Abstract
This review provides an overview of menopausal hormone therapy and pulmonary disease risk, with a focus on the effect of hormone replacement therapy (HRT) on pulmonary function and its relation to lung diseases. This summary is based on authors' knowledge in the field of HRT and supplemented by a PubMed search using the terms "menopause hormone therapy," "asthma”, "lung cancer”, "chronic obstructive pulmonary disease", “lung function”, and “pulmonary hypertension". Available evidence indicates that there is limited research on the role of sex hormones in the susceptibility, severity, and progression of chronic respiratory diseases. However, some studies suggest that the hormonal changes that occur during the menopausal transition may have an impact on pulmonary function and respiratory diseases. Women are in need of convenient access to a safe and effective modality for personalized HRT based on an artificial intelligence (AI)-driven platform that will enable them to receive personalized hormonal treatment through frequent, convenient, and accurate measurements of hormone levels in peripheral blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

1. Female sex hormones play a critical role in preserving lung function in middle-aged women. Studies have shown that long-term HRT is associated with a decreased loss of lung function over a 20-year period. |
2. The increasing health and economic impact of respiratory conditions highlights the importance of targeted interventions during HRT to prevent permanent damage to lung function. |
3. The ongoing discussion centers around the connection between female reproductive hormones and conditions like asthma and obstructive pulmonary disease (COPD), alongside the effects of hormone replacement therapy (HRT) on the occurrence of lung cancer and pulmonary hypertension (PH). It is our belief that consistent observation is crucial to guarantee the secure and efficient application of HRT. |
4. We are of the opinion that women should have convenient access to a secure and efficient avenue for personalized hormone replacement therapy (HRT). One viable option involves utilizing an AI-powered platform. Such a platform should empower women to undergo hormonal treatment by consistently, conveniently, and precisely monitoring their hormone levels. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24126312.
Introduction
Menopausal hormone therapy (MHT), previously known as hormone replacement therapy (HRT), was once considered a highly effective treatment for various postmenopausal symptoms, such as vasomotor symptoms, vaginitis, and osteoporosis. About 80 years ago, the Food and Drug Administration approved the use of conjugated equine estrogen as a form of systemic exogenous estrogen to alleviate these symptoms. Historically, mixtures of conjugated estrogens (Fig. 1) have been the most common agents for postmenopausal women. In the 1960s and 1970s, the use of estrogen replacement therapy (ERT) (i.e., estrogens alone) significantly increased among postmenopausal women due to the immediate and noticeable symptom relief. However, around 1980, epidemiological studies revealed that ERT treatment increased the incidence of endometrial carcinoma, leading to the introduction of HRT, which includes a progestin. Over the years, side effects have prompted a substantial reduction in estrogen dose for HRT. Despite these complications, the benefits of estrogen therapy in alleviating vasomotor symptoms, preventing bone fractures, and improving urogenital atrophy have resulted in its continued use in postmenopausal women [1].
In 1998, the Women's Health Initiative (WHI) began the largest randomized study to date, aimed to assess the effects of HRT on common causes of death and disability in postmenopausal women, including cardiovascular disease, cancer, and osteoporosis. However, the WHI trial suffered from an incomplete experimental design, leading to ambiguous results. As a result, the widespread use of HRT significantly declined. Although the epidemiological data did not strongly support clear harm to women's health, many symptomatic postmenopausal women experiencing symptoms were left without an effective treatment option. Despite this, the unwarranted use of HRT continues in various parts of the world. Subsequent studies have reinforced the notion that HRT provides significant benefits to symptomatic women who begin treatment within 10 years of menopausal onset or to those under the age of 60 years. Presently, HRT is administered in the form of estrogen alone for women without a uterus or as a combination of estrogen and progesterone for women with a uterus. Studies, including the WHI, have played a significant role in raising awareness about the risks associated with HRT, particularly in relation to cardiovascular diseases, stroke, venous thromboembolism, and breast cancer. While millions of women worldwide receive HRT as a preventive measure for chronic diseases like osteoporosis, concerns have emerged regarding its use in the presence of other disorders. The relationship between female reproductive hormones and asthma, obstructive pulmonary disease (COPD), as well as the impact of HRT on the incidence of lung cancer and pulmonary hypertension (PH), remains a subject of ongoing debate. Although numerous studies and clinical trials have explored the association between menopausal HRT, limited research has been conducted to investigate the connection between HRT and lung diseases. This review aims to examine the gender in lung diseases, explore the potential mechanisms of action of HRT, and assess the association between HRT and the most commonly occurring lung diseases.
