Abstract
Background
Dental adhesives with immobilized antibacterial agents are formulated to combat bacterial invasion along the tooth-restoration interface. This study aims to evaluate the antibacterial effect of synthesized quaternary ammonium compound (QAC) incorporated into commercial dental adhesive.
Methods
QAC was synthesized from 2-(Dimethylamino) ethyl methacrylate and 1-Bromobutane and characterized using CHN (Carbon, Hydrogen, Nitrogen), FTIR (Fourier transform infrared) and H+NMR (Proton nuclear magnetic resonance) analyses. The synthesized QAC was assessed for its cytotoxicity and its antibacterial activity against S. mutans using disc diffusion method, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), time-kill kinetics test, and TEM imaging. The QAC was added to the primer of a commercially available adhesive (OptiBond XTR) at two concentrations; 20 and 40 mg mL−1 representing the MIC and MBC, respectively. The antibacterial properties of the experimental adhesives, commercial antibacterial adhesive Clearfil SE Protect containing 12-methacryloyloxydodecylpyridinium bromide (MDPB), and commercial vehicle (OptiBond XTR) were compared using time-kill kinetics test. Statistical analysis by ANOVA followed by tukey post-hoc test (P < 0.05).
Results
Disc diffusion and time-kill kinetics tests showed potent antibacterial action of QAC, both in the unpolymerized and the cured forms. MIC and MBC were 20 and 40 mg mL−1 respectively. There was no statistically significant difference between experimental adhesives and Clearfil Protect with more than 99% reduction in bacterial count, while OptiBond XTR showed no bacterial killing up for up to 10 h.
Conclusions
The synthesized QAC added to a commercially available adhesive imparted antibacterial properties, thus providing an affordable adhesive system to the local market.
Article Highlights
-
Quaternary ammonium compounds impart antibacterial action to a commercially available adhesive (OptiBond XTR).
-
The experimental adhesive shows comparable antibacterial activity to Clearfil SE Protect adhesive.
-
It is feasible to formulate an antibacterial adhesive with simple procedures and reduced cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adhesive restorative systems have made a breakthrough in dentistry especially in the field of conservative dentistry. Continuous development of adhesive dental materials enabled the use of highly esthetic restorations and the application of minimally invasive techniques since they provide immediate bond strength. However, considering the long-term success of the restoration, it is important to study the strength and the durability of the tooth-restoration adhesive junction [1]. The adhesive junction comprises some challenges, such as polymerization shrinkage stresses, bacterial invasion, and degradation at the tooth-restoration interface [2]. As most systematic survival studies of resin composites and dental adhesives indicate, secondary caries is the foremost reason for resin-based restoration failure and life span reduction. Secondary caries between dental adhesive resins and the tooth structure is considered the primary reason for the failure of polymer-based bonded restorations [3]. Hence, there are trials to formulate dental adhesives with antimicrobial abilities to fight bacterial invasion and growth at the interface [4, 5].
To improve the long-term service of dental restorations, various antibacterial agents were added to experimental and commercial dental bonding agents [2]. An antibacterial agent interferes with the growth of bacteria, to fight both the residual bacteria in the tooth cavity and the new invading bacteria at the margins, thus, minimizing the risk of secondary caries [6].
Many trials were made to create adhesives with antibacterial properties, including the incorporation of chlorhexidine, glutaraldehyde, and nano-sized metallic particles such as Silver, Titanium and Copper [7]. However, the low-molecular-weight antimicrobial agents have the limitation of the possible toxicity, short-term effects, and the difficulty of controlling their rate of diffusion [8]. Also, the release of antimicrobial agents has a side effect on the mechanical properties of the adhesive layer [9].
These leachable agents were replaced by polymerizable antibacterial agents, which are immobilized in the resin matrix after curing, ensuring a more prolonged and durable antimicrobial action while maintaining the mechanical properties of the dental bonding agents [10, 11].
Such antibacterial monomers include cationic quaternary ammonium compounds (QAC), which show antimicrobial activity against both Gram-positive and Gram-negative bacteria, fungi, parasites, and enveloped viruses [12]. It was reported that QACs were used in 1970 in mouth rinses to inhibit oral biofilm formation [13, 14]. QACs were also incorporated in dental composite materials in 1994 by Imazato et al. to help inhibit plaque accumulation and secondary caries [9]
One such QAC is 12-methacryloyloxydodecylpyridinium bromide (MDPB), which was synthesized by combining a polymerizable methacrylate group with an antibacterial quaternary ammonium group (dodecylpyridinium bromide) resulting in a compound which is rather hydrophobic [15]. It was initially synthesized in 1993 and introduced into a commercially available dental adhesive system—in 2003—known as Clearfil SE Protect Bond primer bottle (5% MDPB), and it has been used successfully in clinical practice. One study proved its bacteriostatic activity and antiadhesion property against oral streptococci especially S. mutans [16,17,18]. In contrast, there was no statistically significant difference between Clearfil Protect Bond and a dental adhesive without antibacterial function (All-Bond SE) either in enamel demineralization or in dental biofilm formation, which suggests that Clearfil Protect Bond was unable to inhibit secondary caries in situ [19].
