Abstract
This paper reviews plants and microorganisms removal of heavy metals from contaminated sites through bioaccumulation. Increased industrial activities have led to the uncontrolled release of metals into the environment, resulting in a global increase in metal pollution. Heavy metals are also consumed from the surface of glasses over a prolonged period of use. Heavy metal pollution is a serious problem that can have wide-ranging and long-lasting impacts on human health and the environment. Therefore, effective removal and remediation of heavy metal pollution are crucial to protect human and ecological health. Traditional methods of heavy metal removal, such as chemical treatment and physical removal, can be costly and can also have negative impacts on the environment. The utilization of plants and microorganisms for bioremediation of metal-polluted environments has proven effective for removing metals through accumulation and/or detoxification. This method is effective, economical, versatile, and environmentally friendly. Bioaccumulation utilizes plants and microorganisms to absorb and remove heavy metals from contaminated sites. This method is not only cost-effective but also helps to minimize the environmental impact of heavy metal pollution. Additionally, bioaccumulation can be used in combination with other techniques, such as phytostabilization and phytodegradation, to further improve the efficiency of heavy metal removal. The paper also discusses the use of plants and microorganisms in the removal of heavy metals from water and soil through biomagnification and bioconcentration. Techniques such as phytoaccumulation, phytostimulation, phytodegradation, phytovolatilization, phytostabilization, and phytofiltration are also discussed as effective ways of remediation of heavy metal contaminated sites.
Article highlights
-
In addition to common heavy metal pollution sources, research has shown heavy metals to be consumed from glass surfaces over prolonged use.
-
The use of plants and microorganisms are cost effective and ecofriendly in the bioaccumulation of heavy metals.
-
Bioaccumulation in combination with techniques like phytostabilization and phytodegradation achieves better heavy metal removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heavy Metal pollution has become a growing global problem for the environment and public health [1]. Heavy metal accumulation has been reported in the atmosphere, the marine environment, and the soil while impacting drinking water sources and raising the hazardous concentration of metal pollutants in foods [2]. The increase in industrialization and urbanization has accelerated the emission and pollution of metal from anthropogenic operations. Mining, smelting and metal treatment, oil and gas, wastewater, road traffic, and waste dumping sites have contributed to metal pollution, deteriorating the environment and human health [3]. In 1963, local people near Minamata Bay, Japan, consumed mercury-contaminated shellfish. The mercury exposure resulted from chemicals released and discharged from a chemical industry near the bay [2]. A high mercury concentrated wastewater dumped into the sea affected marine food chains, including shellfish and other seafood, which built up high mercury quantity that became harmful to people who ate these marine foods [4]. The exponential increase in the various commercial, domestic, agricultural, and technical uses of metal have dramatically increased human exposure [5].
Glasses are employed in a variety of applications and might be regarded as a major source of exposure to heavy metals in the environment. Metal oxides including B2O3-Ag3PO4-ZnO-Na2O [6], Cr2O3 [7], BaO [8] have been synthesized from radiation shielding glasses. An experiment on 72 new and used drinking glasses, including tumblers, wine and beer glasses, and jars, found cadmium and lead present on the glass surfaces and, in some instances, on the rims, with concentration levels of lead sometimes 1000 times higher than the acceptable level [9, 10]. While simulating persistent use, paint flakes frequently came away from the glass, showing that the heavy metals may be consumed over a prolonged period of use.
Heavy metals have also been found in the aquatic environment; they are very soluble in marine environments and are readily consumed by living organisms [11]. They have been reported in the gills, liver, and muscle tissues of different fish species in polluted marine ecosystems [12]. Metals are present in factory wastewater, through which they get to the soil, accumulates over time in soil along wastewater channels and in the organisms that inhabit such channels [13]. Once in the food chain, metals can end up in the human body. Exposure to metals can be through inhaling polluted aerosols and dust particles and the consumption of polluted food and water [11]. There is currently an increase in global concerns about their potential adverse health effects and long-term impacts on biosystems. However, a small quantity of these metals are prevalent in our surroundings, and dietary, when consumed at very low concentration by the living organism, are not harmful because some are essential for good health, including cobalt, iron, copper, and zinc but at higher amount becomes toxic to the ecosystem and to humans [14].
Heavy metals are a class of elements with a high density and are toxic at low concentrations [15]. Metals, once absorbed, can accumulate in the body of a living organism to levels sufficient to become toxic. Because of their toxicity, metals seriously threaten species exposed to such pollutants [16]. Bioaccumulation is the accumulation of pollutants by organisms from dietary sources (trophic transfer) and the abiotic environment (i.e., air, water, soil). As a result, this mechanism involves the sorption of toxins in species quicker than their removal [17]. Bioaccumulation with plants and microorganisms can be harnessed for bioremediation of sites polluted with metals pollutants [16]. Bioaccumulation is in two stages: the metal ion adsorption onto cell surface known as biosorption method, and the metal species active transport within the cells [18].
Metals can be taken up into the intracellular space by microorganisms via importer complexes that form a lipid bilayer translocation route (i.e., import system).In addition, proteins and peptide ligands can sequester metals inside the intracellular space [19]. The ability of microorganisms to accumulate, use and eliminate pollutants is determined by the environmental conditions’ suitability for their metabolism and growth, such as pH, moisture and temperature [20]. The accumulation and concentration of pollutants from a polluted environment by plant roots are known as phytoaccumulation. The contaminants are subsequently translocated and deposited in the aerial parts of the plant (leaves and shoots). Pollutant absorption by plant roots, accompanied by translocation and accumulation in shoots and leaves, is referred to as phytoabsorption, phytosequestration, or phytoaccumulation [21]. Plants’ aerial portions (shoots and leaves) can be harvested and burnt for energy, with metals reclaimed from the ash as recycling. This approach has been widely used for phytoremediation and elimination of metals such as lead, zinc, copper, nickel, and cadmium utilizing plants such as sunflower and Thlaspi caerulescens [22].
Bioremediation is progressively becoming the normal approach for restoring heavy-metal-polluted soils since it is much more environmentally friendly and cost-effective than traditional physical and chemical treatments, which are typically very expensive and unsuccessful when the concentrations of the metal pollutants are low, as well as producing considerable levels of toxic sludge [16, 23]. The impacts of metals on the environment are discussed in this paper, and how metal pollution can be effectively remediated through bioaccumulation by microorganisms and plants. The advantages, potential, and limitations of bioaccumulation are also highlighted.