Data Sources
A comprehensive literature search was performed using keywords related to hormone replacement therapy, post-menopausal women, asthma, chronic obstructive pulmonary disease, pulmonary hypertension, and lung function. A systematic search was performed in PubMed and Embase databases from inception to December 31, 2022. Initially, titles of the identified studies were screened, and abstracts of the relevant studies were read. We selected only full-text English language articles describing HRT effect on women with different lung diseases. Disagreements were settled by discussion and consensus among the authors. Relevant results are summarized and discussed in this review. Two reviewers (EE and MGK) independently screened research sources to identify publications that described the use of HRT in relation to lung diseases. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Gender Disparity in Lung Disease
The sexual dimorphism of lung disease is influenced by a complex and dynamic interplay of factors, including lung structure, hormones, and development. Sexual dimorphism characterized by differences between males and females can be observed in various disease types across different organ systems. In the case of respiratory diseases, differences in lung structure between males and females may contribute to patterns of sexual dimorphism. Female lungs tend to be smaller, with fewer respiratory bronchioles and smaller airways [2], while male lungs have a larger luminal area for the airways in relation to lung size [3]. These structural disparities may play a role in the observed sexual dimorphism in respiratory diseases. Additionally, females undergo a complex sex hormone cycle that changes over the course of development and maturation, which further contributes to the differences. Although numerous studies have reported statistical differences between sexes in relation to respiratory diseases, there is a lack of comprehensive research on the specific role of sex hormones in influencing the susceptibility, severity, and progression of chronic respiratory diseases. Numerous studies have revealed gender disparities in the prevalence and severity of various lung pathologies. For instance, idiopathic pulmonary fibrosis is found to be twice as common in males [4], while cystic fibrosis and PH exhibit greater severity in women [5]. The interplay between sex and diseases becomes particularly evident in chronic lung diseases like asthma, COPD, and lung cancer. Recent research has started to uncover the potential influence of sex hormones on these diseases, offering new opportunities for investigation and treatment.
Female Reproductive Hormones and Hormone Replacement Therapy
The menopausal transition is characterized by a notable hormonal shift, marked by decreased levels of estrogen and progesterone [6]. These sex steroids are known to affect numerous cellular pathways and functions beyond nonreproductive tissues, leading to the suggestion that reduced estrogen levels could have applications for lung disease [7]. While a considerable number of studies have been conducted on this topic, the precise impact of the menopausal transition on lung disorders remains unclear. During this transition, low estrogen levels commonly give rise to an array of disruptive symptoms, including hot flashes, night sweats, and the genitourinary symptoms of menopause [8]. To alleviate these symptoms, systemic exogenous estrogen administration in the form of conjugated equine estrogen was approved by the Food and Drug Administration around 80 years ago. Today, HRT is administered in the form of estrogen alone for women without a uterus or as a combination of estrogen and progesterone for women with a uterus. As HRT therapy can also serve as a preventive measure for chronic diseases like osteoporosis, millions of women worldwide receive it. However, controversy exists regarding the potential benefits and harms of HRT. While it provides relief for menopausal symptoms, there are concerns about its association with other disorders. HRT has long been considered a biologically plausible treatment for menopausal symptoms, but the potential side effects have not been thoroughly assessed until recently [9]. Studies, such as the one from WHI, have raised awareness about the risks associated with HRT, particularly in relation to cardiovascular diseases, stroke, venous thromboembolism, and breast cancer [10]. However, there has been relatively less attention paid to the effects of HRT on respiratory disease.
Potential Mechanisms of Action of Hormone Replacement Therapy
The hypothalamic–pituitary–gonadal (HPG) axis plays a crucial role in regulating reproductive function. This hormonal cascade starts with the hypothalamus, where gonadotropin-releasing hormone (GnRH) protein is released. GnRH then stimulates the pituitary gland, leading to the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) proteins [11]. These hormones, in turn, act on the gonads to regulate the synthesis of various sex hormones, including small molecule hormones estrogen, progesterone, testosterone and protein hormones activin, inhibin, and follistatin [12]. Changes to the HPG axis can have significant effects on reproduction [13]. For instance, during the post-menopausal period, there is a significant decline in sex hormone synthesis, which results in the loss of hypothalamic feedback inhibition. This leads to an increase in GnRH and gonadotropin (LH, FSH) levels after ovarian senescence. The absence of estrogen and progesterone production by the ovaries during menopause disrupts the delicate balance within the HPG axis, causing changes in hormonal regulation and reproductive function [14]. While the effects of estrogen on the female reproductive system are well established, recent research has uncovered its specific impact on the cardiovascular system, particularly the vasculature. Studies have suggested that estrogen in the dose used for hormone replacement has beneficial effects on the circulatory system; however, the overall conclusions from clinical studies remain largely equivocal [15]. On one hand, estrogen has been found to reduce atherosclerosis and inflammatory processes in the vasculature, suggesting a protective role [16]. Additionally, estrogen may act as an antioxidant, further contributing to cardiovascular health [17]. However, these effects account for only a portion of the total cardiovascular benefits associated with estrogen. Further research is needed to better understand the interplay between estrogen and cardiovascular function.
Estrogen acts as a vasodilator and hypotensive agent, promoting vascular relaxation through the stimulation of endothelium-derived vasodilatory substances, such as nitric oxide (NO), or by acting directly on the vascular smooth muscle (VSM). These cardiovascular effects of estrogen have been associated with potential benefits in extending both the quality and quantity of life. However, it is important to note that estrogen can also have lethal consequences in other tissues, though further clarification on the specific risks is warranted [18].
