Abstract
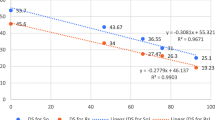
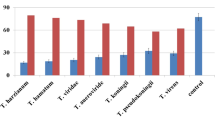
Sclerotium rolfsii Sacc. is one of the important soil borne pathogen causing stem rot of groundnut prevalent in all growing area worldwide. The present study aimed on the identification of native Trichoderma isolates, and its efficacy against the stem rot pathogen in groundnut at field level. Thirty-five isolates of Trichoderma spp. isolated from the groundnut rhizosphere were comparatively evaluated for their biocontrol potential against S. rolfsii Sacc. and growth promoting traits in groundnut. The morphological studies of the 35 isolates were supported molecularly by amplifying of ITS region and classified into four species namely, T. asperellum, T. citrinoviride, T. longibrachiatum and T. harzianum which were further subjected to biocontrol efficacy tests. The highly efficient representative isolates namely, T. harzianum Thar23, T. asperellum Tasp49, T. longibrachiatum Tlongi5 and T. citrinoviride Tcitri2 were evaluated to produce lytic enzymes and growth promoting traits. The comparative study of these isolates revealed that, T. harzianum Thar23 produced significant (P < 0.05) amount of lytic enzymes viz., chitinase (31.36 U/ml), β 1, 3 glucanase (4.1 U/ml) and protease (2.76 U/ml). T. harzianum Thar23 promotes plant growth traits namely germination efficacy (31.48%), increase in the shoot length (42%) and root length (42.43%), improved vigor index, and increased relative water content (25.56%). Soil application, seed treatment and drenching with the powder formulation of Thar23 in field for the years 2019 and 2020 significantly (P < 0.05) reduced stem rot disease incidence to 59.45% and 53.79% and increased pod yield to 2.85 t/ha and 2.68 t/ha respectively. T. harzianum isolate Thar23 will help the groundnut growers for eco-friendly management of stem rot disease and increased yield.




Similar content being viewed by others
References
Abdul-Baki AA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria 1. Crop Sci 13(6):630–633
Agrawal T, Kotasthane AS (2012) Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. Springerplus 1(1):1–10
Annual Report (2015–16) ICAR-directorate of groundnut research, Junagadh-362001, Gujarat, India
Aycock R (1966) Stem rot and other diseases caused by Sclerotium rolfsii or the status of Rolfs’ fungus after 70 years. Raleigh (NC): North Carolina Agricultural Experiment Station Bulletin, pp 132–202
Bell DK, Wells HD, Markham CR (1982) In vitro antagonism of Trichoderma spp. against six fungal plant pathogens. Phytopathology 72:379–382
Bissett J (1984) A revision of the genus Trichoderma I sect. Longibrachiaticum sect. nov. Can J Bot 2:924–931
Boat MAB, Sameza ML, Iacomi B, Tchameni SN, Boyom FF (2020) Screening, identification and evaluation of Trichoderma spp. for biocontrol potential of common bean damping-off pathogens. Biocontrol Sci Technol 30(3):228–242
Cai F, Druzhinina IS (2021) In honour of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Divers 107(1):1–69
Culling KW (1992) Design and testing of a plant specific PCR primer for ecological evolutionary studies. Mol Ecol 1:233–240
Dennis L, Webster J (1971) Antagonistic properties of some species of Trichoderma. III. Hyphal production. Trans Br Mycol Soc 57:363–369
Devi P, Prabhakaran N, Kamil D, Choudhary SP (2021) Polyphasic taxonomy of Indian Trichoderma species. Phytotaxa 502(1):1–27
Elad Y, Chet I, Henis Y (1981) A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 9:59–67
Elamathi E, Malathi P, Viswanathan R, Sundar AR (2018) Expression analysis on mycoparasitism related genes during antagonism of Trichoderma with Colletotrichum falcatum causing red rot in sugarcane. J Plant Biochem Biotechnol 27:351–361
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Guigon-Lopez C, Vargas-Albores F, Guerrero-Prieto V, Ruocco M, Lorito M (2015) Changes in Trichoderma asperellum enzyme expression during parasitism of the cotton root rot pathogen Phymatotrichopsis omnivora. Fungal Biol 119(4):264–273
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Jambhulkar PP, Raja M, Singh B, Katoch S, Kumar S, Sharma P (2022) Potential native Trichoderma strains against Fusarium verticillioides causing post flowering stalk rot in winter maize. Crop Prot 152:105838
Kamala T, Devi SI, Sharma KC, Kennedy K (2015) Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed Res Int 1–21