In another attempt, 2-(Dimethylamino) ethyl methacrylate (DMAEMA) was used as a precursor to synthesize two different quaternary monomers which are compatible with existing dental dimethacrylate-based monomers [10, 20], as DMAEMA is often used as a co-initiator in dental adhesives [21]. It was also used for the synthesis of several cationic antibacterial monomers which were investigated to assess their antimicrobial property against oral bacteria [22,23,24,25,26]. Moreover, four different QACs including dimethylaminobutyl methacrylate (DMABM), showed promising results against S. aureus and E. coli bacteria [27]. It should be noted that the incorporation of QACs into the adhesives had no adverse effect on their micro tensile bond strength [28,29,30]
To our knowledge, there are limited commercially available adhesive systems with antibacterial activity for clinical use in dental applications, such as Clearfil SE Protect (containing MDPB) and GLUMA 2Bond (containing 5% glutaraldehyde) [31]. However, the biocompatibility of GLUMA 2Bond is questionable due to the cytotoxicity of the aldehyde moiety [31]. Clearfil SE Protect is the only commercially available adhesive to date with antibacterial QAC (MDPB), which is rather expensive and not available in all markets. Moreover, it is important to formulate a QAC that is compatible with the adhesive composition, has low cytotoxicity, and needs a simple procedure for synthesis. Therefore, our aim was to formulate an affordable adhesive with comparable antibacterial characteristics. This was done through the synthesis and characterization of an antibacterial QAC, its incorporation into a commercially available adhesive system (Optibond), and assessment of its antibacterial properties, compared to the commercially available (MDPB-containing) antibacterial adhesive. Incorporation of QACs into Optibond has been performed in a previous study using Benzalkonium Chloride QAC, but the study did not evaluate the antibacterial activity of the resultant adhesive [32]. In the current study, the null hypothesis tested that incorporation of QAC into dental adhesive Optibond has no antibacterial effect.
2 Methods
The chemicals used for the synthesis of the QAC were 2-(Dimethylamino) ethyl methacrylate (Alfa Aesar, ThermoFisher, Kandel, Germany), 1-Bromobutane (Fisher Scientific, Loughborough, Leics, UK) and absolute ethyl alcohol as a solvent (Sphinx, Egypt). OptiBond XTR Universal Adhesive (Kerr Corporation, California, USA) was used as a vehicle for the experimental adhesive to which QAC was added. It is a two-step, self-etch adhesive system supplied in two 5 mL bottles (fig. S1). Clearfil SE Protect (Kuraray Medical Inc, Okayama, Japan) (fig. S2) is a two-step, self-etch antimicrobial adhesive. The compositions of both adhesive systems are shown in table S1. Clearfil SE Protect was used as the positive control (as a comparator against the experimental adhesive regarding the antibacterial activity), while Optibond without the QAC was considered the negative control in the antibacterial test.
2.1 Synthesis and characterization of the quaternary ammonium monomer
The QAC was obtained through the Menschutkin reaction [10]. In a tared beaker, 2-(N,N-dimethylamino) ethyl methacrylate DMAEMA (1.57 g, 10 mmol) and 1-Bromobutane (butyl bromide) (1.37 g, 10 mmol) were mixed, and 3 g ethanol were added as a solvent. Using a magnetic stir bar, the beaker was stirred for 24 h at 60 °C. Afterward, the solvent and residual reagents were removed by evaporation [14]. The resultant monomer was a clear, colorless, viscous fluid that turned white and showed increased viscosity and tackiness with time (fig. S3). The following characterization tests for the synthesized QAC were performed using one sample each.
2.1.1 CHN elemental analysis
Elemental analysis of the synthesized (QAC) was done using Automatic Analyzer CHNS (Vario EL III – elementar Analysensysteme GmbH – Germany) to determine the percentage of Carbon, Hydrogen, and Nitrogen found in the sample, compared to the calculated values.
2.1.2 Analysis using Fourier transform infrared spectroscopy (FTIR)
The functional groups of the starting materials and the quaternary ammonium salt were identified by FTIR spectroscopy in a KBr pellet (NICOLET 380 FT-IR, Thermo-scientific, China). FTIR spectra of the starting materials and the synthesized monomer were collected in the 4000 cm−1 to 400 cm−1 region with a wavenumber expanded uncertainty of 0.5 cm−1 [10].
2.1.3 Analysis using proton nuclear magnetic resonance (1H NMR)
Proton NMR analysis was performed to identify the molecular structure of the synthesized QAC. High-resolution 400 MHz 1H NMR spectra were acquired on Bruker NMR spectrometer (Bruker, Germany). Deuterated water (D2O) was used as a solvent.
2.1.4 Cytotoxicity testing
Cell viability against the synthesized QAC was assessed by sulforhodamine B (SRB) assay according to the method described by [33, 34]. The Human Skin Fibroblast (HSF) cell line was used (Nawah Scientific Inc., El Mokattam, Cairo, Egypt). It is a normal cell line, Passage no. 4. The test was performed in triplicate.
The QAC was examined at different concentrations in complete media (10, 20, 40, 60, and 100 μg mL−1). A solution of SRB at 0.4% was used for staining the proteins of viable cells [35]. The percentage of the viable cells exposed to the QAC was calculated with respect to the negative control, and the lethal concentration 50 (LC50) was determined [36, 37].
2.2 Antimicrobial activity of the synthesized QAC
2.2.1 Test microorganism and growth conditions
A standard strain of Streptococcus mutans ATCC 25175 was used, grown in Trypticase soy broth/agar medium supplemented with 3 g L−1 yeast extract (TSY) for 24 h at 37 °C under anaerobic conditions in a CO2 incubator [38].
2.2.2 Susceptibility testing using the disc diffusion method
The susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) recommendation [39]. A suspension of S. mutans of 1.5 × 108 colony-forming unit per milliliter (CFU mL−1) was adjusted spectrophotometrically (T80 UV/VIS double beam Spectrophotometer, PG instruments Ltd, LEICESTERSHIRE, UK) to get a culture density equivalent to a 0.5 McFarland standard [40]. Then the culture was diluted to 106 CFU mL−1 and was surface inoculated onto the TSY agar plates using a sterile swap. The synthesized QAC was assessed at two concentrations (20 and 10 mg mL−1). Clindamycin antibiotic disc (15μg) served as the positive control. TSY agar plates were incubated in a CO2 incubator at 37 °C for 24 h. The inhibition zone was measured around each disc. The test was performed in triplicate.
