Abstract
Prolactin, a pituitary hormone that was discovered about 80 years ago and is primarily known for its functions in mammary gland development and lactation, is now known to participate in numerous functions across different phylogenetic groups. Fundamentally known for its secretion from lactotroph cells in adenohypophysis region of pituitary gland, newer studies have demonstrated a number of extrapituitary sites which secrete prolactin, where it acts in an autocrine, paracrine, and endocrine manner to regulate essential physiological and biochemical processes. These sites include lymphocytes, epithelial cells of lactating mammary glands, breast cancer cells of epithelial origin, and the placenta. The placenta is one of the most important organs secreting prolactin; however, its role in placental biology has not to date been reviewed comprehensively. This review elaborates upon the various facets of prolactin hormone, including prolactin production and its post-translational modifications and signaling. Major emphasis is placed on placental prolactin and its potential roles, ranging from the role of prolactin in angiogenesis, preeclampsia, maternal diabetes, and anti-apoptosis, among others.


Similar content being viewed by others
References
Riddle O, Bates RW, Dykshorn SW (1933) The preparation, identification and assay of prolactin - a hormone of the anterior pituitary In. Am J Physiol 105:191–216. https://doi.org/10.1152/ajplegacy.1933.105.1.191
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19(3):225–268. https://doi.org/10.1210/edrv.19.3.0334
Morishige WK, Rothchild I (1974) Temporal aspects of the regulation of corpus luteum function by luteinizing hormone, prolactin and placental luteotrophin during the first half of pregnancy in the rat. Endocrinol 95(1):260–274. https://doi.org/10.1210/endo-95-1-260
Gunnet JW, Freeman ME (1983) The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev 4(1):44–61. https://doi.org/10.1210/edrv-4-1-44
Bridges RS, DiBiase R, Loundes DD, Doherty PC (1985) Prolactin stimulation of maternal behavior in female rats. Sci 227(4688):782–784. https://doi.org/10.1126/science.3969568
Loretz CA, Bern HA (1982) Prolactin and osmoregulation in vertebrates An update. Neuroendocrinol 35(4):292–304. https://doi.org/10.1159/000123397
Shennan DB (1994) Regulation of water and solute transport across mammalian plasma cell membranes by prolactin. J Dairy Res 61(1):155–166. https://doi.org/10.1017/S0022029900028156
Buskila D, Shoenfeld Y (1996) Prolactin, bromocriptine and autoimmune diseases. Isr J Med Sci 32(1):23–27
Csaba G (2014) Hormones in the immune system and their possible role. A critical review. Acta Microbiol Immunol Hung 61(3):241–260. https://doi.org/10.1556/amicr.61.2014.3.1.
Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR (2006) Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab 17(3):110–116. https://doi.org/10.1016/j.tem.2006.02.005
Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, Weiner RI, Martial JA (1999) Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci U S A 96(4):1246–1251. https://doi.org/10.1073/pnas.96.4.1246
Ferrara N, Clapp C, Weiner R (1991) The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinol 129(2):896–900. https://doi.org/10.1210/endo-129-2-896
Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI (1993) The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinol 133(3):1292–1299. https://doi.org/10.1210/endo.133.3.7689950
Lee J, Majumder S, Chatterjee S, Muralidhar K (2011) Inhibitory activity of the peptides derived from buffalo prolactin on angiogenesis. J Biosci 36(2):341–354. https://doi.org/10.1007/s12038-011-9073-6
Griffond B, Deray A, Jacquemard C, Fellmann D, Bugnon C (1994) Prolactin immunoreactive neurons of the rat lateral hypothalamus: immunocytochemical and ultrastructural studies. Brain Res 635(1–2):179–186. https://doi.org/10.1016/0006-8993(94)91437-0
DeVito WJ (1988) Distribution of immunoreactive prolactin in the male and female rat brain: effects of hypophysectomy and intraventricular administration of colchicine. Neuroendocrinol 47(4):284–289. https://doi.org/10.1159/000124926
Montgomery DW, LeFevre JA, Ulrich ED, Adamson CR, Zukoski CF (1990) Identification of prolactin-like proteins synthesized by normal murine lymphocytes. Endocrinol 127(5):2601–2603. https://doi.org/10.1210/endo-127-5-2601
Nolin JM, Witorsch RJ (1976) Detection of endogenous immunoreactive prolactin in rat mammary epithelial cells during lactation. Endocrinol 99(4):949–958. https://doi.org/10.1210/endo-99-4-949
Ginsburg E, Vonderhaar BK (1995) Prolactin synthesis and secretion by human breast cancer cells. Cancer Res 55(12):2591–2595
Kasai K, Shik SS, Yoshida Y (1982) Production and localization of human prolactin in placenta and decidua in early and at term normal pregnancy. Int J Biol Res Pregnancy 3(1):25–29
Tabarelli M, Kofler R, Wick G (1983) Placental hormones: I Immunofluorescence studies of the localization of chorionic gonadotrophin, placental lactogen and prolactin in human and rat placenta and in the endometrium of pregnant rats. Placenta 4(4):379–387. https://doi.org/10.1016/s0143-4004(83)80041-1.