2 Heavy metals
Heavy Metals are elements that occur naturally with a high atomic mass and a density at least five times that of water [24]. Metals have gained much attention in the recent decade as potential environmental pollutants. Lead, cadmium, vanadium, cobalt, chromium, copper, iron, arsenic, nickel, manganese, tin, zinc, and mercury are some examples that have been reported in various literature [25, 26]. Heavy Metals such as chromium, cadmium, mercury, lead, nickel, and thorium can be dangerous in elemental or combined form [11]. The toxicity levels of several selected metals for humans are as follows: Cobalt ˂ Aluminium ˂ Chromium ˂ Lead ˂ Nickel ˂ Zinc ˂ Copper ˂ Cadmium ˂ Mercury [27]. Metals toxicity in humans is determined by their concentration, emission rate, and duration of exposure. Mercury, Cadmium, and Lead are three metals which have attracted increased attention in recent decades.
Most of these metals pollutants are easily stored in plants, through which they enter the food chain, and are passed to humans, causing catastrophic illnesses and diseases [28]. High concentrations of metals may interchange essential ions via antagonism in enzymes and/or chlorophyll, resulting in oxidative stress. The major indications of metal toxicity are reduced plant growth and a decrease in photosynthetic activity [29]. Some metals have been confirmed to be mutagenic, carcinogenic, teratogenic, allergenic, or endocrine disrupting. They have also been reported to be hepatotoxic and/or nephrotoxic and its detrimental impacts on the immune system can lead to heart rhythm problems, neurological and behavioural changes, particularly in children, as well as damage to the bone marrow, central nervous system, and causing osteoporosis [24].
3 Sources of heavy metals
Geogenic, mining, agricultural, chemical, domestic effluents, and atmospheric sources have all been identified as sources of different metals in the environment [30]. Point sources of emissions, including mining, foundries, smelters, as well as other metal-based manufacturing activities, are particularly polluted with metals. Heavy metal pollution can also be caused by rapidly developing industrial areas, high metal waste disposal, mine tailings, leaded gasoline and paints, application of fertilizer, pesticides, sewage sludge, animal manures, wastewater drainage, combustion of coal residues, atmospheric deposition and petrochemical spillage [31]. Scientists classified metal sources into natural and anthropogenic categories (Fig. 1).
4 Conventional methods for removal of heavy metal
The advancement of technologies for eliminating metals from the environment has been a focus for many years. Ultra-filtration, chemical precipitation, solvent extraction, reverse osmosis, electrodeposition, ion exchange electrowinning, and adsorption have been the most commonly used procedures for eliminating metals [32, 33].
Techniques such as electrocoagulation, coagulation/flocculation, electro-deposition, and electro-floatation have also been employed to remove metals from polluted water resources in addition to these traditional methods [17, 34]. However, systems as mentioned above have drawbacks such as sludge production, inadequate metal removal, high reagent and energy needs, membrane fouling and aggregation of metal precipitates. There have been issues of efficacy with these methods when faced with low metal concentrations, low metal selectivity, and high operating or start-up costs. These procedures may be costly or ineffective, especially where metal ions are in solutions containing between 1 and 100 mg of dissolved metal ions per liter [35].
5 Bioremediation
Since the beginning of the 21st century, there has been a growing awareness of the need to safeguard the natural environment. Biotechnological methods, which rely on the inherent characteristics of microbes and plants to absorb and accumulate metals, have proven viable alternative to physicochemical methods [36]. Bioremediation, particularly phytoremediation procedures, is used as a simple and appealing alternative technology due to its low energy, low cost, and high efficiency [21].
Bioremediation is a process that detoxifies pollutants in soil and other environments primarily by using microbes, plants, or microbial or plant enzymes. It employs biological mechanisms inherent in plants and microorganisms to eliminate harmful pollutants and return the environment to its natural or original state [37]. Microbes mobilize the metals from the polluted environment through chelation, leaching, redox transformation of harmful metals, and methylation. Eliminating these metal ions cannot be absolute; rather, the activity changes the oxidation state and makes it less harmful [38]. Plants play an essential function in the biological process of phytoremediation because they break down, reduce, degrade, and eliminate pollutants through various plant parts, including the leaves, root, stomata, shoot, and cell wall [39]. Bioremediation efficiency depends on some parameters, including shortage of essential nutrients like nitrogen and phosphorus [40], temperature, pH, contact time of biomass, age of biomass [41], the type of organisms used, environmental conditions at the polluted site and the concentration of the pollutant [42].
The key principles of bioremediation are to lower the solubility of pollutants in the environment by altering pH, redox processes, and pollutant adsorption from a polluted environment [43]. Redox processes involve chemical transformation of hazardous pollutants to less hazardous, more stable, less mobile, or inactive substances [44, 45]. The pH values are an important characteristic that affect pollutant adsorption, with adsorptive capacity increasing with decreasing pH [16].
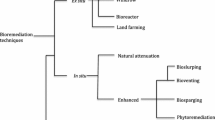
Metals can be captured and accumulated from an aqueous solution by all microbial species. Metal absorption is linked to a microbial mechanism that allows for the uptake of components essential for growth and metabolic functions. The ability of biomass to bind and accumulate toxic metals can be used in the development of efficient and cheap technology for removing environmental pollutants [36]. Summarily, Plants and Microbes can be employed in the biological methods for the removal of metals (Fig. 2).
6 Bioaccumulation
The procedure by which living organisms absorb pollutants can either be through direct contact with polluted media or indirectly through the ingestion of food containing the pollutant [46]. Bioaccumulation occurs when the amount of pollutant absorption surpasses the pollutant removal rate. As a result, the pollutant becomes trapped inside the organism and accumulates [47]. Heavy metal bioaccumulation is the process through which toxic metals or chemical compounds become bonded inside a cell structure. The bioaccumulation of metal is influenced by several exposure routes (diet and solution) and geochemical impacts on bioavailability. Bioaccumulation of metals is particularly useful as an exposure indicator since metals are not metabolized.
On the other hand, bioaccumulation is a helpful integrative indicator of organisms’ exposure to chemical exposure in polluted environments [48]. The bioactivity of biomass is vital in the process of bioaccumulation. Cells must be alive to absorb pollutants through metabolic processes [47]. During bioaccumulation, metal ions are absorbed by the entire cell. Metals enter the cells of living organisms via the same pathways as nutrients do. Unicellular organisms absorb metals and the essential nutrients they need to survive, such as calcium and magnesium.
There are two stages of bioaccumulation. In the first stage, metal ions are attached to the cell surface. This first stage is metabolically inactive. Metal ions are then carried into the cell. This procedure’s second stage is only achievable when the cells are metabolically active. The amount of biomass increases if optimal conditions for organism growth are maintained in the second stage. This enables the binding of more significant amounts of metal ions [36].