Kaur R, Kalia A, Lore JS, Kaur A, Yadav I, Sharma P, Sandhu JS (2021) Trichoderma sp. endochitinase and β-1,3-glucanase impede Rhizoctonia solani growth independently, and their combined use does not enhance impediment. Plant Pathol 70:1388–1396
Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB (2014) Unravelling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol 98:533–544
Kimura MA (1980) Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotides sequences. J Mol Evol 2:87–90
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2012) Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J Microbiol 52(2):137–144
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the fungal genus Trichoderma—a multigene approach. Mycol Res 106:757–767
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Li Y, Sun R, Yu J, Saravanakumar K, Chen J (2016) Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J Microbiol 56(3):318–327
Ma J, Tsegaye E, Li M, Wu B, Jiang X (2020) Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 10(8):1–13
Macena AMF, Kobori NN, Mascarin GM, Vida JB, Hartman GL (2020) Antagonism of Trichoderma-based biofungicides against Brazilian and North American isolates of Sclerotinia sclerotiorum and growth promotion of soybean. Biocontrol 65:235–246
Malolepsza U, Nawrocka J, Szczech M (2017) Trichoderma virens 106 inoculation stimulates defense enzyme activities and enhances phenolics levels in tomato plants leading to lowered Rhizoctonia solani infection. Biocontrol Sci Technol 27(2):180–199
Manzar N, Singh Y, Kashyap AS, Sahu PK, Rajawat MVS, Bhowmik A, Sharma PK, Saxena AK (2021) Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolour L.) from Tarai and hill regions of India. Biol Control 152:104474
Muthu KA, Sharma P (2011) Molecular and morphological characters: an appurtenance for antagonism in Trichoderma spp. Afr J Biotechnol 10(22):4532–4543
Nofal AM, El-Rahman MA, AbdelghanyTM A-M, M, (2021) Mycoparasitic nature of Egyptian Trichoderma isolates and their impact on suppression Fusarium wilt of tomato. Egypt J Biol Pest Control 31:103
Pandian RTP, Raja M, Kumar A, Sharma P (2016) Morphological and molecular characterization of Trichoderma asperellum strain Ta13. Indian Phytopathol 69:297–303
Persoon CH (1794) Dispositamethodicafungorum. RomersneuesMagazin der Botanik 1:81–128
Raja M, Sharma RK, Jambhulkar PP, Sharma KR, Sharma P (2021) Biosynthesis of silver nanoparticles from Trichoderma harzianum Th3 and its efficacy against root rot complex pathogen in groundnut. Mater Today Proc 43(5):3140–3143
Rifai MA (1969) A revision of the genus Trichoderma. Mycol Papers 116:1–56
Sallam N, Eraky AM, Sallam A (2019) Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol Biol Rep 46(4):4463–4470
Samuels GJ, Lieckfeldt E, Nirenberg HI (1999) Trichoderma asperellum, a new species with warted conidia, and redescription T. viride. Sydowia 51:71–88
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87(3):787–799
Sharma P, Saini MK, Deep S, Kumar V (2012) Biological control of groundnut root rot in farmer’s field. J Agric Sci 4(8):48–59
Sharma P, Sharma M, Raja M, Shanmugam V (2014) Status of Trichoderma research in India: a review. Indian Phytopathol 67(1):1–19
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tian X, He M, Wang Z, Zhang J, Song Y, He Z, Dong Y (2015) Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul 77(3):343–356
Tulasne LR, Tulasne C (1865) Selecta fungorumcarpologia. Jussu, Paris
Vinale F, Sivasithamparam K (2020) Beneficial effects of Trichoderma secondary metabolites on crops. Phytother Res 34(11):2835–2842
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Acknowledgements
The authors are thankful to Head, Department of Plant Pathology, Sri Karan Narendra Agriculture University, Jobner for proving research facilities. This present study is part of first author’s Ph.D. programme and thankful to Head, Department of Biosciences, Manipal University Jaipur for necessary support during the research work.
Funding
Funding was provided by Indian Council of Agricultural Research (F. No. Agril. Edn. /9/11/2016/ES/HRD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raja, M., Sharma, R.K., Jambhulkar, P.P. et al. Comparative evaluation of native Trichoderma species from groundnut rhizosphere against stem rot caused by Sclerotium rolfsii Sacc.. Indian Phytopathology 76, 459–471 (2023). https://doi.org/10.1007/s42360-023-00610-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00610-3