.org/10.11674/zwyf.18283
Mei S, Lin K, Williams DV, Liu Y, Dai H, Cao F (2022) Cadmium Accumulation in Cereal Crops and Tobacco: A Review. Agronomy 12:1952. https://doi.org/10.3390/agronomy12081952
Mirkarimi SR, Ardakani Z, Rostamian R (2021) Economic and environmental assessment of tobacco production in Northern Iran. Ind Crops Prod 161:113171. https://doi.org/10.1016/j.indcrop.2020.113171
Mishra T, Pandey VC (2019) Phytoremediation of Red Mud Deposits through Natural Succession. In: Pandey VC, Bauddh K (eds) Phytomanagement of Polluted Sites. Elsevier, pp 409–424. https://doi.org/10.1016/b978-0-12-813912-7.00016-8
Moghtaderi T, Shakeri A, Rodríguez-Seijo A (2020) Potentially Toxic Element Content in Arid Agricultural Soils in South Iran. Agronomy 10:564. https://doi.org/10.3390/agronomy10040564
Oliver D, Hannam R, Tiller KG, Wilhelm N, Merry RH, Cozens G (1994) The effects of zinc fertilization on cadmium concentration in wheat grain. J Environ Qual 23:705–711. https://doi.org/10.2134/jeq1994.00472425002300040013x
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney RS (eds) Method of soil analysis: part 2. Chemical and microbiological properties. Agronomy monographs no. 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 403–430
Rassaei F, Hoodaji M, Abtahi SM (2020) Cadmium speciation as influenced by soil water content and zinc and the studies of kinetic modeling in two soils textural classes. Int Soil Water Conserv Res 8:286–294. https://doi.org/10.1016/j.iswcr.2020.05.003
Rastegari Mehr M, Shakeri A, Amjadian K, Khalilzadeh Poshtegal M, Sharifi R (2021) Bioavailability, distribution and health risk assessment of arsenic and heavy metals (HMs) in agricultural soils of Kermanshah Province, west of Iran. J Environ Health Sci Engineer 19:107–120. https://doi.org/10.1007/s40201-020-00585-7
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol 218:407–411. https://doi.org/10.1111/nph.14907
Rehman MZU, Rizwan M, Rauf A, Ayub MA, Ali S, Qayyum MF, Waris AA, Naeem A, Sanaullah M (2019) Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 226:454–462. https://doi.org/10.1016/j.chemosphere.2019.03.182
Rezapour S, Atashpaz B, Moghaddam SS, Damalas CA (2019) Heavy metal bioavailability and accumulation in winter wheat (Triticum aestivum L.) irrigated with treated wastewater in calcareous soils. Sci Total Environ 656:261–269. https://doi.org/10.1016/j.scitotenv.2018.11.288
Rizwan M, Ali S, Rehman MZU, Maqbool A (2019) A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ Sci Pollut Res 26:6279–6289. https://doi.org/10.1007/s11356-019-04174-6
Rodrigo-Comino J, López-Vicente M, Kumar V, Rodríguez-Seijo A, Valkó O, Rojas C et al (2020) Soil Science Challenges in a New Era: A Transdisciplinary Overview of Relevant Topics. Air. Soil Water Res 13 https://doi.org/10.1177/1178622120977491
Rodríguez-Seijo A, Lago-Vila M, Andrade ML, Vega FA (2016) Pb pollution in soils from a trap shooting range and the phytoremediation ability of Agrostis capillaris L. Environ Sci Pollut Res 23:1312–1323. https://doi.org/10.1007/s11356-015-5340-7
Rodríguez-Seijo A, Soares C, Ribeiro S, Amil BF, Patinha C, Cachada A, Fidalgo F, Pereira R (2022) Nano-Fe2O3 as a tool to restore plant growth in contaminated soils - Assessment of potentially toxic elements (bio)availability and redox homeostasis in Hordeum vulgare L. J Hazard Mater 425:127999. https://doi.org/10.1016/j.jhazmat.2021.127999
Rojas-Cifuentes GA, Johnson BL, Berti MT, Norvell WA (2012) Zinc Fertilization Effects on Seed Cadmium Accumulation in Oilseed and Grain Crops Grown on North Dakota Soils. Chil J Agric Res 72:117–124. https://doi.org/10.4067/S0718-58392012000100019
Sadiku OO, Rodríguez-Seijo A (2022) Metabolic and genetic derangement: a review of mechanisms involved in arsenic and lead toxicity and genotoxicity. Arh Hig Rada Toksikol 73:244–255. https://doi.org/10.2478/aiht-2022-73-3669
Sharma RK, Agrawal M, Agrawal SB (2007) Interactive Effects of Cadmium and Zinc on Carrots: Growth and Biomass Accumulation. J Plant Nutr 31:19–34. https://doi.org/10.1080/01904160701741727
Slamet-Loedin IH, Johnson-Beebout SE, Impa SM, Tsakirpaloglou N (2015) Enriching rice with Zn and Fe while minimizing Cd risk. Front Plant Sci 6:121. https://doi.org/10.3389/fpls.2015.00121
Sozubek B, Belliturk K, Saglam M (2015) Effect of Zinc Application on Cadmium Uptake of Maize Grown in Alkaline Soil. Commun Soil Sci Plant Anal 46:1244–1248. https://doi.org/10.1080/00103624.2015.1033534