Lee DW, Markoff E (1986) Synthesis and release of glycosylated prolactin by human decidua in vitro. J Clin Endocrinol Metab 62(5):990–994. https://doi.org/10.1210/jcem-62-5-990
Harigaya T, Komori M, Kawakami T, Watanabe H, Abe M (1997) Expression of prolactin gene in mouse placenta during late pregnancy: detection of mRNA and its translation product. Endocr J 44(1):155–161. https://doi.org/10.1507/endocrj.44.155
Freeman ME, Kanyicska B, Lerant A, Nagy G (2000) Prolactin: structure, function, and regulation of secretion. Physiol Rev 80(4):1523–1631. https://doi.org/10.1152/physrev.2000.80.4.1523
Ignacak A, Kasztelnik M, Sliwa T, Korbut RA, Rajda K, Guzik TJ (2012) Prolactin--not only lactotrophin. A “new” view of the “old” hormone. J Physiol Pharmacol 63(5):435–443
Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW (1996) Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev 17(6):639–669. https://doi.org/10.1210/edrv-17-6-639
Jabbour HN, Critchley HO (2001) Potential roles of decidual prolactin in early pregnancy. Reproduction 121(2):197–205. https://doi.org/10.1530/rep.0.1210197
Truong AT, Duez C, Belayew A, Renard A, Pictet R, Bell GI, Martial JA (1984) Isolation and characterization of the human prolactin gene. EMBO J 3(2):429–437
Berwaer M, Martial JA, Davis JR (1994) Characterization of an up-stream promoter directing extrapituitary expression of the human prolactin gene. Mol Endocrinol 8(5):635–642. https://doi.org/10.1210/mend.8.5.8058071
Berwaer M, Monget P, Peers B, Mathy-Hartert M, Bellefroid E, Davis JR, Belayew A, Martial JA (1991) Multihormonal regulation of the human prolactin gene expression from 5000 bp of its upstream sequence. Mol Cell Endocrinol 80(1–3):53–64. https://doi.org/10.1016/0303-7207(91)90142-f
Sinha YN (1995) Structural variants of prolactin: occurrence and physiological significance. Endocr Rev 16(3):354–369. https://doi.org/10.1210/edrv-16-3-354
Mittra I (1980) A novel “cleaved prolactin” in the rat pituitary: part I. Biosynthesis, characterization and regulatory control. Biochem Biophys Res Commun 95(4):1750–1759. https://doi.org/10.1016/s0006-291x(80)80101-x.
Sinha YN, Gilligan TA (1984) A cleaved form of prolactin in the mouse pituitary gland: identification and comparison of in vitro synthesis and release in strains with high and low incidences of mammary tumors. Endocrinol 114(6):2046–2053. https://doi.org/10.1210/endo-114-6-2046
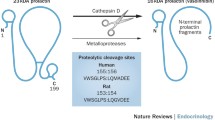
Piwnica D, Touraine P, Struman I, Tabruyn S, Bolbach G, Clapp C, Martial JA, Kelly PA, Goffin V (2004) Cathepsin D processes human prolactin into multiple 16K-like N-terminal fragments: study of their antiangiogenic properties and physiological relevance. Mol Endocrinol 18(10):2522–2542. https://doi.org/10.1210/me.2004-0200
Piwnica D, Fernandez I, Binart N, Touraine P, Kelly PA, Goffin V (2006) A new mechanism for prolactin processing into 16K PRL by secreted cathepsin D. Mol Endocrinol (Baltimore, Md.) 20(12):3263–3278. https://doi.org/10.1210/me.2006-0044.