7 Biomagnification
Biomagnification is a condition in which the concentration of the pollutant in an organism surpasses the concentration of its food when the organism’s diet is the primary exposure route. Food web biomagnification is the progressive increase in pollutant concentrations with growing animal trophic status and is used to explain the trophic accumulation of pollutants within food webs [49]. Contaminants enter the food web through bioaccumulation, which occurs when they accumulate in an organism. In contrast, biomagnification occurs when they are transferred from a trophic level to another (and thus increase concentration) in a food web.
8 Bioconcentration
Bioconcentration is an organism’s ability to concentrate a chemical from its surroundings [50]; it is the aggregation of a material due to passing from one phase to another [51]. Bioconcentration occurs when the amount of a chemical within an organism exceeds that of its environment (air or water). In contrast, biomagnification occurs when the chemical or pollutant concentration increases as it advances through one trophic level to another.
9 Microbial bioaccumulation of heavy metals
Microbial bioaccumulation of metal is an active process. Various microorganisms, including bacteria, algae, and fungi, have been used to bioaccumulate metals from polluted environments [52]. Microorganisms used for bioaccumulation should be tolerant to one or more pollutants at increasing levels. Furthermore, they may possess biotransformational abilities, converting the harmful chemical to a less toxic or nontoxic form, allowing the organism to minimize the pollutants toxicity and keeping it contained [19]. Many environmental microbial species exhibit high metal accumulation levels in cell walls or the areas bounded by cytoplasm. This deposit might account for up to 6% of the cell dry mass, and when examined in the context of the water or soil environment, this event can result in a temporary decrease in the concentrations of metal ions [53].
Zolgharnein et al. [54] demonstrated the uptake of metal ions - copper, zinc, cadmium, and lead by Pseudomonas aeruginosa. They also stated that metal uptake by bacteria is a combination of surface phenomena and diffusion. Pseudomonas putida accumulated cadmium intracellularly and periplasmically, indicating the presence of metal binding and/or efflux systems within the cells that mediate resistance to toxicity of metals [55]. Metal ion buildup in the cytoplasm [56] and periplasm [57] has been described in E. coli because of metallothioneins expression. Bacillus can accumulate lead (Pb) with an efficiency of 57% [58]. Tsukamurella paurometabola, Cupriavidus taiwanensis, and Pseudomonas aeruginosa have been used in the bioaccumulation of Cd and Zn. Limcharoensuk [59] experiment showed that Zn2+ and Cd2+ were adsorbed on these bacteria cell walls. According to Aslam et al. [60], indigenous bacterial strains (Klebsiella pneumoniae, Stenotrophomonas sp., and Staphylococcus sp.) have the potential to accumulate metals (Chromium, Nickel, Lead) in the remediation of a polluted environment. These bacteria were able to tolerate 700–1000 µg/ml of Nickel, 500–1000 µg/ml of Chromium, and 1000–1600 µg/ml of Lead. The metal binding ability of the E. coli cell wall has also been demonstrated [61, 62].
Different fungal species (Aspergillus tereus, Fusarium oxysporum, Cladosporium cladosporioides, Penicillium spp., Gliocladium roseum, Trichoderma koningii, and Talaromyces helices) have been studied for their metal bioaccumulation potentials [63]. Paecilomyces marquandii has shown a more significant influence on growing and eliminate zinc. During growth medium incubation, the zinc level of P. marquandii mycelium reached 10–20 mg/g dry weight. Fusarium flocciferum fungus can absorb copper, cadmium, and nickel. Living mycelium pellets of Phanerochaete chrysosporium fungi adsorb copper cation (II) in aqueous solutions [63]. The strain of Trichoderma atroviride isolated from sewage sludge can tolerate metals, including copper, cadmium and zinc and ingest these elements. This fungus can survive at high metal concentrations, which appears to be due to the natural selection of resistant cells [64].
10 Heavy metal toxicity to microorganisms
Heavy Metals can alter the biochemical and physiological characteristics of microorganisms, chromium and cadmium can cause denaturation and oxidative damage in microbes, as well as reduce the ability of microorganisms to absorb and eliminate metals [52]. Heavy metals can be toxic to microorganisms, causing DNA structural destruction and damage to the cell membrane. Heavy metals displacements from their native binding sites or ligand interactions cause this toxicity to microbes [65]. Changing the structure of the nucleic acid, producing functional disruption altering cell membranes limiting the activity of enzymes and oxidative phosphorylation all impact the morphology, metabolism and development of microorganisms [66]. Copper has been reported to damage DNA lipids, cytoplasmic molecules, and other proteins [67]. Aluminum (Al) can stabilize superoxide radicals, which damages the DNA[68]. Metals may disrupt critical enzymatic processes by causing configurational changes in enzymes through competitive or non-competitive substrates interactions [69]. Metals can also produce ion imbalance by sticking to the surface of cells and gaining entrance into cells through transmembrane carriers or ion channels [70].
11 Phytoaccumulation
Phytoaccumulation is a phytoremediation technique that have been used in the treatment of heavy metal contaminated sites by different researchers [31, 71,72,73]. Phytoaccumulation is of important scientific study because of its utilization in phytoprospection (metal ores are detected through the local flora investigation), phytostabilization (minimizing the concentration of soil pollutants by root sequestration), phytoremediation (metal removal from soil pollutants), or phytomining (application of plant to mine relevant beneficial metals from polluted soil [74]. Phytoaccumulation involves using plants to absorb pollutants from water or the soil, then translocate and accumulate these pollutants in the aboveground plant biomass [75]. The phytoaccumulation of metals involves several steps: (i) metal mobilization in the rhizosphere, (ii) uptake of metal by plant roots, (iii) translocation of metal ion from roots to the aerial parts of the plant, and (iv) sequestration and compartmentation of metal ion in plant tissues [76]. Phytoaccumulation has been the most popular phytoremediation approach for removing metals and metalloids pollutants from soil in recent years [77]. Several factors influence phytoaccumulation efficiency, including (a) plant type used, (b) the level of plant tolerance to high metal concentrations, and (c) the ability of plants to rapidly absorb and move metals from roots to the exposed surfaces above the root [16].