2.2.3 Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by broth microdilution method
MIC and MBC of the synthesized QAC were determined using broth microdilution assay according to the CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards [41]. Serial dilutions of the QAC starting from 40 mg mL−1 to 78 μg mL−1 were prepared in the wells of the 96-well microplate. A suspension of S. mutans in TSY broth at 106 CFU mL−1 was added to the wells. Positive control (bacteria and plain TSY medium) and negative control (plain TSY medium, to confirm the sterility of the medium) were prepared. The MIC was defined as the lowest concentration inhibiting the visible growth after incubation anaerobically at 37 °C for 24 h.
For the determination of MBCs, 20μl aliquots from each well showing no visible turbidity were diluted and transferred onto TSY agar plates. The plates were incubated overnight at 37 °C anaerobically in a CO2 incubator. Viable colony count on agar medium was performed. MBC was defined as the concentration causing > 99.9% (> 3-log cycle) reduction of the initially inoculated colony counts [42]. MIC and MBC determinations were performed in triplicate.
2.2.4 Kill kinetics assay (Time-kill test) of QAC
Time-kill kinetics of the QAC against S. mutans was performed using concentrations equal to 2 × MIC, 3 × MIC, and 4 × MIC of the monomer [43, 44]. An inoculum of 106 CFU mL−1 was added to the wells containing the mentioned QAC concentrations, as well as positive and negative controls, and incubated at 37 °C for 12 h. Aliquots of 20μl were withdrawn at time intervals of one hour for up to 12 h. The aliquoted samples were diluted appropriately and inoculated onto TSY agar plates, then incubation for 24 h anaerobically at 37 °C was performed and the number of recovered viable cells (CFU mL−1) was determined (fig. S4). The test was performed in triplicate [24, 45, 46].
2.2.5 Bacterial cell imaging by the transmission electron microscope (TEM)
TEM was used to monitor the structural changes in bacterial cells exposed to the antibacterial QAC. An overnight culture of S. mutans in TSY broth was prepared, mixed with the synthesized QAC (20 mg mL−1) and incubated anaerobically in a CO2 incubator at 37 °C for 12 h. Another culture was prepared without QAC to serve as positive control. A drop (2–5 μL) was pipetted from each suspension (the positive control and the QAC-treated bacteria suspension) and spread on a sheet of parafilm. The electron microscope grid (carbon-coated 400-mesh copper grids) was made directly on the specimen and stained with 2% Phosphotungstic acid (PTA) stain for 30 s. The prepared grids are taken for imaging by TEM (JEM-1400Flash Electron Microscope, JEOL Ltd., Tokyo, Japan).
2.3 Preparation of the experimental adhesive system.
The synthesized QAC was added to the primer bottle of the OptiBond XTR adhesive with concentrations 20 and 40 mg mL-1 (0.57% and 1.1% by weight), representing the MIC and MBC, respectively. Each bottle was sonicated using an ultrasonic processor (Hielscher UP50H, Hielscher Ultrasonics Germany) to ensure homogenous mixing.
2.4 Sample size calculation
In a previous study by Ma et al. in 2012 [25] the reduction in the number of viable bacteria (%) within group 1 (adhesive with antibacterial quaternary ammonium compound) and group 2 (MDPB-containing dental adhesive) was normally distributed with mean and standard deviation of 99.45 (0.59) and 32.80 (13.12) respectively. Therefore, we will need to study 3 subjects per group to be able to reject the null hypothesis that the means of the experimental and control groups are equal with probability (power) 0.8. The Type I error probability associated with this test of this null hypothesis is 0.05. The sample size was calculated using G*Power version 3.1.9.2 for windows using independent t test.
2.5 Kill kinetics assay of the experimental and commercially available adhesives
The rate of bacterial killing of experimental adhesive systems (containing either 20 or 40 mg mL−1 of the QAC) was compared against the commercially available antibacterial adhesive system, Clearfil SE Protect Bond (containing 5% MDPB monomer) as the positive control, while the commercially available vehicle OptiBond XTR was considered the negative control, to demonstrate that the primer itself is not antimicrobial. The adhesives were applied and cured according to the manufacturer’s instructions into the wells of a 48-well plate (fig. S5).
An amount of 500μL of S. mutans suspension in TSY broth with 106 CFU mL−1 inoculum size was added to the wells containing the polymerized adhesives and incubated at 37 °C for 12 h in a CO2 incubator. Aliquots of 20μl samples were withdrawn every hour for up to 12 h. The cell count of surviving bacteria was determined by the plate count method as mentioned before. The test was performed in triplicate (n = 3) [24, 45, 46].
To express the change (reduction or increase) in the microbial population compared to a starting inoculum, the percentage decrease was determined for each time point as follows [47]:
And the Log reduction was calculated as follows [47]:
2.6 Statistical analysis
Data for kill kinetics assay were expressed as mean and standard deviation of three replicates and were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey post-hoc test. Differences were considered statistically significant at P < 0.05. The statistical variables were evaluated using the Medcalc software, version 19 for windows (MedCalc Software Ltd, Ostend, Belgium).
3 Results
3.1 Synthesis and characterization of the QAC
3.1.1 CHN elemental analysis:
The elemental analysis data of QAC by CHN analyzer is shown in Table 1. These experimental values (found) given by the analyzer were consistent with the theoretical value (calculated) according to the estimated structure of the synthesized QAC (Fig. 1).