Markoff E, Sigel MB, Lacour N, Seavey BK, Friesen HG, Lewis UJ (1988) Glycosylation selectively alters the biological activity of prolactin. Endocrinol 123(3):1303–1306. https://doi.org/10.1210/endo-123-3-1303
Sinha YN, DePaolo LV, Haro LS, Singh RN, Jacobsen BP, Scott KE, Lewis UJ (1991) Isolation and biochemical properties of four forms of glycosylated porcine prolactin. Mol Cell Endocrinol 80(1–3):203–213. https://doi.org/10.1016/0303-7207(91)90157-n
Haro LS, Lee DW, Singh RN, Bee G, Markoff E, Lewis UJ (1990) Glycosylated human prolactin: alterations in glycosylation pattern modify affinity for lactogen receptor and values in prolactin radioimmunoassay. J Clin Endocrinol Metab 71(2):379–383. https://doi.org/10.1210/jcem-71-2-379
Oetting WS, Tuazon PT, Traugh JA, Walker AM (1986) Phosphorylation of prolactin. J Biol Chem 261(4):1649–1652. https://doi.org/10.1016/S0021-9258(17)35989-6
Brooks CL, Kim BG, Aphale P, Kleeman BE, Johnson GC (1990) Phosphorylated variant of bovine prolactin. Mol Cell Endocrinol 71(2):117–123. https://doi.org/10.1016/0303-7207(90)90248-7
Wang YF, Walker AM (1993) Dephosphorylation of standard prolactin produces a more biologically active molecule: evidence for antagonism between nonphosphorylated and phosphorylated prolactin in the stimulation of Nb2 cell proliferation. Endocrinol 133(5):2156–2160. https://doi.org/10.1210/endo.133.5.8404666
Ho TW, Greenan JR, Walker AM (1989) Mammotroph autoregulation: the differential roles of the 24K isoforms of prolactin. Endocrinol 124(3):1507–1514. https://doi.org/10.1210/endo-124-3-1507
Coss D, Kuo CB, Yang L, Ingleton P, Luben R, Walker AM (1999) Dissociation of Janus kinase 2 and signal transducer and activator of transcription 5 activation after treatment of Nb2 cells with a molecular mimic of phosphorylated prolactin. Endocrinol 140(11):5087–5094. https://doi.org/10.1210/endo.140.11.7104
Chen TJ, Kuo CB, Tsai KF, Liu JW, Chen DY, Walker AM (1998) Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinol 139(2):609–616. https://doi.org/10.1210/endo.139.2.5758
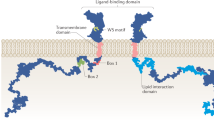
Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C (1994) Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA 91(12):5232–5236. https://doi.org/10.1073/pnas.91.12.5232
Swaminathan G, Varghese B, Fuchs SY (2008) Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia 13(1):81–91. https://doi.org/10.1007/s10911-008-9068-6
Goffin V, Binart N, Touraine P, Kelly PA (2002) Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67. https://doi.org/10.1146/annurev.physiol.64.081501.131049
Berlanga JJ, Fresno Vara JA, Martín-Pérez J, García-Ruiz JP (1995) Prolactin receptor is associated with c-src kinase in rat liver. Mol Endocrinol 9(11):1461–1467. https://doi.org/10.1210/mend.9.11.8584023
Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M (1997) Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem 272(4):2050–2052. https://doi.org/10.1074/jbc.272.4.2050
Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K (1997) Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16(23):6926–6935. https://doi.org/10.1093/emboj/16.23.6926
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA (1997) Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11(2):167–178. https://doi.org/10.1101/gad.11.2.167
Binart N, Helloco C, Ormandy CJ, Barra J, Clément-Lacroix P, Baran N, Kelly PA (2000) Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinol 141(7):2691–2697. https://doi.org/10.1210/endo.141.7.7568
Shah GV, Sheth AR (1979) Is prolactin involved in sperm capacitation? Med Hypotheses 5(8):909–914. https://doi.org/10.1016/0306-9877(79)90079-3
Fukuda A, Mori C, Hashimoto H, Noda Y, Mori T, Hoshino K (1989) Effects of prolactin during preincubation of mouse spermatozoa on fertilizing capacity in vitro. J In Vitro Fert Embryo Transf 6(2):92–97. https://doi.org/10.1007/BF01130733
Bartke A (1980) Role of prolactin in reproduction in male mammals. Fed Proc 39(8):2577–2581
Binart N, Imbert-Bollore P, Baran N, Viglietta C, Kelly PA (2003) A short form of the prolactin (PRL) receptor is able to rescue mammopoiesis in heterozygous PRL receptor mice. Mol Endocrinol (Baltimore, Md.) 17(6):1066–1074. https://doi.org/10.1210/me.2002-0181.