Moreover, estrogen cardiovascular effects are time-dependent, as its initiation of HRT more than 10 years after menopause onset was no longer beneficial. To address this intriguing paradox, careful monitoring and personalized treatment dosing may be necessary. It is crucial to consider individual factors when determining the appropriate estrogen therapy for each patient.
Furthermore, these changes in hormone levels can have systemic effects on various physiological processes, including respiratory function. Understanding the broader implications of estrogen’s effects on the cardiovascular system in relation to other physiological systems is an area of ongoing research.
The Effect of Hormone Replacement Therapy on Pulmonary Function
There is evidence suggesting that sex steroids, such as estrogen and progesterone, may have a positive impact on pulmonary functions in young women [19]. The relationship between HRT and pulmonary functions in postmenopausal women remains an area of limited understanding. Menopause is often associated with the exacerbation of preexisting asthma and may even coincide with the clinical onset of asthma. Several studies have investigated the impact of HRT and pulmonary function tests (PFT) in postmenopausal women, yielded conflicting results. Some studies that exclusively utilized estrogen for HRT, with a relatively small number of asthma cases, reported a negative effect of estrogen on lung function [20]. However, another study employing a cross-over mechanism found that among women with asthma using estrogen for HRT, interruption or resumption of the drug did not significantly affect respiratory function [21]. Moreover, a prospective randomized controlled trial observed significant increases in measured and percent-predicted forced expiratory volume (FEV1) and forced vital capacity (FVC) values after HRT application, particularly continuous combined estrogen and progesterone therapy [22]. The population-based European Community Respiratory Health Survey, conducted across multiple countries, examined the association between HRT and lung function in postmenopausal women. In this study, spirometry data were collected for 1075 women at baseline and after a follow-up period of 20 years. The study focused on women who were non-menopausal at baseline but became postmenopausal during the follow-up period. The findings of this study indicated that long-term oral HRT was significantly associated with decreased loss of lung function over the 20-year period. These sex steroids play an important role in the preservation of lung function in middle-aged women. The accelerated decline in lung function observed with menopause is likely attributed to the decreasing levels of estrogen [23].
HRT and Different Lung Diseases. Clinical Implications
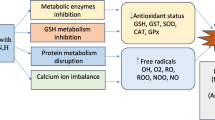
A growing body of evidence suggests that the hormonal changes that occur during the menopausal transition may have an impact on pulmonary function, lung vessels, and respiratory diseases (Fig. 2). Although the exact mechanisms by which the decline in estrogen and progesterone production affects respiratory health are not well understood, it has been proposed that sex hormones may act directly on airway and immune cells to modulate respiratory disease pathways [24]. Diminished pulmonary function is an important predictor of mortality and morbidity [25]. Poor lung function is a hallmark of chronic respiratory diseases such as asthma and COPD, which are characterized by decreased lung function and represent a significant portion of worldwide public health and economic burdens. With an aging population and increased life expectancy, an increasing number of women are spending a significant proportion of their lives in the postmenopausal state. Therefore, it is crucial to comprehend the impact of significant hormonal changes of menopause on respiratory health. This understanding can help the development of appropriate prevention and management strategies for women at risk of chronic respiratory diseases. The rising health and economic impacts of respiratory conditions underscore the importance of targeted interventions and optimal disease management to prevent permanent impairment of lung function. To deliver safe hormonal supplement to support post-menopausal health, we believe that HRT monitoring and a personalized treatment are essential.
Lung Cancer
The impact of estrogens on the growth of tumors in female reproductive organs by interacting with estrogen receptors is well established, as evidenced by many studies [26,27,28]. However, the role of estrogens in lung cancer remains unclear. It is believed that exogenous hormones may play a role in lung cancer development, however, the biologic mechanisms underlying hormone metabolism and lung cancer are not well understood. Lung cancer is one of the most diagnosed cancers and the leading cause of cancer death globally. Women appear to be more susceptible than men to developing lung adenocarcinoma, particularly among non-smokers, and have better survival than men worldwide [29]. Furthermore, estrogen and progesterone receptor expression in healthy lung cells and tumor cells have been confirmed, and that estrogens may promote lung tumorigenesis while progesterone inhibits it (Fig. 3). However, the influence of hormone changes and exposures on lung cancer risk and outcomes are not fully investigated. It is possible that hormone changes and exposures in women may play a role as indicated by Moore KA and colleagues [30], which found among 15,000 individuals that estrogen exposure appeared to have a protective effect on lung cancer-specific mortality with differences seen in premenopausal and postmenopausal women, and between postmenopausal women and men. Additionally, there is evidence that growth of progesterone receptor-positive non-small-cell lung cancers grow with progesterone receptor stimulation [31]. Studies evaluating the use of HRT and its impact on incident lung cancer have yielded conflicting results. Many of these studies have limitations such as not examining individual HRT formulations, duration of use, limited adjustment for the confounding effects of tobacco use, and/or limited to nonsmokers [32, 33]. We believe that frequent estrogen level monitoring during HRT for lung cancer patients can increase our knowledge about the pathogenetic influence of estrogen of the disease progress and survival.