3.1.2 Analysis using FTIR spectroscopy
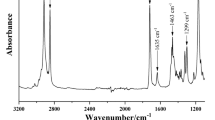
FTIR spectra of the synthesized QAC and its starting reagents (DMAEMA and Bromobutane) showed the disappearance of the C–Br absorption bands from Bromobutane (667 cm−1, 570 cm−1) as well as the N(CH3)2 bands (2952 cm−1, 2771 cm−1) from DMAEMA. The prepared QAC displayed absorbance bands corresponding to the aliphatic methyl groups in the 3000 cm−1 to 2800 cm−1 region (2964 cm−1, 2877 cm−1). Also, the carbonyl ester group was evident in the 1750 cm−1 to 1725 cm−1 region (1720 cm−1) (Fig. 2).
3.1.3 Analysis using 1H NMR
The peak assignments δ (ppm) 1H NMR were as follows (Fig. 3A):
1H NMR (400 MHz, D2O) δ 0.86 (t, J = 7.6 Hz, 3H, CH3), 0.91–0.95 (m, 2H, CH2), 1.06–1.10 (m, 4H, CH2), 1.84 (s, 3H, CH2=C–CH3), 3.08 (s, 6H, N(CH3)2), 3.67–3.69 (m, 2H, OCH2CH2), 4.52–4.54 (m, 2H, OCH2), 5.68 (s, 1H, C=CH2), 6.06 (s, 1H, C=CH2). This 1H-NMR analysis confirmed the structure of the synthesized QAC (Fig. 3B).
3.1.4 Cytotoxicity testing
The results are expressed as the viability % of HSF subjected to different concentrations of QAC, compared to the viability of the negative control (Fig. 4). Cell viability showed a mild decrease with increasing monomer concentration to reach HSF viability of approximately 85% at 100 μg mL-1. The median lethal concentration LC50 for the QAC was 51.79 μg mL-1.
3.2 Antimicrobial activity of the synthesized QAC
3.2.1 Susceptibility test using the disc diffusion method
The synthesized QAC showed antimicrobial activity against the tested strain of S. mutans ATCC 25175, which was more potent with a concentration 20 mg mL-1 than 10 mg mL-1 as evidenced by the diameter of the inhibition zone, 11 and 7.5 mm respectively (Fig. 5), However, clindamycin showed the highest level of inhibition resulting in an inhibition zone of 32.5 mm (table S2).
3.2.2 MIC and MBC by broth microdilution method
The synthesized QAC showed average MIC and MBC values of 20 and 40 mg mL−1, respectively, against S. mutans. The MIC (20 mg mL−1) resulted in a one-log cycle reduction of the bacterial count, equivalent to 90% inhibition of the growth of the initially inoculated count.
3.2.3 Kill kinetics assay (Time-kill test)
The time-kill kinetics profile of the synthesized antibacterial monomer against S. mutans at concentrations equivalent to 2 × MIC, 3 × MIC, and 4 × MIC showed a reduction in number of viable cells after 2 h at all tested concentrations. While the bacteria in the positive control group showed continuous viability and growth. Moreover, a complete bacterial death was observed after 10 h at QAC concentrations equivalent to 3 × MIC, and 4 × MIC (60 and 80 mg mL−1, respectively). Bacteria treated with 2 × MIC (40 mg mL−1) of the QAC showed complete death at 12 h (Fig. 6).
3.2.4 Bacterial cell imaging by TEM
For the untreated bacteria, the TEM micrograph showed actively growing bacteria having several initial membrane invaginations for septum formation and well-defined cell wall (Fig. 7A). While the bacteria treated with QAC appeared longer than usual with no sign of membrane invagination, and the dividing septum was not evident at all, moreover, a distorted cell wall appeared (Fig. 7B).
3.3 Kill kinetics assay of the experimental and commercially available adhesives
The tested groups were the experimental adhesives containing 20 and 40 mg mL−1 QAC, denoted OB20 and OB40 respectively, MDPB-containing adhesive Clearfil Protect, OptiBond adhesive without an antibacterial agent, and positive control bacteria group. The time kill kinetics profile for the tested adhesives is shown in comparison to the positive control bacteria group, which showed continuous viability and growth as represented by Log10 CFU mL−1 (Fig. 8).
Kill kinetics of experimental adhesives and commercial controls. “Black line” represents the effect of the commercial Clearfil Protect, “Green line” and “Red line” represent the kill effect of the experimental adhesive systems, supplemented with 40 and 20 mg mL−1 QAC respectively (OB40 and OB20), “Orange line” represents the kill effect of the commercial OptiBond XTR adhesive system without an antibacterial agent. “Blue line” represents the growth rate of the bacterial positive control. Data is presented as Mean and standard deviation of Log bacterial count
The percentage bacterial count showed a significant decrease with time for OB20, OB40 and Clearfil Protect (P = 0.006, P = 0.047 and P = 0.026) respectively after 10 h, while for Optibond the bacterial count increased with time (P = 0.661). After 10 h there was no statistically significant difference between OB20, OB40 and Clearfil Protect with more than -99% reduction in the bacterial count, however, the 3 groups differed significantly from Opibond (Table 2).
4 Discussion
Insoluble antimicrobial agents incorporated into dental adhesives aim to target microorganisms along the tooth restoration interface [48]. A compound with a methacrylate functional group that copolymerizes with the comonomer mixture of the adhesive will improve antibacterial activity and prevent leakage and losing therapeutic effects over time [49]. These agents include QACs which have shown successful antibacterial action against oral pathogens [50].
The classical Menshutkin reaction provides a successful and simple approach to producing antibacterial QACs with minimal post-reaction purification [13, 14, 51].