Healy DL, Burger HG, Muller HK (1978) Hypothesis: placental membranes produce prolactin. Mol Cell Endocrinol 11(1):1–6. https://doi.org/10.1016/0303-7207(78)90027-8
Tabarelli M, Kofler R, Berger P, Wick G (1983) Placental hormones: II. Immunofluorescence studies of the localization of prolactin/placental lactogen- and human chorionic gonadotrophin-receptors in human and rat placenta. Placenta 4(4):389–396. https://doi.org/10.1016/s0143-4004(83)80042-3.
Wu WX, Brooks J, Millar MR, Ledger WL, Saunders PT, Glasier AF, McNeilly AS (1991) Localization of the sites of synthesis and action of prolactin by immunocytochemistry and in-situ hybridization within the human utero-placental unit. J Mol Endocrinol 7(3):241–247. https://doi.org/10.1677/jme.0.0070241
Rana M, Basu-Modak S (2016) Immunohistochemical analysis of heme oxygenases, prolactin and VEGF in the mouse placenta. IJSR 5(11):712–716
Tomita K, McCoshen JA, Fernandez CS, Tyson JE (1982) Immunologic and biologic characteristics of human decidual prolactin. Am J Obstet Gynecol 142(4):420–426. https://doi.org/10.1016/s0002-9378(16)32383-3
Takahashi H, Nabeshima Y, Ogata K, Takeuchi S (1984) Molecular cloning and nucleotide sequence of DNA complementary to human decidual prolactin mRNA. J Biochem 95(5):1491–1499. https://doi.org/10.1093/oxfordjournals.jbchem.a134757
DiMattia GE, Gellersen B, Duckworth ML, Friesen HG (1990) Human prolactin gene expression. The use of an alternative noncoding exon in decidua and the IM-9-P3 lymphoblast cell line. J Biol Chem 265(27):16412–16421
Golander A, Barrett J, Hurley T, Barry S, Handwerger S (1979) Failure of bromocriptine, dopamine, and thyrotropin-releasing hormone to affect prolactin secretion by human decidual tissue in vitro. J Clin Endocrinol Metab 49(5):787–789. https://doi.org/10.1210/jcem-49-5-787
Handwerger S, Richards R, Markoff E (1991) Autocrine/paracrine regulation of prolactin release from human decidual cells. Ann N Y Acad Sci 622:111–119. https://doi.org/10.1111/j.1749-6632.1991.tb37855.x
Fluhr H, Krenzer S, Deperschmidt M, Zwirner M, Wallwiener D, Licht P (2006) Human chorionic gonadotropin inhibits insulin-like growth factor-binding protein-1 and prolactin in decidualized human endometrial stromal cells. Fertil Steril 86(1):236–238. https://doi.org/10.1016/j.fertnstert.2005.12.031
Chao HS, Poisner AM, Poisner R, Handwerger S (1994) Lipopolysaccharides inhibit prolactin and renin release from human decidual cells. Biol Reprod 50(1):210–214. https://doi.org/10.1095/biolreprod50.1.210
Kanda Y, Jikihara H, Markoff E, Handwerger S (1999) Interleukin-2 inhibits the synthesis and release of prolactin from human decidual cells. J Clin Endocrinol Metab 84(2):677–681. https://doi.org/10.1210/jcem.84.2.5450
Brosens JJ, Hayashi N, White JO (1999) Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinol 140(10):4809–4820. https://doi.org/10.1210/endo.140.10.7070
Brar AK, Kessler CA, Handwerger S (2002) An Ets motif in the proximal decidual prolactin promoter is essential for basal gene expression. J Mol Endocrinol 29(1):99–112. https://doi.org/10.1677/jme.0.0290099
Jiang Y, Hu Y, Zhao J, Zhen X, Yan G, Sun H (2011) The orphan nuclear receptor Nur77 regulates decidual prolactin expression in human endometrial stromal cells. Biochem Biophys Res Commun 404(2):628–633. https://doi.org/10.1016/j.bbrc.2010.12.027