Paulo et al. [71] commented on the potential of phytoaccumulation for high efficiency and potential economic benefit in the recovery of metal and energy production. Continuous phytoextraction and Induced phytoextraction are the two approaches used in phytoaccumulation. Continuous phytoextraction takes advantage of indigenous plants that can naturally collect high levels of metals (hyper-accumulators). Induced phytoextraction increases the accumulation of metals by plants using chemical compounds such as chelates to improve metal bioavailability and root uptake [28]. Chelating agents including aminocarboxylic acids and synthetic carboxylic and their salts are used [78]. Soil-applied sorbent materials promote partial immobilization of metal ions in the environment. Antonkiewicza and Para [78] revealed that applying dihydrazone to a heavy metal-polluted soil increased plant absorption of Cd, Cu, Pb, and Zn, implying that this compound might be used in the phytoremediation of these metal pollutants from the soil.
Plants employed in phytoaccumulation should generally have the following characteristics: tolerance to high metal concentrations, rapid growth, a high biomass yield, a root system that is extensive, easy to cultivate and harvest, high extraction ability with high metal accumulation in aerial tissues, very resistant to pests and infections, and be unattractive to herbivores (so as to prevent metals from entering the food chain) [76, 79, 80]. For phytoremediation, preference is given to trees ahead of crop plants because they are not edible, which implies that metals are less likely to enter the food chain [81].
Over 450 species of plants from at least 45 angiosperm families, from annual herbs to perennial trees and shrubs, have been identified as metal hyperaccumulators, including Fabaceae, Brassicaceae, Asterraceae, Euphorbiaceae, and Lamiaceae [82]. Phytoaccumulation potential of some plants has been demonstrated as follows: Yang et al. [83] showed the potential of three Napier grass cultivars (Pennisetum purpureum) in the absorption of cadmium and zinc in field experiments. Ghazaryan et al. [84] examined Melilotus officinalis and Amaranthus retroflexus ability to remediate copper and molybdenum polluted soils. Their findings revealed that A. retroflexus accumulate copper and molybdenum in the shoots, whereas M. officinalis prefers zinc storage in the roots. After 2 months before transplantation in field settings, Jacobs et al. [75] discovered zinc concentrations in the leaves of Noccaea caerulescensis exceeding 300 g Cadmium ha−1. Khalid et al. [85] used pot tests to investigate Alternanthera bettzickiana’s phytoaccumulation capacity to Nickel and Copper, finding that after 8 weeks of treatment, A. bettzickiana accumulated two times more copper in shoots than the control. Fourati et al. [86] found that nickel was deposited at higher concentrations in the aboveground section of Sesuvium portulacastrum. Trifolium alexandrinum was used as a promising option for phytoextraction of cadmium, lead, copper, and zinc due to its rapid growth, high biomass, resilience to pollution, and several harvests in one single growing season [87]. The successful outcome and effectiveness of phytoremediation rely on the plant’s root and shoot structures, the root plant’s accumulation rate, the existence of root activity facilitating various operation and microbial synthesis groups, and interactivities in the rhizosphere [88]. Thus, study on phytoremediation methods has drawn more attention to other areas on selection, root and shoot biomass, soil management and advancement of bioavailability, uptake of pollutants and degradation potentiality, conventional breeding, genetic engineering to multiple pollutant tolerance, and genetic engineering of microbes [88].
12 Limitations of phytoaccumulation
A plant’s capacity to tolerate high metal concentrations may result in metal accumulation in the harvestable part; this is a problem because of the possible pollution of the food chain [89]. If pollutant concentrations are too high, plants may die. When harvested plants contain large quantities of metals, disposal can be a challenge [90].
Most plant hyperaccumulators are slow growers with little plant biomass, which decreases the efficiency of the metal accumulation [82]. Thus, to boost the efficiency of phytoaccumulation, plants with high growth rates and biomass (e.g., maize, sorghum, and alfalfa) are occasionally utilized in soil remediation exercises with metal-chelating compounds [91].
Chelating compounds limit precipitation and metal sorption by forming metal chelate complexes, increasing metals’ bioavailability. One significant limitation of employing chelates in phytoaccumulation is the possibility of metal leakage into groundwater. This is because the application of chelates increases metals availability in the soil solution. Furthermore, chelates can be harmful to plants and soil microorganisms when employed in high amounts [91].
In most situations, plants absorb metals readily available in soil solutions. Although some metals are soluble in plant absorption forms, others are insoluble and hence not available for plant uptake [92]. Treatment is limited due to the depth of the pollutants. Plant root depth determines the treatment zone. It usually only affects shallow soils, groundwater and streams. However, researchers discovered that using trees (instead of smaller plants) allows them to remediate more profound pollution since the roots of trees can reach more soil depth [90].
Depending on the location, phytoaccumulation success may be seasonal. Other climatic factors will have an impact on its efficacy. As a result, phytoremediation is often restricted to areas with a low concentration of pollutants and areas with pollution in shallow soils, groundwater, and streams [90].
In general, the solubility/availability of metals for plant absorption and the suitability of a location for phytoaccumulation are additional elements to consider (along with plant suitability) before adopting phytoaccumulation for soil remediation [93].
13 Phytostimulation
Phytostimulation is the degradation of the soil’s contaminants with the increased activity of microorganisms in the plant’s rhizosphere [94]. This technique may also improve biodegradation in the rhizosphere. The root system is associated with symbiotic soil plants and microbes in phytostimulation for the degradation of contaminants. Rhizodegradation is another term for phytostimulation. Microbial activity in the root system has been stimulated in several ways. Roots containing indigenous microbial species exude compounds such as acetates, amino acids, carbohydrates and enzymes [95]. Oxygen is carried to the rhizosphere by the root system for aerobic transformation [96]. Mycorrhizal fungi, due to unique enzymatic pathways degrade organic contaminants that bacteria cannot transform [97]. The availability of organic carbon is increased by root biomass [98], and plants improve the habitat and activity of microbial populations.
14 Phytodegradation
In phytodegradation, plants store, absorb, and degrade contaminants within their tissues [99]. Phytodegradation, also referred to as phytotransformation, is a phytoremediation process where contaminants are degraded within plants by the metabolic processes that occur in plants [100]. A few organic pollutants are beneficial to plant growth. Such organic pollutants are first reduced into simpler forms before being incorporated into plant tissues, thereby increasing plant growth [101].
15 Phytovolatilization
In phytovolatilization, plants adsorb pollutants from the growth matrix and volatilize the contaminants into the atmosphere in volatile form via transpiration [102]. Phytovolatilization occurs when plants absorb water and organic pollutants. Water travels through the plants vascular system from the plant roots to the leaves, the pollutants are changed and modified along this path. Phytovolatilization has been used to remove toxic mercury by converting it into a less toxic elemental mercury [102]. It has also been used to remove Arsenic from polluted soils [103].