The multifunctional monomer N,N-dimethylaminoethyl methacrylate (DMAEMA) has several reactive groups (ether, double bond and amino groups) and can copolymerize with other vinyl monomers with good biological compatibility [27, 52]. It has been used as the basis for QAC formulation which proved high antibacterial activity both in vitro and in vivo [20, 51]. It is well known that the alkyl chain length of a QAC is one factor that influences its antibacterial activity, where an increase in antibacterial efficiency was observed by increasing chain length from 3 to 16, but it decreased when reached 18 [51]. For the current study, the reaction of DMAEMA with butyl bromide resulted in QAC with four carbon atoms. This is considered a relatively short length of alkyl group, with more exposed positively charged groups enhancing the antibacterial effectiveness in the polymerized form compared to long chain compounds [53].
The results of CHN elemental analysis match the basic structure of the synthesized monomer, with the molecular formula (C12H24NO2Br) (fig. S3). Spectra from FTIR spectroscopy showed the disappearance of the C–Br band from bromobutane, and the disappearance of N(CH3)2 bands from DMAEMA, which is characteristic of the quaternization reaction [10]. Results of 1H NMR further confirmed the structure of the synthesized QAC and were in accordance with other studies [27].
The cytotoxicity test results have shown a favorable response of HSF cells to the QAC, where the cell viability was 85% using SRB assay. Previous studies used fibroblasts when testing the cytotoxicity of QACs [54, 55]. Moreover, the high value of LC50 (51.79 μg. mL−1) indicated good biocompatibility, where bisphenol A-glycidyl methacrylate (BisGMA), one of the primary components in dental adhesives, showed LC50 values ranging between [56]. Studies also reported that DMAE-CB exhibited LC50 values between 2 and 5 μg. mL−1. As the LC50 values of Bis-GMA, the most used monomer in dental materials, were reported to be μg. mL.−1 [57]
Streptococcus mutans was chosen for the antimicrobial testing as it is one of the main pathogens in the development of primary and secondary dental caries [45, 58, 59]. Inhibition zone measurement was carried out to test the quaternary ammonium monomer’s antibacterial abilities to estimate the potent concentrations against a certain bacterial strain [60].
In this study, the MIC and MBC values of the synthesized QAC agree with previous studies where a similar QAC, dimethyl ammonium ethyl dimethacrylate) was tested [56]. The value of MIC calculated for the synthesized monomer against S. mutans was so close to a similar monomer (DMAEDM) studied before [56]. Other researchers studied the antibacterial properties of QAC and the MIC value was 15 mg mL−1 [24]. Moreover, the MIC (20 mg mL−1) caused the death of 90% of the bacterial colonies, with suppression of growth to the remaining colonies, so even the MIC showed considerable antibacterial action against S. mutans.
As regards the bactericidal activity of the synthesized QAC in its unpolymerized form, it showed potent action at a concentration of 40 mg mL−1, where more than 99.9% of the bacterial culture was dead, which is beneficial in the clinical application as the primer will initially get in contact with the cut dentin, causing disinfection of the cavity [38, 58]. The MBC values reported in previous studies against S. mutans range between 31mg mL−1 and 62 mg mL−1 [24].
It should be noted that it was not feasible to compare the synthesized QAC with MDPB present in the commercial adhesive Clearfil Protect due to the difficulty in isolating the monomer from the adhesive.
The TEM images of the untreated bacteria revealed its normal bead-like appearance previously described in many studies [23, 61]. While for the bacteria treated with the QAC, an altered morphology could be seen in accordance with a similar study [23] and only a few cells were observed, whose appearance was altered into elongated cells with distorted membranes, such description of the affected cells was observed by other researchers [62]. It is understood that the mechanism of action of antibacterial QAC is mainly through binding to bacterial membranes and causing bacterial lysis [13, 63]. That is because the positively charged quaternary amine (N+) is adsorbed to the negatively charged bacterial cell wall and binds with the phosphate part of the cell wall through an ionic interaction. It then penetrates the cell wall and attaches to the cytoplasmic membrane leading to its disruption. This causes a disturbance in the electric balance and denaturation of proteins resulting in the leakage of intracellular components and the death of bacteria, moreover, the bacterial cell could explode under its own osmotic pressure [64,65,66]. It should be noted that S. mutans belongs to Gram-positive bacteria, where there is a thick peptidoglycan cell wall covering the inner cytoplasmic layer. The QAC can penetrate such layer, due to their alkyl groups, which are lipophilic groups enhancing the bactericidal ability [67, 68].
The primer of the parent commercial adhesive, Optibond, was used to serve as a vehicle to incorporate the synthesized antimicrobial QAC, since the primer is applied directly to the exposed cavity wall [58].
Time-kill test monitors the progressive death of bacteria exposed to the antibacterial monomer in relation to time [39]. Upon observing the time-kill assay of the unpolymerized QAC and the experimental adhesive after polymerization, it was seen that the adhesive system with 40 mg mL−1 QAC showed the total killing of S. mutans bacteria after only 9 h, which is faster than unpolymerized QAC (12 h). It was stated by other researchers that the polymerized QACs possess stronger antibacterial activities than their monomer counterparts, due to the higher charge density of the polymer, which increases the binding sites of the antimicrobial active groups to the negatively charged bacterial surface, resulting in multiple points of disruption of the cell membrane [52, 53, 69].
Optibond was used as a negative control to exclude the effect of the solvent, it was found that the bacterial count increased after 10 h of exposure (Table 2). On the other hand, the experimental adhesives with both QAC concentrations (OB20 and OB40), as well as the commercially available MDPB-containing adhesive (Cearfil Protect), showed a statistically significant reduction in the bacterial count with time reaching almost 100% bacterial death after 10 h (Table 2), thus the null hypothesis was rejected. There was no statistically significant difference between the positive control antibacterial adhesive (Clearfil Protect) and the experimental adhesives even when the concentration of 20 mg mL−1 was used, which is equivalent to the MIC value.