Asthma
Asthma is a chronic disease of the airways characterized by a history of respiratory symptoms including wheezing, shortness of breath, repetitive coughing, and chest tightness, plus variable expiratory airflow limitation, all of which may vary over time and in intensity. Asthma is commonly linked to inflammation and increased sensitivity of the airways in response to specific or non-specific triggers, though these factors alone may not be necessary or sufficient for an asthma diagnosis [34]. Adult-onset asthma is often progressive and the cause of considerable morbidity. The major increase in asthma prevalence observed in most developed countries in recent years suggests that it may in part be due to environmental factors [35]. Although asthma is a multifactorial disorder, which affects people of all ages, there is clear evidence that there are significant age dependency and sex-related differences in the clinical manifestations of the disease [36]. Differences in asthma incidence during the life cycle suggest that reproductive hormones influence the development of asthma and its severity. Adult asthma and hospital admissions for asthma are more prevalent in women than in men, and asthma severity has been shown to vary during the menstrual cycle [37]. HRT has long been suspected to play a role in asthma in women [38] (Fig. 4). In addition, some women may experience asthma exacerbations because of HRT, and certain publications suggest the existence of a subgroup of asthma patients who experience poorer symptom control while on HRT, possibly due to specific variations in the estrogen receptor gene [39]. Several animal models suggest that both progesterone and estrogen may directly affect the lungs by reducing contractility and increasing relaxation of bronchial smooth muscle [40]. Further, estrogen withdrawal increases bronchial airway contraction [41]. Progesterone, which is responsible for maintaining relaxation of smooth muscle in the main organs, has been found to reduce the contractility of bronchial smooth muscle. Synthetic progesterone may also have androgenic properties and depress responses to histamine, thus causing improvements in pulmonary function among oral contraceptive women [42]. Studies examining oral contraceptive usage in women with moderate-to-severe asthma are scarce, characterized by small sample sizes, and lack adequate control for potential confounding factors like dose of medication usage, allergic status, allergen exposure, and allergic reaction. Currently, no studies have compared lung symptomatology or pulmonary function based on the dosage or type of oral contraceptive medication or HRT. Nonetheless, many physicians believe that oral contraceptive usage could potentially alleviate menstrual fluctuations in both pulmonary function and symptomatology by providing a consistent hormonal environment. Within the national longitudinal cohort of perimenopausal and postmenopausal individuals, there is a lack of data on this topic. These results were overall similar for peri- and postmenopausal women [43]. HRT might play an important role in the development of asthma in mature women and clinicians prescribing HRT should be aware that new airway symptoms might develop, and that patients with pre-existing asthma might get worse following HRT initiation [44]. Based on these studies, we are suggesting that asthma patients can also benefit from HRT if frequent and accurate hormonal blood levels are monitored.
COPD
Chronic obstructive pulmonary disease (COPD) is a widespread global ailment affecting millions of individuals and ranking as the fourth leading cause of death worldwide. As reported by the Global Burden of Disease study, an estimated 74.5 million adults were living with COPD in 2015, and it was responsible for more than 3 million deaths [45, 46]. With the aging population and ongoing exposure to risk factors for COPD, its burden is projected to escalate in the forthcoming decades. Moreover, COPD frequently coexists with other medical conditions that can influence the progression of the disease. Many individuals endure the effects of COPD for extended periods and succumb prematurely to COPD itself or its associated comorbidities. Common comorbidities linked to COPD include heart problems, pulmonary hypertension, impaired exercise capacity, weight loss, respiratory infections, etc. These factors contribute to a deteriorated health status and poor prognosis. Unfortunately, these comorbidities often go undiagnosed in many low-income regions. While medical treatment for COPD tends to focus primarily on the primary organ dysfunction, it is essential to recognize and treat these comorbidities, as they are potentially remediable.
The incidence of COPD in women has been reported to be increasing [47]. Although smoking is the main risk factor for COPD, females tend to develop COPD faster than males even though they smoke fewer cigarettes [48]. In non-smokers, females make up two-thirds of cases with COPD [49]. Research has shown that cell proliferation contributes to the intimal thickening of pulmonary arteries in smokers and non-smokers people with COPD [50]. The injury of vessel’s smooth muscle and endothelial cells in of smokers and individuals with COPD has been linked to the potential growth-promoting effects of estrogen. The metabolism of estrogen in the lungs and respiratory tree can result in oxidative stress, which further contributes to the proliferation of these cells. Estrogens undergo hydroxylation to form catechol estrogens, which act as substrates in redox reactions catalyzed by cytochrome P450 [51]. Consequently, the potentiation of oxidative stress by estrogen may increase the susceptibility of female smokers to COPD and worsen the course of the disease. Various animal models have been utilized to explore potential sex-related disparities in COPD risk. Chronic exposure of mice to cigarette smoke has induced emphysematous-like changes in the structure of alveoli, with these changes occurring more rapidly in females compared to males. This information holds significance in understanding the mechanisms involved in the development and progression of COPD [52]. It is important to note that cigarette smoke is composed of more than 4000 different chemicals that undergo two different phases of metabolism. Metabolites from cigarette smoke are toxic, and the lungs may suffer oxidant damage as a result. Estradiol up-regulates cytochrome P450 enzymes, making female lungs more susceptible to oxidant damage in response to cigarette smoke. Animal experiments have supported this concept [53]. Female lungs had higher expression of cytochrome P450 enzymes and demonstrated increased accumulation of potent oxidants from other metabolites. The increased cytochrome P450 expression is related to increased levels of estradiol and increased metabolism of cigarette smoke to generate oxidants, suggesting that female sex hormones contribute to oxidative stress and greater airway injury [54].