16 Phytostabilization
Phytostabilization essentially reduces the mobility of pollutants in the soil and their bioavailability to the food chain [104]. Soil amendments such as alkalizing agents, biosolids, organic matter, and phosphates are used to reduce metal solubility in the soil and their leaching to groundwater [105], as well as to prevent their transport. In phytostabilization, plants can change the pollutant factor’s form into non-resoluble or non-transportable in water. Phytostabilization has been employed to remove cadmium and mercury [106].
17 Phytofiltration
Phytofiltration is precipitating or adsorbing pollutants into the roots constituting the root system [107]. The mechanism of phytofiltration is related to the synthesis of some chemicals within the root system, which cause pollutant to be adsorbed since plants may contain phytochelatins to enhance the adsorption affinity of pollutants such as metal ions [108]. Phytofiltration is also known as rhizofilteration. Plant roots are used to degrade contaminants by storing or taking pollutants from an aqueous growth matrix or filtering pollutants from wastewater, surface water and groundwater through roots [109]. This method allows for the use of both aquatic and terrestrial plants. Aside from the natural environment, phytofiltration is also employed in ponds, tanks and basins [110, 111].
18 Conclusion
Bioremediation processes involving the bioaccumulation of metals by plants and microorganisms have gained significant attention in recent years due to their potential in cleaning and recovering metals. However, it is essential to thoroughly investigate the potential of these organisms and conduct comprehensive studies to fully utilize their ability to alleviate heavy-metal pollution. Various physicochemical parameters such as temperature, pH, biomass contact time, and concentration should be taken into account when using plants and microorganisms for bioremediation. It is essential to determine the fate of pollutants collected in tree leaves and wood, and to ensure that plant droppings and products do not introduce toxic compounds to the food chain and cause any negative impact on human or animal health.
Additionally, it is important to consider the impact of bioremediation on the ecosystem and wildlife. Plants and microorganisms used for bioremediation should be non-invasive and native to the area. The risk of transferring pollutants from the contaminated site to a non-contaminated area should also be avoided.
Overall, more research is needed to fully understand and optimize the use of plants and microorganisms in bioremediation. This includes identifying the most effective organisms and conditions for bioremediation, as well as developing safe and sustainable methods for managing and disposing of pollutants collected by plants and microorganisms.
Data availability
Not applicable.
References
Masindi V, Muedi KL (2018) Environmental contamination by Heavy Metals. Heavy Met. https://doi.org/10.5772/intechopen.76082
Siddiquee S, Rovina K, Azad SA, Naher L, Suryani S, Chaikaew P (2015) Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: a review. J Microb Biochem Technol 7(6):384–395. https://doi.org/10.4172/1948-5948.1000243
Armah FA, Obiri S, Yawson DO, Onumah EE, Yengoh GT, Afrifa EK, Odoi JO (2010) Anthropogenic sources and environmentally relevant concentrations of heavy metals in surface water of a mining district in Ghana: a multivariate statistical approach. J Environ Sci Health Part A 45(13):1804–1813. https://doi.org/10.1080/10934529.2010.513296
Tarekegn MM, Salilih FZ (2020) Ishetu AI (2020) Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agricult 10(1080/23311932):1783174
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy Metals and Pesticides toxicity in agricultural soil and plants: ecological risks and Human Health Implications. Toxics 9:42. https://doi.org/10.3390/toxics9030042
Alrowaili ZA, Taha TA, Ibrahim M, Saron KMA, Sriwunkum C, Al-Baradi AM, Al-Buriahi MS (2021) Synthesis and characterization of B2O3-Ag3PO4-ZnO-Na2O glasses for optical and radiation shielding applications. Optik 248:168199. https://doi.org/10.1016/j.ijleo.2021.168199
Al-Buriahi MS, Alajerami YSM, Abouhaswa AS, Alalawi A, Nutaro T, Tonguc B (2020) Effect of chromium oxide on the physical, optical, and radiation shielding properties of lead sodium borate glasses. J Non-cryst Solids 544:120171. https://doi.org/10.1016/j.jnoncrysol.2020.120171
Al-Buriahi MS, Sriwunkum C, Arslan H, Tonguc BT, Bourham MA (2020) Investigation of barium borate glasses for radiation shielding applications. Appl Phys A 126(1):1–9. https://doi.org/10.1007/s00339-019-3254-9
Turner A (2018) High levels of migratable lead and cadmium on decorated drinking glassware. Sci Total Environ 616:1498–1504. https://doi.org/10.1016/j.scitotenv.2017.10.164
Turner A (2019) Heavy metals in the glass and enamels of consumer container bottles. Environ Sci Technol 53(14):8398–8404. https://doi.org/10.1021/acs.est.9b01726
Kinuthia GK, Ngure V, Beti D, Lugalia R, Wangila A, Kamau L (2020) Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep 10:8434. https://doi.org/10.1038/s41598-020-65359-5
Sobhanardakani S, Tayebi L, Farmany A (2011) Toxic metal (pb, hg, and as) contamination of muscle, gill and liver tissues of Otolithes ruber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson and Onchorynchus mykiss. World Appl Sci J 14(10):1453–1456
Kalavrouziotis IK, Koukoulakis P (2016) Wastewater and sludge reuse management in agriculture. EQA-Environmental Qual 20:1–13. https://doi.org/10.6092/issn.2281-4485/6303
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer Sci Bus Media. https://doi.org/10.1007/978-3-540-32714-1_19
Koller M, Saleh HM (2018) Introductory Chapter: Introducing heavy metals, heavy metals. https://doi.org/10.5772/intechopen.97198
Ojuederie OB, Babalola OO (2017) Microbial and Plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504. https://doi.org/10.3390/ijerph14121504
Kanamarlapudi SRK, Chintalpudi VK, Muddada S (2018) Application of biosorption for removal of heavy metals from wastewater. https://doi.org/10.5772/intechopen.77315