Reynolds LP, Redmer DA (2001) Angiogenesis in the placenta. Biol Reprod 64(4):1033–1040. https://doi.org/10.1095/biolreprod64.4.1033
Sibai B, Dekker G, Kupferminc M (2005) Pre-eclampsia. Lancet 365(9461):785–799. https://doi.org/10.1016/S0140-6736(05)17987-2
Leaños-Miranda A, Márquez-Acosta J, Cárdenas-Mondragón GM, Chinolla-Arellano ZL, Rivera-Leaños R, Bermejo-Huerta S, Romero-Arauz JF, Alvarez-Jiménez G, Ramos-León JC, Ulloa-Aguirre A (2008) Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab 93(7):2492–2499. https://doi.org/10.1210/jc.2008-0305
Masumoto A, Masuyama H, Takamoto N, Akahori Y, Hiramatsu Y (2010) Expression of antiangiogenic prolactin fragments in the placentas of women with pregnancy induced hypertension. Acta Med Okayama 64(4):249–255. https://doi.org/10.18926/AMO/40133.
González C, Parra A, Ramírez-Peredo J, García C, Rivera JC, Macotela Y, Aranda J, Lemini M, Arias J, Ibargüengoitia F, de la Escalera GM, Clapp C (2007) Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest 87(10):1009–1017. https://doi.org/10.1038/labinvest.3700662
Moses EK, Freed KA, Higgins JR, Brennecke SP (1999) Alternative forms of a novel aspartyl protease gene are differentially expressed in human gestational tissues. Mol Hum Reprod 5(10):983–989. https://doi.org/10.1093/molehr/5.10.983
Boulot P, Chabbert-Buffet N, d’Ercole C, Diabetes and Pregnancy Group F. a. et al (2003) French multicentric survey of outcome of pregnancy in women with pregestational diabetes. Diabetes Care 26(11):2990–2993. https://doi.org/10.2337/diacare.26.11.2990.
Sisino G, Bouckenooghe T, Aurientis S, Fontaine P, Storme L, Vambergue A (2013) Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim Biophys Acta 1832(12):1959–1968. https://doi.org/10.1016/j.bbadis.2013.07.009
Perimenis P, Bouckenooghe T, Delplanque J, Moitrot E, Eury E, Lobbens S, Gosset P, Devisme L, Duvillie B, Abderrahmani A, Storme L, Fontaine P, Froguel P, Vambergue A (2014) Placental antiangiogenic prolactin fragments are increased in human and rat maternal diabetes. Biochem Biophys Acta 1842(9):1783–1793. https://doi.org/10.1016/j.bbadis.2014.06.026
Faria de Castro L, Alves dos Santos Á, Augusto Casulari L, Ansaneli Naves L, Amorim Amato A (2020) Association between variations of physiological prolactin serum levels and the risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 166:108247
Li M, Song Y, Rawal S, Hinkle SN, Zhu Y, Tekola-Ayele F, Ferrara A, Tsai MY, Zhang C (2020) Plasma prolactin and progesterone levels and the risk of gestational diabetes: a prospective and longitudinal study in a multiracial cohort. Front Endocrinol (Lausanne) 11:83. https://doi.org/10.3389/fendo.2020.00083
Kelly PA, Binart N, Lucas B, Bouchard B, Goffin V (2001) Implications of multiple phenotypes observed in prolactin receptor knockout mice. Front Neuroendocrinol 22(2):140–145. https://doi.org/10.1006/frne.2001.0212
Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G (2007) Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinol 148(5):2326–2334. https://doi.org/10.1210/en.2006-1643
Dudley DJ, Trautman MS, Araneo BA, Edwin SS, Mitchell MD (1992) Decidual cell biosynthesis of interleukin-6: regulation by inflammatory cytokines. J Clin Endocrinol Metab 74(4):884–889. https://doi.org/10.1210/jcem.74.4.1548355