The available evidence concerning the relationship between HRT and COPD is limited. A clinical trial conducted as part of the Cardiovascular Health Study found that older women (aged over 65 years) currently receiving HRT exhibited higher lung function parameters compared to women who were not receiving HRT [55]. Respiratory function indices were also higher among current HRT users in comparison to those who had never used HRT or were past users. These findings demonstrate a potential short-term effect of HRT on the bronchial tree in COPD. However, caution should be exercised when making recommendations for women with previously diagnosed COPD due to inconsistencies in the available results. It is important to note that women with asthma exacerbations and type A and B of COPD who receive HRT are at a heightened risk of developing osteoporosis, breast cancer, and may also face an increased risk of cardiovascular events. Hence, HRT presents potential benefits and risks for these patients beyond its pulmonary effects. Some women may experience exacerbations because of HRT, and certain publications suggest the existence of a subgroup of asthma patients who experience poorer symptom control while on HRT, possibly due to specific variations in the estrogen receptor gene [56]. This may also hold true for COPD. However, further research is needed to clarify the association between HRT and chronic pulmonary diseases. As there is significant interpatient variability in hormonal blood levels following HRT administration, measuring and monitoring of the exact hormonal blood levels during HRT treatment can potentially help in understanding the aforementioned controversial results.
Pulmonary Hypertension
Pulmonary hypertension (PH)) is a severe and potentially fatal disease that primarily affects the pulmonary vasculature. It is characterized by the proliferation of neointimal cells and hypertrophy of smooth muscle cells, leading to the formation of occlusive lesions in the smallest pulmonary arteries [57, 58]. PH is a prevalent condition, with around 250,000 hospitalizations in the United States annually, either as a primary or secondary diagnosis [59, 60]. Although the global prevalence of acquired and congenital pulmonary hypertension remains uncertain, significant progress has been made in comprehending the molecular mechanisms underlying the development of the disease and the development of novel therapeutic strategies over the past decade [61]. Nevertheless, PH continues to be a devastating clinical condition that significantly impacts patients' morbidity and mortality. Previously considered a rare disease, recent studies have reported a prevalence of 15 to 50 cases per million individuals [62, 63]. The World Health Organization (WHO) classifies pulmonary hypertension into five distinct groups. Group 1 comprises pulmonary arterial hypertension (PAH), including idiopathic PAH, heritable PAH, PAH associated with congenital heart disease, liver disease, HIV infection, connective tissue diseases, and drug/toxin-induced PAH. Group 2 encompasses PH due to left heart disease, which includes both systolic and diastolic dysfunction. Group 3 involves PH associated with lung diseases such as chronic obstructive pulmonary disease (COPD), emphysema, and pulmonary fibrosis. Group 4 is characterized by PH resulting from chronic thromboembolic pulmonary hypertension. Finally, Group 5 comprises PH secondary to various miscellaneous diseases, including sarcoidosis, sickle cell anemia, chronic hemolytic anemia, and different metabolic disorders.