Mrvčić J, Stanzer D, Šolić E, Stehlik-Tomas V (2012) Interaction of lactic acid bacteria with metal ions: opportunities for improving food safety and quality. World J Micro-biology Biotechnol 28:2771–2782. https://doi.org/10.1007/s11274-012-1094-2
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43(11):1162–1222. https://doi.org/10.1080/10934529.2011.627044
Verma JP, Jaiswal DK (2016) Book review: advances in biodegradation and bioremediation of industrial waste. Front Microbiol 6:1555. https://doi.org/10.3389/fmicb.2015.01555
Abdel-Shafy HI, El-Khateeb MA, Shehata M (2017) Blackwater treatment via combination of sedimentation tank and hybrid wetlands for unrestricted reuse in Egypt. J Desalin Water Treat 71:145–151. https://doi.org/10.5004/dwt.2017.20538
Vander Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyper-accumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334. https://doi.org/10.1007/s11104-012-1287-3
Ekperusi OA, Aigbodion FI (2015) Bioremediation of petroleum hydrocarbons from crude oil-contaminated soil with the earthworm: Hyperiodrilus africanus. 3 Biotech 5(6):957–965. https://doi.org/10.1007/s13205-015-0298-1
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Molecular, Clinical and Environmental Toxicology. Springer, Basel, pp 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Ebadi GA, Hikmat H (2018) Physicochemical characterization of sediments from Tajan river basin in the northern Iran. Toxicol Environ Chem 100(5–7):540–549. https://doi.org/10.1080/02772248.2018.1460929
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett 16(3):903–917. https://doi.org/10.1007/s10311-018-0734-7
Mansourri G, Madani M (2016) Examination of the level of heavy metals in wastewater of Bandar Abbas Wastewater Treatment Plant. Open J Ecol 6(2):55–61. https://doi.org/10.4236/oje.2016.62006
Nedjimi B (2021) Phytoremediation: a sustainable environmental technology for heavy metals decontamination. Appl Sci 3:286. https://doi.org/10.1007/s42452-021-04301-4
Shi GL, Zhu S, Bai SN, Xia Y, Lou LQ, Cai QS (2015) The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. J Hazard Mater 299:94–102. https://doi.org/10.1016/j.jhazmat.2015.06.009
Singh R, Ahirwar NK, Tiwari J, Pathak J (2018) Review on sources and effect of Heavy Metal in Soil: its bioremediation. Int J Res Appl Natural Soc Sci 2008:1–22
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Notices, vol. 2011, p. 20 https://doi.org/10.5402/2011/402647
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2016) Removal of heavy metals from industrial wastewaters: a review. Chem Biochem Eng Reviews 4:37–59. https://doi.org/10.1002/cben.201600010
Joshi NC (2017) Heavy metals, conventional methods for heavy metal removal, biosorption and the development of low cost adsorbent. Eur J Pharm Med Res 4:388–393
Un UT, Ocal SE (2015) Removal of heavy metals (cd, Cu, Ni) by electrocoagulation. Int J Environ Sci Dev 6:425. https://doi.org/10.7763/IJESD.2015.V6.630
Singh M, Verghese S (2016) Conventional and innovative techniques for removal of heavy metals from electroplating industry waste water. Int J Eng Sci Res Technol 5(10):150–159. https://doi.org/10.22192/ijcrcps.2016.03.09.006
Zabochnicka-Świątek M, Krzywonos M (2014) Potentials of Biosorption and Bioaccumulation processes for heavy metal removal. Pol J Environ Stud 23(2):551–561
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94. https://doi.org/10.3390/ijerph14010094
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol 77(3):229–236. https://doi.org/10.1016/S0960-8524(00)00108-5
Sharma S (2012) Bioremediation: features, strategies and applications. Asian J Pharm Life Sci 2(2):202–212
Nnaji ND, Ughamba KT, Aduba CC, Ogbonna KE, Anyanwu CU (2020) Potato skin: a potential biostimulating agent for used motor oil biodegraders. Int J Environ Agric Biotechnol 5(2):296–309. https://doi.org/10.22161/ijeab.46.44
Shamim S (2018) Biosorption of Heavy Metals, Biosorption, In: Jan Derco and Branislav Vrana (Eds) IntechOpen. https://doi.org/10.5772/intechopen.72099
Freitas EV, Nascimento CW, Souza A, Silva FB (2013) Citric acid-assisted phytoextraction of lead: a field experiment. Chemosphere 92:213–217. https://doi.org/10.1016/j.chemosphere.2013.01.103
Jain S, Arnepalli D (2016) Biominerlisation as a remediation technique: A critical review. In Proceedings of the Indian Geotechnical Conference (IGC2016), Chennai, India, 15–17 December 2016. https://doi.org/10.1007/978-981-13-0899-4_19
Ajiboye TO, Oyewo OA, Onwudiwe DC (2021) Conventional and current methods of toxic metals removal from Water using gC 3 N 4-Based materials. J Inorg Organomet Polym Mater 31:1419–1442. https://doi.org/10.1007/s10904-020-01803-3
Tandon PK, Singh SB (2016) Redox processes in water remediation. Environ Chem Lett 14:15–25. https://doi.org/10.1007/s10311-015-0540-4
US Environmental Protection Agency (USEPA) (2010) Solid waste and emergency response glossary—Bioaccumulation: US Environmental Protection Agency. https://setac.onlinelibrary.wiley.com/doi/epdf/10.1002/ieam.1690
Chojnacka K (2010) Biosorption and bioaccumulation – the prospects for practical applications. Environ Int 36(3):299. https://doi.org/10.1016/j.envint.2009.12.001
Mondal K, Ghosh S, Haque S (2018) A review on contamination, bioaccumulation and toxic effect of cadmium, mercury and lead on freshwater fishes. Int J Zool Stud 3(2):153–159
Drouillard KG (2008) Biomagnification. In: Jorgensen SE, Fath BD (eds) Ecotoxicology vol 1 of encyclopedia of ecology, vol 5. Elsevier, Oxford, pp 441–448
Peake BM, Braund R, Tong AY, Tremblay LA (2016) Impact of pharmaceuticals on the environment. Life-Cycle Pharm Environ 5:121
Schmitz KS (2018) Chapter 4 Life Science. Physical Chemistry. Elsevier Science Publishing Co Inc, Amsterdam, Amsterdam, pp 784–785
Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018) Toxicity and bioremediation of heavy Metals contaminated ecosystem from Tannery Wastewater: a review. J Toxicol. https://doi.org/10.1155/2018/2568038
Kisielowska E, Hołda A, Niedoba T (2010) Removal of heavy metals from coal medium with application of biotechnological methods. Górnictwo I Geoinzynieria 34:93–104