When comparing the time-to-kill of the tested adhesives, the experimental adhesives with 20 and 40 mg mL−1 QAC and MDPB-containing adhesive showed 10, 9, and 7 h respectively (Fig. 8). The different performance between MDPB and the synthesized QAC is probably due two one of two reasons, both are related to the polymer structure. First, the quaternary ammonium group in MDPB is located at the terminal end of the alkyl chain, making it more accessible to interact with the bacterial cell wall [17], while the synthesized QAC has the quaternary ammonium group almost in the middle of the chain, so it is less likely to encounter the bacterial cell wall. The second possible reason is the presence of the pyridine ring in MDPB, where the conjugation tends to pull away the electrons from around the N+, creating a negative inductive effect on the nitrogen atom, rendering it more positive [70].
5 Limitations and recommendations
It is recommended to investigate the long-term antibacterial action of the experimental adhesive containing the synthesized QAC. Further studies are necessary to determine whether the addition of QAC to the adhesive system has any effect on its degree of conversion, mechanical properties, or dentin bond strength. It is also recommended to evaluate the antibacterial activity of multiple QACs synthesized using reagents with variable chain length.
6 Conclusions
The experimental adhesive system used in this study showed promising antibacterial properties, through incorporation of QAC to the commercial adhesive resin, which provides an opportunity for the formulation of an affordable antibacterial dental adhesive system, comparable to the commercially available antibacterial adhesive Clearfil Protect.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Abbreviations
- BisGMA:
-
Bisphenol A-glycidyl methacrylate
- CFU mL−1 :
-
Colony-forming unit per milliliter
- CHN:
-
Carbon, Hydrogen, Nitrogen
- CLSI:
-
Clinical and Laboratory Standards Institute
- CP:
-
Clearfil SE Protect
- DMAEMA:
-
2-(Dimethylamino) ethyl methacrylate
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- FTIR:
-
Fourier transform infrared
- 1H NMR:
-
Proton nuclear magnetic resonance
- HSF:
-
Human Skin Fibroblast
- LC50 :
-
Median lethal concentration
- MBC:
-
Minimum bactericidal concentration
- MDPB:
-
12-Methacryloyloxydodecylpyridinium bromide
- MIC:
-
Minimum inhibitory concentration
- QAC:
-
Quaternary ammonium compound
- SRB:
-
Sulforhodamine B
- TEM:
-
Transmission electron microscope
- TSY:
-
Trypticase soy broth/agar medium supplemented with 3 g L−1 yeast extract
References
Carvalho RM, Manso AP, Geraldeli S, Tay FR, Pashley DH. Durability of bonds and clinical success of adhesive restorations. Dent Mater. 2012;28(1):72–86. https://doi.org/10.1016/j.dental.2011.09.011.
Elgezawi M, Haridy R, Abdalla MA, Heck K, Draenert M, Kaisarly D. Current strategies to control recurrent and residual caries with resin composite restorations: operator- and material-related factors. J Clin Med. 2022. https://doi.org/10.3390/jcm11216591.
Hiers RD, Huebner P, Khajotia SS, Florez FLE. Characterization of experimental nanoparticulated dental adhesive resins with long-term antibacterial properties. Nanomaterials. 2022. https://doi.org/10.3390/nano12213732.
Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HHK. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2013;41(4):345–55. https://doi.org/10.1016/j.jdent.2013.01.004.
Lopes SR, et al. Development of an antibacterial dentin adhesive. Polymers (Basel). 2022. https://doi.org/10.3390/polym14122502.
Cheng L, et al. Developing a new generation of antimicrobial and bioactive dental resins. J Dent Res. 2017;96(8):855–63. https://doi.org/10.1177/0022034517709739.
Chen Z, et al. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater Today Bio. 2023. https://doi.org/10.1016/j.mtbio.2023.100635.
Ebi N, Imazato S, Noiri Y, Ebisu S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater. 2001;17(6):485–91. https://doi.org/10.1016/S0109-5641(01)00006-9.
Ge Y, Wang S, Zhou X, Wang H, Xu H, Cheng L. The use of quaternary ammonium to combat dental caries. Materials. 2015;8(6):3532–49. https://doi.org/10.3390/ma8063532.
Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28(2):219–28. https://doi.org/10.1016/j.dental.2011.10.004.
Chen L, Suh BI, Yang J. Antibacterial dental restorative materials: a review. Am J Dent. 2018;31:6–12.
Jiao Y, Niu L, Ma S, Li J, Tay FR, Chen J. Progress in polymer science quaternary ammonium-based biomedical materials: state-of-the-art, toxicological aspects and antimicrobial resistance. Progress Polym Sci. 2017;71:53–90.
Zhang N, et al. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent Mater. 2015. https://doi.org/10.1016/j.dental.2015.06.010.
Antonucci JM, Zeiger DN, Tang K, Lin-gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Mater. 2013;28(2):219–28. https://doi.org/10.1016/j.dental.2011.10.004.Synthesis.
Imazato S, Chen JH, Ma S, Izutani N, Li F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn Dental Sci Rev. 2012;48(2):115–25. https://doi.org/10.1016/j.jdsr.2012.02.003.
Jacobo C, Torrella F, Bravo-González LA, Ortiz AJ, Vicente A. In vitro study of the antibacterial properties and microbial colonization susceptibility of four self-etching adhesives used in orthodontics. Eur J Orthod. 2014;36(2):200–6. https://doi.org/10.1093/ejo/cjt032.