Mao J, Duan RW, Zhong L, Gibori G, Azhar S (1997) Expression, purification and characterization of the rat luteal 20 alpha-hydroxysteroid dehydrogenase. Endocrinol 138(1):182–190. https://doi.org/10.1210/endo.138.1.4825
LaVoie HA, Witorsch RJ (1995) Investigation of intracellular signals mediating the anti-apoptotic action of prolactin in Nb2 lymphoma cells. Proc Soc Exp Biol Med 209(3):257–269. https://doi.org/10.3181/00379727-209-43901
Travers MT, Barber MC, Tonner E, Quarrie L, Wilde CJ, Flint DJ (1996) The role of prolactin and growth hormone in the regulation of casein gene expression and mammary cell survival: relationships to milk synthesis and secretion. Endocrinol 137(5):1530–1539. https://doi.org/10.1210/endo.137.5.8612482
Tessier C, Prigent-Tessier A, Ferguson-Gottschall S, Gu Y, Gibori G (2001) PRL antiapoptotic effect in the rat decidua involves the PI3K/protein kinase B-mediated inhibition of caspase-3 activity. Endocrinol 142(9):4086–4094. https://doi.org/10.1210/endo.142.9.8381
Pijnenborg R, Bland JM, Robertson WB, Brosens I (1983) Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4(4):397–413. https://doi.org/10.1016/s0143-4004(83)80043-5
Stefanoska I, Jovanović Krivokuća M, Vasilijić S, Ćujić D, Vićovac L (2013) Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 34(9):775–783. https://doi.org/10.1016/j.placenta.2013.06.305
King A, Loke YW (1990) Uterine large granular lymphocytes: a possible role in embryonic implantation? Am J Obstet Gynecol 162(2):308–310. https://doi.org/10.1016/0002-9378(90)90375-h
Fukamatsu Y, Tomita K, Fukuta T (1984) Further evidence of prolactin production from human decidua and its transport across fetal membrane. Gynecol Obstet Invest 17(6):309–316. https://doi.org/10.1159/000299168
Wu WX, Brooks J, Glasier AF, McNeilly AS (1995) The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. J Mol Endocrinol 14(2):255–261. https://doi.org/10.1677/jme.0.0140255
Leontic EA, Tyson JE (1977) Prolactin and fetal osmoregulation: water transport across isolated human amnion. Am J Physiol 232(3):R124-127. https://doi.org/10.1152/ajpregu.1977.232.3.R124
Abramovich DR, Garden A, Jandial L, Page KR (1979) Fetal swallowing and voiding in relation to hydramnios. Obstet Gynecol 54(1):15–20. https://doi.org/10.1097/00006250-197907000-00005
Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, Struman I, Fischer P, Drexler H (2007) Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol 50(24):2354–2355. https://doi.org/10.1016/j.jacc.2007.10.006
Kinet V, Nguyen NQ, Sabatel C, Blacher S, Noël A, Martial JA, Struman I (2009) Antiangiogenic liposomal gene therapy with 16K human prolactin efficiently reduces tumor growth. Cancer Lett 284(2):222–228. https://doi.org/10.1016/j.canlet.2009.04.030
Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, Creatsa M, Christodoulakos G, Alevizaki M, Sfikakis PP, Papamichael C (2009) Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension 54(1):98–105. https://doi.org/10.1161/HYPERTENSIONAHA.109.132100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or experiments performed on animals by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rana, M., Jain, S. & Choubey, P. Prolactin and its significance in the placenta. Hormones 21, 209–219 (2022). https://doi.org/10.1007/s42000-022-00373-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-022-00373-y