Zolgharnein H, Karami K, Assadi MM, Sohrab DA (2010) Investigation of heavy metals biosorption on Pseudomonas aeruginosa strain MCCB 102 isolated from Persian Gulf. Asian J Biotechnol 2(2):99–109. https://doi.org/10.3923/ajbkr.2010.99.109
Rani A, Souche YS, Goel R (2009) Comparative assessment of in situ bioremediation potential of cadmium resistant acidophilic Pseudomonas putida 62BN and alkalophilic Pseudomonas monteilli 97AN strains on soybean. Int Biodeterior Biodegradation 63:62–66. https://doi.org/10.1016/j.ibiod.2008.07.002
Yoshida N, Kato T, Yoshida T, Ogawa K, Yamashita M, Murooka Y (2002) Bacterium-based heavy metal biosorbents: enhanced uptake of cadmium by E. coli expressing a metallothionein fused to b-galactosidase. Biotechniques 32:551–556. https://doi.org/10.2144/02323st08
Naz N, Young HK, Ahmed N, Gadd GM (2005) Cadmium accumulation and DNA homology with metal resistance genes in sulfate-reducing bacteria. Appl Environ Microbiol 71:4610–4618. https://doi.org/10.1128/AEM.71.8.4610-4618.2005
Arifiyanto A, Apriyanti FD, Purwaningsih P, Kalqutny SH, Agustina D, Surtiningsih T, Shovitri M, Zulaika E (2017) Lead (Pb) bioaccumulation; genera Bacillus isolate S1 and SS19 as a case study. AIP Conf Proc. https://doi.org/10.1063/1.4985394
Limcharoensuk T, Sooksawat N, Sumarnrote A, Awutpet T, Kruatrachue M, Pokethitiyook P, Auesukaree C (2015) Bioaccumulation and biosorption of Cd2+ and Zn2+ by bacteria isolated from a zinc mine in Thailand. Ecotoxicol Environ Saf 122:322–330. https://doi.org/10.1016/j.ecoenv.2015.08.013
Aslam F, Yasmin A, Sohail S (2020) Bioaccumulation of lead, chromium, and nickel by bacteria from three different genera isolated from industrial effluent. Int Microbiol 23:253–261. https://doi.org/10.1007/s10123-019-00098-w
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15:65. https://doi.org/10.1186/s12951-017-0308-z
Hoque E, Fritscher J (2019) Multimetal bioremediation and biomining by a combination of new aquatic strains of Mucor hiemalis. Sci Rep. https://doi.org/10.1038/s41598-019-46560-7
Mathew BB, Beeregowda KN, Krishnamurthy TP (2015) Bioaccumulation of heavy metals by fungi. Int J Environ Chem Chromatogr 1(1):15–21
Errasquın EL, Vazquez C (2003) Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 50(1):137–143. https://doi.org/10.1016/S0045-6535(02)00485-X
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14(5):10197–10228. https://doi.org/10.3390/ijms140510197
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047. https://doi.org/10.3390/ijerph13111047
Giner-Lamia J, López-Maury L, Florencio FJ, Janssen PJ (2014) Global transcriptional profiles of the copper responses in the cyanobacterium synechocystis sp. PCC 6803. PLoS ONE 9(9):e108912. https://doi.org/10.1371/journal.pone.0108912
Booth SC, Weljie AM, Turner RJ (2015) Metabolomics reveals differences of metal toxicity in cultures of Pseudomonas pseudoalcaligenes KF707 grown on different carbon sources. Front Microbiol 6:827. https://doi.org/10.3389/fmicb.2015.00827
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal-PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269. https://doi.org/10.1016/j.aquatox.2014.05.026
Chen S, Yin H, Ye J, Peng H, Liu Z et al (2014) Influence of co-existed benzo[a]pyrene and copper on the cellular characteristics of Stenotrophomonas maltophilia during biodegradation and transformation. Bioresour Technol 158:181–118. https://doi.org/10.1016/j.biortech.2014.02.020
Paulo JCF, Pratas J, Varun M, D’Souza R, Paul SM (2013) Phytoremediation of soils contaminated with metals and metalloids at mining areas: potential of native flora. https://doi.org/10.5772/57469
Abdel-Shafy HI, Mansour SM (2018) Phytoremediation for the Elimination of Metals, Pesticides, PAHs, and Other Pollutants from Wastewater and Soil. https://doi.org/10.1007/978-981-13-1187-1_5
Ayuba DK, Tella IO, Oluwole M, Aliba N (2020) Phytoextraction and Phytoaccumulation Potentials of Chrysopogonzizanioides (L. Roberty) and Eucalyptus camaldulensis (Dehnh) in the Bioattenuation of petroleum hydrocarbon polluted soil. IOSR J Environ Sci Toxicol Food Technol 14(8):28–37. https://doi.org/10.9790/2402-1408022837
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Jacobs A, Drouet T, Noret N (2018) Field evaluation of cultural cycles for improved cadmium and zinc phytoextraction with Noccaea caerulescens. Plant Soil 430:381–394. https://doi.org/10.1007/s11104-018-3734-2
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals – concepts and applications. Chemosphere 91:869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721. https://doi.org/10.1016/j.chemosphere.2016.12.116
Antonkiewicz J, Para A (2016) The use of dialdehyde starch derivatives in the phytoremediation of soils contaminated with heavy metals. Int J Phytoremediation 18(3):245–250. https://doi.org/10.1080/15226514.2015.1078771
Sakakibara M, Ohmori Y, Ha NTH, Sano S, Sera K (2011) Phytoremediation of heavy metal contaminated water and sediment by Eleocharis acicularis. Clean: Soil Air Water 39:735–741. https://doi.org/10.1002/clen.201000488
Shabani N, Sayadi MH (2012) Evaluation of heavy metals accumulation by two emergent macrophytes from the polluted soil: an experimental study. Environmentalist 32:91–98. https://doi.org/10.1007/s10669-011-9376-z
Burges A, Alkorta I, Epelde L, Garbisu C (2018) From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int J Phytorem 20:384–397. https://doi.org/10.1080/15226514.2017.1365340
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci 9:1476. https://doi.org/10.3389/fpls.2018.01476
Yang W, Gu J, Zhou H, Huang F, Yuan T, Zhang J, Wang S, Yi SZ, H. and, Liao B (2020) Effect of three Napier grass varieties on phytoextraction of Cd- and Zn-contaminated cultivated soil under mowing and their safe utilization. Environ Sci Pollut Res 27:16134–16144. https://doi.org/10.1007/s11356-020-07887-1
Ghazaryan K, Movsesyan A, Minkina HS, Sushkova TM, S. N. and, Rajput VD (2019) The identification of phytoextraction potential of Melilotus officinalis and Amaranthus retroflexus growing on copper-and molybdenum-polluted soils. Geochem Health Environ. https://doi.org/10.1007/s10653-019-00338-y