Although PH affects both sexes, it is more frequently observed in females than in males, with a ratio of 4.1:1. The exact mechanisms responsible for this gender disparity remain unclear, but one potential explanation is the damaging effect of estrogens on the pulmonary vasculature. Many women are exposed to estrogens through hormone replacement therapy. However, conflicting data exist regarding the role of HRT in PH. Therefore, it is important to specify the PH group in which the studies were conducted. With an improved understanding of the specific genetic mutations associated with PAH, heritable PAH includes patients with identified mutations in the bone morphogenic protein receptor type 2 (BMPR2) gene, activin receptor-like kinase type 1 (ALK-1) gene, and endoglin. Research on heritable PAH has demonstrated that estrogens suppress the expression of wild-type BMPR2 in model systems by binding to the BMPR2 promoter via the estrogen receptor-α [64]. The concept that estrogens increase susceptibility to PAH by reducing BMPR2 expression aligns with previous data showing reduced BMPR2 expression in PAH lungs, even among individuals with normal genotypes [65]. Furthermore, specific estrogen metabolites may promote the proliferation of pulmonary vascular cells more than others, adding additional complexity to the relationship between estrogens, sex, and susceptibility to PAH [66]. While a small retrospective study found an association between HRT and a lower rate of PH due to systemic sclerosis [67], another report described the rapid onset of heritable PH following HRT [68]. Many physicians do not recommend routine use of HRT unless there is a compelling reason to continue. We believe that the actual level of estrogen during HRT use can explain these differences depending on the PH group. A randomized, double-blind, placebo-controlled trial of anastrozole in patients with idiopathic PAH demonstrated that reducing estradiol levels did not have an effect on the primary outcome of PH, nor did it impact circulating biomarkers, functional class, or health-related quality of life [69]. In vivo studies have indicated that estrogen plays a critical role in regulating endothelial cell proliferation and function [70]. Estrogen has been shown to induce the expression of vascular endothelial growth factor (VEGF), reduce protein synthesis necessary for apoptosis, and upregulate protective genes involved in cell death pathways, potentially contributing to both apoptosis resistance and endothelial cell proliferation [71]. Given our current understanding of PH pathology, which involves the transformation of pulmonary vascular endothelium into a highly proliferative and apoptosis-resistant phenotype [72], there is a growing interest in the impact of estrogen exposure on PH, particularly in groups 1 and 5 of the PH classification. Notably, an intriguing study revealed that 71% of premenopausal and 86% of postmenopausal women with PH (with 56% having primary heritable or idiopathic PH and 27% having connective tissue disease) reported previous or current use of hormone therapy for more than 10 years [73].
In conclusion, PH is a debilitating disease that predominantly affects women. While basic scientific research has provided limited and conflicting information regarding the effects of sex hormones on the pulmonary vasculature, recent evidence strongly suggests that altered levels and metabolism of estrogen likely play a significant role in the pathophysiology of PH, particularly in idiopathic and heritable forms of the disease. Further research focusing on the influence of sex hormones, gender-specific therapy responses, genomics, and sexual dimorphism in pulmonary function is crucial for advancing our understanding and management of this devastating condition.
Future Direction
Women experience more than a third of their lifetimes within the peri- and post-menopausal phases. Notably, trends suggest that by 2030, approximately 1.2 billion women worldwide will find themselves in these crucial life stages. The female journey from puberty to menopause encompasses profound hormonal transformations, yet the current landscape of hormonal treatment lacks the foundation of data-driven precision medicine. Hormone replacement therapy (HRT) stands as a central approach to managing menopausal symptoms. This therapeutic method involves the introduction of hormone supplements to restore equilibrium to systemic hormonal levels, thereby enhancing and sustaining both emotional and physical well-being.
Numerous health conditions, including depression, diabetes, fatigue, cardiovascular disease, insomnia, migraine headaches, obesity, osteoporosis, and cancer, can arise from disruptions in hormonal balance. While the benefits of HRT are evident, it is imperative that hormone supplementation be administered in a regulated and closely monitored manner. Excessive usage of hormones can lead to adverse side effects and potentially grave health hazards. Furthermore, the ideal levels of hormones and individual sensitivity to hormone therapy exhibit substantial variance from person to person. Consequently, the establishment of personalized treatment protocols, incorporating predetermined dosages for achieving optimal balance, emerges as a pivotal necessity.
Artificial intelligence (AI) and machine learning technology are making significant strides in the field of medicine, primarily due to their unparalleled capability to provide highly accurate predictions. AI-powered systems have demonstrated their prowess in forecasting clinical data [74]. Notably, predictive analytic algorithms have already been employed to guide decision-making at critical junctures of ovarian stimulation [75]. Furthermore, a predictive model has been developed to furnish tailored estimations of the cumulative probabilities of successful live births before embarking on in vitro fertilization (IVF) [76]. While our investigation yielded no data regarding the nexus of AI and hormone replacement therapy (HRT), we hold the conviction that AI has the potential to wield a substantial influence in this domain. With the global population aging, the demand for efficient and effective hormone replacement treatments is mounting. To address this burgeoning need, researchers and medical practitioners can explore the capacity of AI to redefine the landscape of HRT. Envisioning a future where personalized computer-assisted HRT is the norm, we anticipate a more exacting and controlled approach to maintaining hormonal equilibrium within the body. Through the utilization of AI-driven systems, medical professionals and patients can administer hormones with enhanced precision and consistency, ensuring the administration of optimal dosages tailored to individual requirements. Furthermore, this approach can be attuned to each patient's distinctive hormonal profile, ushering in a more personalized dimension to the treatment process.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Loose DS, Stancel GM. Estrogens and Progestins. In: Brunton LL, Lazo JS, Parker KL, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill; 2006. p. 1541–72.
Chiarella SE, Cardet JC, Prakash YS. Sex, Cells, and Asthma. Mayo Clin Proc. 2021;96(7):1955–69.
Sheel AW, Dominelli PB, Molgat-Seon Y. Revisiting dysanapsis: sex-based differences in airways and the mechanics of breathing during exercise. Exp Physiol. 2016;101(2):213–8.