Szerement J, Szatanik-Kloc A, Mokrzycki J, Mierzwa-Hersztek M (2022) Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J Soil Sci Plant Nutr 22:1129–1159. https://doi.org/10.1007/s42729-021-00719-2
Tavakkoli L, Khanjani N (2016) Environmental and occupational exposure to cadmium in Iran: a systematic review. Rev Environ Health 31:457–463. https://doi.org/10.1515/reveh-2016-0042
Tkalec M, Štefanić PP, Cvjetko P, Šikić S, Pavlica M, Balen B (2014) The Effects of Cadmium-Zinc Interactions on Biochemical Responses in Tobacco Seedlings and Adult Plants. PLoS One 9:e87582. https://doi.org/10.1371/journal.pone.0087582
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Usman K, Al-Ghouti MA, Abu-Dieyeh MH (2019) The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci Rep 9:5658. https://doi.org/10.1038/s41598-019-42029-9
Vasiliadou S, Dordas C (2009) Increased concentration of soil cadmium affects on plant growth, dry matter accumulation, Cd, and Zn uptake of different tobacco cultivars (Nicotiana tabacum L.). Int J Phytoremediation 11:115–130. https://doi.org/10.1080/15226510802378400
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38. https://doi.org/10.1097/00010694-193401000-00003
Wang C, Xu W, Li H, Zhou K, Liu J, Zhang M (2013) Effects of zinc on physiologic characterization and cadmium accumulation and chemical forms in different varieties of pepper. Wuhan Univ J Nat Sci 18:541–548. https://doi.org/10.1007/s11859-013-0970-4
Wang H, Xu C, Luo Z, Zhu H, Wang S, Zhu Q, Huang D, Zhang Y, Xiong J, He Y (2018) Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environ Sci Pollut Res 25:29287–29294. https://doi.org/10.1007/s11356-018-2938-6
Wu F, Zhang G (2002) Alleviation of cadmium-toxicity by application of zinc and ascorbic acid in barley. J Plant Nutr 25:2745–2761. https://doi.org/10.1081/PLN-120015536
Zhang S, Song J, Cheng Y, Liu G, Wallace AR (2018) Trace metal(loid)s exposure through soil–tobacco–human pathway: Availability in metal-contaminated agricultural soils, transfer models and health risk assessment. Ecotox Environ Saf 148:1034–1041. https://doi.org/10.1016/j.ecoenv.2017.11.043
Zhen S, Shuai H, Xu C, Lv G, Zhu X, Zhang Q, Zhu Q, Núñez-Delgado A, Conde-Cid M, Zhou Y, Huang D (2021) Foliar application of Zn reduces Cd accumulation in grains of late rice by regulating the antioxidant system, enhancing Cd chelation onto cell wall of leaves, and inhibiting Cd translocation in rice. Sci Total Environ 770:145302. https://doi.org/10.1016/j.scitotenv.2021.145302
Zhou J, Zhang C, Du B, Cui H, Fan X, Zhou D, Zhou J (2020) Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars. Environ Pollut 265:115045. https://doi.org/10.1016/j.envpol.2020.115045
Zoffoli HJ, do Amaral-Sobrinho NM, Zonta E, Luisi MV, Marcon G, Tolón-Becerra A (2013) Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s Southern Region. Environ Monit Assess 185:2423–2437. https://doi.org/10.1007/s10661-012-2721-y
Acknowledgements
Andrés Rodríguez Seijo (ARS) wants to acknowledge MCIN/AEI/UVigo for their contract JdCi under the “Actuación financiada por IJC2020-044197-I/MCIN/AEI/10.13039/501100011033 y por la Unión Europea NextGenerationEU”/PRTR”. ARS want also acknowledges the financial support of the Consellería de Cultura, Educación e Universidade (Xunta de Galicia) is also recognized through the contract ED431C 2021/46-GRC granted to the research group BV1 of the University of Vigo. Funding for open access charge: Universidade de Vigo/CISUG.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
R.C.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft, Writing—review and editing. T.M.: formal analysis, methodology; writing-editing manuscript; A.R.S.: formal analysis, writing-editing manuscript. R.A.: Conceptualization, Supervision, funding acquisition, writing-editing manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent to Publish
Not applicable
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cham, R., Moghtaderi, T., Rodríguez-Seijo, A. et al. Single and Combined Effect of Cd and Zn on Growth, Metal Accumulation and Mineral Nutrition in Tobacco Plants (Nicotiana tabacum L.). J Soil Sci Plant Nutr 23, 4521–4531 (2023). https://doi.org/10.1007/s42729-023-01368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01368-3