Khalid A, Farid M, Zubair M, Rizwan M, Iftikhar U, Ishaq HK, Farid S, Latif U, Hina K, Ali S (2020) Efficacy of Alternanthera bettzickiana to remediate copper and cobalt contaminated soil physiological and biochemical alterations. Int J Environ Res 14:243–255. https://doi.org/10.1007/s41742-020-00251-8
Fourati E, Wali M, Vogel-Mikuš K, Abdelly C, Ghnaya T (2016) Nickel tolerance, accumulation and subcellular distribution in the halophytes Sesuvium portulacastrum and Cakile maritima. Plant Physiol Biochem 108:295–303. https://doi.org/10.1016/j.chemosphere.2016.12.116
Ali H, Naseer M, Sajad MA (2012) Phytoremediation of heavy metals by Trifolium alexandrinum. Int J Environ Sci 2:1459–1469. https://doi.org/10.6088/ijes.00202030031
Wenzel WW (2009) Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 321(1):385–408. https://doi.org/10.1007/s11104-008-9686-1
Morkunas I, Woźniak A, Mai VC, Rucińska-Sobkowiak R, Jeandet F (2018) The role of heavy metals in plant response to biotic stress. Molecules 23(9):2320. https://doi.org/10.3390/molecules23092320
Center for Public Environmental Oversight (CPEO) n.d. Phytoremediation. Viewed 2, June, 2021. http://www.cpeo.org/techtree/ttdescript/phytrem.htm
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci. https://doi.org/10.1155/2014/752708
Gupta N, Ram H, Kumar B (2016) Mechanism of zinc absorption in plants: uptake, transport, translocation and accumulation. Rev Environ Sci Biotechnol 15:89–109. https://doi.org/10.1007/s11157-016-9390-1
DalCorso G, Fasani E, Manara A, Visioli G, Furini A (2019) Heavy metal pollutions: state of the Art and Innovation in Phytoremediation. Int J Mol Sci 20(14):3412. https://doi.org/10.3390/ijms20143412
Zahoor M, Irshad M, Rahman H, Qasim M, Afridi SG, Qadir M, Hussain A (2017) Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol Environ Saf 142:139–149. https://doi.org/10.1016/j.ecoenv.2017.04.005
Rohrbacher F, St-Arnaud M (2016) Root exudation: the ecological driver of hydrocarbon rhizoremediation. Agronomy 6(1):19. https://doi.org/10.3390/agronomy6010019
Duca D, Lorv J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 106(1):85–125. https://doi.org/10.1007/s10482-013-0095-y
Fan X, Song F (2014) Bioremediation of atrazine: recent advances and promises. J Soils Sediments 14(10):1727–1737. https://doi.org/10.1007/s11368-014-0921-5
Cárdenas-Aguiar E, Gascó G, Paz-Ferreiro J, Méndez A (2017) The effect of biochar and compost from urban organic waste on plant biomass and properties of an artificially copper polluted soil. Int Biodeterior Biodegrad 124:223–232. https://doi.org/10.1016/j.ibiod.2017.05.014
Tangahu BV, Abdullah S, Basri SR, Idris H, Anuar M, N., Mukhlisin M, (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. https://doi.org/10.1155/2011/939161
Singh T, Singh DK (2017) Phytoremediation of organochlorine pesticides: Concept, method, and recent developments. Int J Phytoremediat 19(9):834–843. https://doi.org/10.1080/15226514.2017.1290579
Tampouris S, Papassiopi N, Paspaliaris I (2001) Removal of contaminant metals from fine grained soils, using agglomeration, chloride solutions and pile leaching techniques. J Hazard Mater 84(2–3):297–319. https://doi.org/10.1016/S0304-3894(01)00233-3
Moreno FN, Anderson CWN, Stewart RB, Robinson BH, Nomura R, Gomshei M (2004) Mercury phytoextraction and phytovolatilisation from hg-contaminated artisanal mine sites. Phytoremediat Mercur Mine Wastes. 2004a, pp. 147–159
Sakakibara M, Watanabe A, Inoue M, Sano S, Kaise T (2010), January Phytoextraction and phytovolatilization of arsenic from As-contaminated soils by Pteris vittata. In Proceedings of the annual international conference on soils, sediments, water and energy. Vol. 12, No. 1, p. 26
Cui H, Li H, Zhang S, Yi Q, Zhou J, Fang G, Zhou J (2020) Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 242:125252. https://doi.org/10.1016/j.chemosphere.2019.125252
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of as, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag 28(1):215–225. https://doi.org/10.1016/j.wasman.2006.12.012
Zgorelec Z, Bilandzija N, Knez K, Galic M, Zuzul S (2020) Cadmium and mercury phytostabilization from soil using Miscanthus× giganteus. Sci Rep 10(1):1–10. https://doi.org/10.1038/s41598-020-63488-5
Khan MA, Ullah N, Khan T, Jamal M, Shah NA, Ali H (2019) Phytoremediation of electronic waste: a mechanistic overview and role of plant secondary metabolites. Electron Waste Pollut. https://doi.org/10.1007/978-3-030-26615-8_16
Singh NP, Santal AR (2015) Phytoremediation of heavy metals: the use of green approaches to clean the environment. Phytoremediation. Springer, Cham, pp 115–129. https://doi.org/10.1007/978-3-319-10969-5_10
Chourasia S, Khanna I, Gera N, Chinthala S (2014) Reduction of pollutants from RO reject using phytoremediation: proposed methodology. Strategic Technologies of Complex Environmental Issues-A Sustainable Approach, 202
Sánchez-Galván G, Olguín EJ, Melo FJ, Jiménez-Moreno D, Hernández VJ (2022) Pontederia sagittata and Cyperus papyrus contribution to carbon storage in floating treatment wetlands established in subtropical urban ponds. Sci Total Environ 832:154990. https://doi.org/10.1016/j.scitotenv.2022.154990
Olguín EJ, García-López DA, González-Portela RE, Sánchez-Galván G (2017) Year-round phytofiltration lagoon assessment using Pistia stratiotes within a pilot-plant scale biorefinery. Sci Total Environ 592:326–333. https://doi.org/10.1016/j.scitotenv.2017.03.067
Acknowledgements
We thankful to the invited reviewers for their insightful comments.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: NDN and HO; data curation: NDN, HO, CU; writing original draft: NDN and HO; writing review and editing: HO, TM, CU.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nnaji, N.D., Onyeaka, H., Miri, T. et al. Bioaccumulation for heavy metal removal: a review. SN Appl. Sci. 5, 125 (2023). https://doi.org/10.1007/s42452-023-05351-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05351-6