Han MK, Murray S, Fell CD, Flaherty KR, Toews GB, Myers J, et al. Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31(6):1183–8.
Lam GY, Goodwin J, Wilcox PG, Quon BS. Sex disparities in cystic fibrosis: review on the effect of female sex hormones on lung pathophysiology and outcomes. ERJ Open Res. 2021;7(1):00475–2020.
Su HI, Freeman EW. Hormone changes associated with the menopausal transition. Minerva Ginecol. 2009;61:483–9.
Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24.
Igbal J, Zaidi M. Understanding estrogen action during menopause. Endocrinology. 2009;150:3443–5.
Lee SR, Cho MK, Cho YJ, Chun S, Hong SH, Hwang HR, et al. The 2020 menopausal hormone therapy guidelines. J Menopausal Med. 2020;26:69–98.
Hodis HN, Mack WJ. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: it is about time and timing. Cancer J. 2022;28:208–23.
Perrett RM, McArdle CA. Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol (Lausanne). 2013;4:180.
Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: understanding advantages and limitations. J Hum Reprod Sci. 2014;7:170–4.
Bizzari C, Cappa M. Ontogeny of hypothalamus–pituitary–gonadal axis and minipuberty: An ongoing debate? Front Endocrinol (Lausanne). 2020;11:187.
Blair JA, McGee H, Bhatta S, Palm R, Casadesus G. Hypothalamic–pituitary–gonadal axis involvement in learning and memory and Alzheimer’s disease: more than “just” estrogen. Front Endocrinol (Lausanne). 2015;6:45.
Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:133–8.
Xie F, Li X, Xu Y, Cheng D, Xia X, Lv X, et al. Estrogen mediates an atherosclerotic-protective action via estrogen receptor Alpha/SREBP-1 signaling. Front Cardiovasc Med. 2022;9: 895916.
Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54–70.
Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials. 2012;7:47–70.
Sathish V, Martin YN, Prakash YS. Sex steroid signaling: Implications for lung diseases. Pharmacol Ther. 2015;150:94–108.
Pata O, Atiş S, Utku Oz A, Yazici G, Tok E, Pata C, et al. The effects of hormone replacement therapy type on pulmonary functions in postmenopausal women. Maturitas. 2003;46(3):213–8.
Lieberman D, Kopernic G, Porath A, Levitas E, Lazer S, Heimer D. Influence of estrogen replacement therapy on airway reactivity. Respiration. 1995;62(4):205–8.
Hepburn MJ, Dooley DP, Morris MJ. The effects of estrogen replacement therapy on airway function in postmenopausal, asthmatic women. Arch Intern Med. 2001;161(22):2717–20.
Cevrioglu AS, Fidan F, Unlu M, Yilmazer M, Orman A, Fenkci IV, et al. The effects of hormone therapy on pulmonary function tests in postmenopausal women. Maturitas 2004 Nov 15;49(3):221–7
Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13(1):92–9.
Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health. 1999;53(4):230–4.
Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62(7):2141–50
Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res. 1990;50(20):6632–5.
Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol. 2002;188(1–2):125–40.
Jin K, Wu M, Zhou JY, Yang J, Han RQ, Jin ZY, et al. Tobacco smoking modifies the association between hormonal factors and lung cancer occurrence among post-menopausal Chinese women. Transl Oncol. 2019;12(6):819–27.
Moore KA, Mery CM, Jaklitsch MT, Estocin AP, Bueno R, Swanson SJ, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann Thorac Surg. 2003;76(6):1789–95.
Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, et al. Progesterone receptor in non-small cell lung cancer–a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65(14):6450–8.
Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18(5):607–12.
Schwartz AG, Wenzlaff AS, Prysak GM, Murphy V, Cote ML, Brooks SC, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non-small-cell lung cancer in women. J Clin Oncol. 2007;25(36):5785–92.
Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J. 2008;32:487–502.
Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246.
Ticconi C, Pietropolli A, Piccione E. Estrogen replacement therapy and asthma. Pulm Pharmacol Ther. 2013;26:617–23.
Fuseini H, Newcomb DC. Mechanism driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17:19.
Tattersfield AE. Is postmenopausal HRT a risk factor for adult-onset asthma? Thorax. 2010;65(4):282–4.
Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28.
Foster PS, Goldie RG, Paterson JW. Effect of steroids on beta-adrenoceptor-mediated relaxation of pig bronchus. Br J Pharmacol. 1983;78(2):441–5.
Skobeloff EM, Spivey WH, Silverman R, Eskin BA, Harchelroad F, Alessi TV. The effect of the menstrual cycle on asthma presentations in the emergency department. Arch Intern Med. 1996;156(16):1837–40.
Matsuo N, Shimoda T, Matsuse H, Kohno S. A case of menstruation-associated asthma: treatment with oral contraceptives. Chest. 1999;116(1):252–3.
Nwaru BI, Shah SA, Tibble H, Pillinger R, McLean S, Ryan D, et al. Hormone replacement therapy and risk of severe asthma exacerbation in perimenopausal and postmenopausal women: 17-year national cohort study. J Allergy Clin Immunol Pract. 2021;9(7):2751–60.