Imazato S, Russell RR, McCabe JF. Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent. 1995;23(3):177–81. https://doi.org/10.1016/0300-5712(95)93576-N.
Pinto CF, et al. In situ antimicrobial activity and inhibition of secondary caries of self-etching adhesives containing an antibacterial agent and/or fluoride. Am J Dent. 2015;28(3):167–73.
de Vasconcelos SMLC, de Melo MAS, Wenceslau JPMS, Zanin ICJ, Beltrao HCP, Fernandes CAO, Rodrigues LKA. In situ assessment of effects of the bromide-and fluoride-incorporating adhesive systems on biofilm and secondary caries. J Contemp Dental Practice. 2014;15(2):142.
Liu Y, et al. Evaluation of the ability of adhesives with antibacterial and remineralization functions to prevent secondary caries in vivo. Clin Oral Investig. Apr.2022;26(4):3637–50. https://doi.org/10.1007/s00784-021-04334-4.
Pratap B, Gupta RK, Bhardwaj B, Nag M. Resin based restorative dental materials: characteristics and future perspectives. Jpn Dental Sci Rev. 2019;55(1):126–38. https://doi.org/10.1016/j.jdsr.2019.09.004.
Li F, et al. Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res. 2009;88(4):372–6. https://doi.org/10.1177/0022034509334499.
Xiao YH, Ma S, Chen JH, Chai ZG, Li F, Wang YJ. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J Biomed Mater Res B Appl Biomater. 2009;90B(2):813–7. https://doi.org/10.1002/jbm.b.31350.
Xiao YH, et al. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J Oral Sci. 2008;50(3):323–7.
Ma S, et al. Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent Mater J. 2012;31(1):150–6. https://doi.org/10.4012/dmj.2011-180.
Li F, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37(4):289–96. https://doi.org/10.1016/j.jdent.2008.12.004.
Lu G, Wu D, Fu R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. Reactive Funct Polym. 2007;67:355–66. https://doi.org/10.1016/j.reactfunctpolym.2007.01.008.
Bapat RA, et al. Recent update on applications of quaternary ammonium Silane as an antibacterial biomaterial: a novel drug delivery approach in dentistry. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.927282.
Zhang Y, Chen Y, Hu Y, Huang F, Xiao Y. Quaternary ammonium compounds in dental restorative materials. Dental Mater J. 2018;37(2):183–91. https://doi.org/10.4012/dmj.2017-096.
Garcia IM, Rodrigues SB, de Souza Balbinot G, Visioli F, Leitune VCB, Collares FM. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin Oral Investig. 2020;24(2):777–84. https://doi.org/10.1007/s00784-019-02971-4.
Ramburrun P, Pringle NA, Dube A, Adam RZ, D’souza S, Aucamp M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials. 2021. https://doi.org/10.3390/ma14123167.
Flury S, Lussi A, Peutzfeldt A. Long-term bond strength of two benzalkonium chloride-modified adhesive systems to eroded dentin. Biomed Res Int. 2017. https://doi.org/10.1155/2017/1207208.
Allam RM, et al. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol Lett. 2018;291:77–85. https://doi.org/10.1016/j.toxlet.2018.04.008.
Skehan P, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12.
Garcia IM, Rodrigues SB, DeSouzaBalbinot G, Visioli F, Leitune VCB, Collares FM. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin Oral Investig. 2020;24(2):777–84. https://doi.org/10.1007/S00784-019-02971-4/FIGURES/3.
Inc. https://www.aatbio.com/tools/lc50-calculator. AAT Bioquest, “LC50 Calculator.” Accessed: Dec. 21, 2023. [Online]. Available: “Quest GraphTM LC50 Calculator.” AAT Bioquest, Inc., 21 Dec. 2023, https://www.aatbio.com/tools/lc50-calculator.
M. E. Hilburn, Encyclopedia of toxicology. Encyclopedia of Toxicol, 1147–1149, 2014.
Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22(6):527–32. https://doi.org/10.1016/j.dental.2005.05.009.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–9. https://doi.org/10.1016/j.jpha.2015.11.005.
André CB, et al. Dentine bond strength and antimicrobial activity evaluation of adhesive systems. J Dent. 2015;43(4):466–75. https://doi.org/10.1016/j.jdent.2015.01.004.
Loose M, Naber KG, Coates A, Wagenlehner FME, Hu Y. Effect of different media on the bactericidal activity of colistin and on the synergistic combination with azidothymidine against mcr-1-positive colistin-resistant Escherichia coli. Front Microbiol. 2020;11(January):1–8. https://doi.org/10.3389/fmicb.2020.00054.
Parvekar P, Palaskar J, Metgud S, Maria R, Dutta S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent. 2020;7(1):105–9. https://doi.org/10.1080/26415275.2020.1796674.
Appiah T, Boakye YD, Agyare C. Antimicrobial activities and time-kill kinetics of extracts of selected ghanaian mushrooms. Evid Based Complem Alternat Med. 2017. https://doi.org/10.1155/2017/4534350.
Zhang L, Ismail MM, Rocchetti G, Fayek NM, Lucini L, Saber FR. the untargeted phytochemical profile of three meliaceae species related to in vitro cytotoxicity and anti-virulence activity against MRSA isolates. Molecules. 2022. https://doi.org/10.3390/MOLECULES27020435.
Huang L, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56(4):367–73. https://doi.org/10.1016/j.archoralbio.2010.10.011.
Imazato S, Ebi N, Tarumi H, Russell RRB, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20(9):899–903. https://doi.org/10.1016/S0142-9612(98)00247-6.
Ngene A, Ohaegbu C, Idu E, Odo E. Time-kill kinetics and antibacterial activity of ethanolic extract of Allium sativum. Microbes Infect Dis. 2023. https://doi.org/10.21608/mid.2023.175501.1417.