Hansen ESH, Aasbjerg K, Moeller AL, Gade EJ, Torp-Pedersen C, Backer V. Hormone replacement therapy and development of new asthma. Chest. 2021;160(1):45–52.
Ingadottir AR, Beck AM, Baldwin C, Weekes CE, Geirsdottir OG, Ramel A, et al. Two components of the new ESPEN diagnostic criteria for malnutrition are independent predictors of lung function in hospitalized patients with chronic obstructive pulmonary disease (COPD). Clin Nutr 2018;37(4):1323–1331
Vestbo J. Clinical assessment, staging, and epidemiology of chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc. 2006;3(3):252–6.
Han MK, Postma D, Mannino DM, Giardino ND, Buist S, Curtis JL, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176(12):1179–84.
Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335(13):931–7.
Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–43.
Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–8.
Liehr JG, Roy D, Ari-Ulubelen A, Bui QD, Weisz J, Strobel HW. Effect of chronic estrogen treatment of Syrian hamsters on microsomal enzymes mediating formation of catecholestrogens and their redox cycling: implications for carcinogenesis. J Steroid Biochem. 1990;35(5):555–60.
Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 2007;293(2): L272–8.
Van Winkle LS, Gunderson AD, Shimizu JA, Baker GL, Brown CD. Gender differences in naphthalene metabolism and naphthalene-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;282(5): L1122–34.
Sin DD, Cohen SB, Day A, Coxson H, Paré PD. Understanding the biological differences in susceptibility to chronic obstructive pulmonary disease between men and women. Proc Am Thorac Soc. 2007;4(8):671–4.
Carlson CL, Cushman M, Enright PL, Cauley JA, Newman AB. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med. 2001;163(2):423–8.
Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346(13):967–74.
Stenmark KR, Frid MG, Graham BB, Tuder RM. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc Res. 2018;114(4):551–64.
Tobal R, Potjewijd J, van Empel VPM, Ysermans R, Schurgers LJ, Reutelingsperger CP, et al. Vascular remodeling in pulmonary arterial hypertension: the potential involvement of innate and adaptive immunity. Front Med (Lausanne). 2021;22(8): 806899.
Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2010;122(2):164–72.
Harder EM, Small AM, Fares WH. Primary cardiac hospitalizations in pulmonary arterial hypertension: trends and outcomes from 2001 to 2014. Respir Med. 2020;161: 105850.
Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360: j5492.
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30.
Beshay S, Sahay S, Humbert M. Evaluation and management of pulmonary arterial hypertension. Respir Med. 2020;171: 106099.
Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, Phillips Iii JA, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6.
Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–8.
White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–98.
Beretta L, Caronni M, Origgi L, Ponti A, Santaniello A, Scorza R. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand J Rheumatol. 2006;35(6):468–71.
Morse JH, Horn EM, Barst RJ. Hormone replacement therapy: a possible risk factor in carriers of familial primary pulmonary hypertension. Chest. 1999;116(3):847.
Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, et al. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 2017; 195:360–368.
Applanat MP, Buteau-Lozano H, Herve MA, Corpet A. Vascular endothelial growth factor is a target gene for estrogen receptor and contributes to breast cancer progression. Adv Exp Med Biol. 2008;617:437–44.
Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17beta protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008;18(4):491–9.
Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(6):558–64.
Sweeny L, Voelkel NF. Estrogen exposure, obesity and thyroid disease in women with severe pulmonary hypertension. Eur J Med Res. 2009;14(10):433–42.
Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94–98.
Letterie G, Mac Donald A. Artificial intelligence in in vitro fertilization: a computer decision support system for day-to-day management of ovarian stimulation during in vitro fertilization. Fertil Steril. 2020;114(5):1026–1031.
McLernon DJ, Raja EA, Toner JP, Baker VL, Doody KJ, Seifer DB, Sparks AE, Wantman E, Lin PC, Bhattacharya S, Van Voorhis BJ. Predicting personalized cumulative live birth following in vitro fertilization. Fertil Steril. 2022;117(2):326–338.
Acknowledgements
We would like to gratefully acknowledge the Aveta.Life company, which is a science and clinical-based women’s health company, helping women achieve optimal health throughout their life journey. Also, we would like to thank Amihai Rottenstreich, MD for helpful discussion and revision.
Funding
This study and the journal’s Rapid Service Fee were funded by Aveta.Life Inc.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article conception and design, drafting, revising, and editing of the manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Efrat Eliyahu has received research support from Aveta.Life Inc., Lina Freage-Kahn, Henry Grage, Bazalel Arkush, Nataly Shtraizent, and Tuvia Barak received consultation fees from Aveta.Life Inc. The other co-authors have no conflicts to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eliyahu, E., Katz, M.G., Vincek, A. et al. Effects of Hormone Replacement Therapy on Women's Lung Health and Disease. Pulm Ther 9, 461–477 (2023). https://doi.org/10.1007/s41030-023-00240-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00240-0