Hardan L, et al. The bond strength and antibacterial activity of the universal dentin bonding system: a systematic review and meta-analysis. Microorganisms. 2021. https://doi.org/10.3390/microorganisms9061230.
Collares FM, Leitune VCB, Franken P, Parollo CF, Ogliari FA, Samuel SMW. Influence of addition of [2-(methacryloyloxy)ethyl] trimethylammonium chloride to an experimental adhesive. Braz Oral Res. 2017;31: e31. https://doi.org/10.1590/1807-3107bor-2017.vol31.0031.
Featherstone JDB. Dental restorative materials containing quaternary ammonium compounds have sustained antibacterial action. J Am Dental Assoc. 2022;153(12):1114–20. https://doi.org/10.1016/j.adaj.2022.09.006.
Rego GF, et al. Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent Mater. 2017;33(10):1149–56. https://doi.org/10.1016/j.dental.2017.07.010.
Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces-2: How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28(32):4870–9. https://doi.org/10.1016/j.biomaterials.2007.06.012.
Lv X, et al. Construction of a quaternary ammonium salt platform with different alkyl groups for antibacterial and biosensor applications. RSC Adv. 2018;8(6):2941–9. https://doi.org/10.1039/c7ra11001d.
Mokeem LS, Balhaddad AA, Garcia IM, Collares FM, Melo MAS. Benzyldimethyldodecyl ammonium chloride doped dental adhesive: impact on core’s properties, biosafety, and antibacterial/bonding performance after aging. J Funct Biomater. 2022. https://doi.org/10.3390/jfb13040190.
Sai M, Le-qun S, Yu-hong X, Fang L, Li H, Shen L, Ji-hua C. The cytotoxicity of methacryloxylethyl cetyl ammonium chloride, a cationic antibacterial monomer, is related to oxidative stress and the intrinsic mitochondrial apoptotic pathway. Brazil J Med Biol Res. 2011;44:1125–33. https://doi.org/10.1590/S0100-879X2011007500130.
Li F, Weir MD, Fouad AF, Xu HHK. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 2013;41(10):881–91. https://doi.org/10.1016/j.jdent.2013.07.006.
Chai Z, Li F, Fang M, Wang Y, Ma S, Xiao Y, Chen J. The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J Oral Rehabil. 2011;38(11):849–56. https://doi.org/10.1111/j.1365-2842.2011.02212.x.
Chen C, et al. Primer containing dimethylaminododecyl methacrylate kills bacteria impregnated in human dentin blocks. Int J Oral Sci. 2016;8(4):239–45. https://doi.org/10.1038/ijos.2016.43.
Wang Y, Ding Y, Deng J, Nie R, Meng X. Antibacterial one-step self-etching dental adhesive with silver nanoparticles synthesized in situ. J Dent. 2023. https://doi.org/10.1016/j.jdent.2023.104411.
Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. 2009. www.atcc.org
Wang S, et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of streptococcus mutans biofilm. Int J Mol Sci. 2014;15(7):12791–806. https://doi.org/10.3390/ijms150712791.
Aqawi M, Sionov RV, Gallily R, Friedman M, Steinberg D. Anti-bacterial properties of cannabigerol toward streptococcus mutans. Front Microbiol. 2021;12(April):1–15. https://doi.org/10.3389/fmicb.2021.656471.
X. Xu, Y. F. Y. W. S. L. Zezhang T. Wen, Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B Appl Biomater, 29(6), 997–1003, 2012
Collares FM, Leitune VCB, Franken P, Parollo CF, Ogliari FA, Samuel SMW. Influence of addition of [2-(methacryloyloxy)ethyl] trimethylammonium chloride to an experimental adhesive. Braz Oral Res. 2017;31:1–6. https://doi.org/10.1590/1807-3107BOR-2017.vol31.0031.
Namba N, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater. 2009;25(4):424–30. https://doi.org/10.1016/j.dental.2008.08.012.
Aljehani W, et al. An overview of antibacterial dental restorative materials. J Healthcare Sci. 2023;03(02):96–102. https://doi.org/10.52533/JOHS.2023.30201.
Kenawy ER, Abdel-Hay FI, El-Magd AA, Mahmoud Y. Biologically active polymers: VII. Synthesis and antimicrobial activity of some crosslinked copolymers with quaternary ammonium and phosphonium groups. React Funct Polym. 2006;66(4):419–29. https://doi.org/10.1016/j.reactfunctpolym.2005.09.002.
Kenawy E-R, Mahmoud YA-G. Biologically active polymers. Macromol Biosci. 2003;3(2):107–16. https://doi.org/10.1002/mabi.200390016.
Zhou H, Li F, Weir MD, Xu HHK. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J Dent. 2013;41(11):1122–31. https://doi.org/10.1016/j.jdent.2013.08.003.
Gilkerson WR, Ezell JB. Ion-solvent interaction. importance of the dipole moment and basicity of the ligand. J Am Chem Soc. 2002;89(4):808–15. https://doi.org/10.1021/JA00980A013.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Synthesis and characterization of QAC, and preparation of the experimental adhesives were done by MEl-D and El-RK. For the antibacterial testing, adhesive samples were prepared by MEl-D and NA. H, while the preparation of the bacterial cultures and the antibacterial testing were performed by MM. I. The first draft of the manuscript was written by MEL-D and revised by MM. I, El-RK, and NA. H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Deeb, M., Ismail, M.M., Kenawy, ER. et al. Evaluation of antibacterial activity of an experimental dental adhesive containing synthesized quaternary ammonium compound: in vitro study. Discov Appl Sci 6, 120 (2024). https://doi.org/10.1007/s42452-024-05756-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05756-x