Abstract
Over the past 22 years, annual GOLD Reports have documented important changes in guidance and recommendations for uniformly treating patients with chronic obstructive pulmonary disease (COPD) with the goal of improving outcomes in patients suffering from this condition. The most recent GOLD Report, released in 2023, shows continued refinement in several areas, including more precise definitions of COPD and exacerbations of COPD, a new set of parameters to assess exacerbation severity, an updated COPD assessment tool, updated guidelines for initial and follow-up treatment, new information regarding the association between pharmacological triple therapy and reduction in mortality, and new discussions of inhaler device choice and adherence to COPD medications. Whereas we do not address all of the new or updated material in GOLD’s 2023 Report, we summarize key changes in GOLD’s recommendations regarding inhalation therapy for stable COPD and frame these changes in the context of previous GOLD recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) is internationally recognized for the development of evidence-based strategy documents, most notably the annual GOLD Reports, for COPD diagnosis, management, and prevention |
The most recent (2023) GOLD Report has several updates relevant to COPD treatment, including updated definitions of COPD, recent associations of inhaled pharmacological therapy with mortality, expanded discussion related to inhaled drug delivery and adherence to inhaled medication, and updated recommendations for initial and follow-up pharmacological treatment |
We summarize key features of these updates in the context of previous GOLD recommendations |
Introduction
The annual Global Initiative for Chronic Obstructive Lung Disease (GOLD) reports for chronic obstructive pulmonary disease (COPD) diagnosis, management, and prevention are developed by expert committees for healthcare professionals on the basis of the available scientific evidence. The GOLD reports are used to implement effective management programs based on local healthcare systems around the world. Over the past 22 years, annual GOLD Reports have documented important changes in available pharmaceuticals, knowledge regarding effective dosages and potential side effects, the various combinations of drugs used to relieve symptoms, and various inhaler device options to apply these innovations to the care of patients with COPD [1]. The most recent GOLD Report, released in 2023 [2], shows continued refinement in several areas, including more precise definitions of COPD and exacerbations of COPD, a new set of parameters to assess exacerbation severity, an updated COPD assessment tool, updated guidelines for initial and follow-up treatment, updated information regarding the association between pharmacological treatment and mortality, and new discussions of inhaler device choice and adherence to COPD medications. Whereas we do not address all of the new or updated material in GOLD’s 2023 Report, we summarize key changes in GOLD’s recommendations regarding inhalation therapy for stable COPD and frame these changes in the context of previous GOLD recommendations. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by either of the authors.
Evolving Definitions of COPD
Definition of COPD
Defining a disease, or health-related condition, has implications regarding its prevalence, prevention, and treatment, and also how it is considered and analyzed for the purpose of research [3, 4]. In the appropriate clinical setting, the presence of airflow limitation (ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) < 0.7 after bronchodilator measured by spirometry) was considered to be confirmatory for COPD. Disease etiology and its natural history often provide a useful framework for conceptualizing and addressing illness [5], and these are the basis for the recent change in the COPD definition endorsed by GOLD [2, 4]. The first GOLD Report in 2001 defined COPD as:
A disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases [6].
Over the next two decades of GOLD reports, the definition of COPD evolved to include: (1) the concept of COPD as a preventable and treatable disease, (2) respiratory symptoms in addition to airflow limitation as important components of the disease, and (3) the fact that certain host factors and comorbidities may play a role in the development of COPD and its impact on the health of patients:
COPD is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases and influenced by host factors including abnormal lung development. Significant comorbidities may have an impact on morbidity and mortality [7].
As noted by Celli et al. [4], the latter definition still suffers from limitations that may have a negative influence on the research, prevention and treatment of COPD. For example, as reflected in the above definitions, COPD continues to be considered mainly a consequence of cigarette smoking. However, in many parts of the world, particularly for women, factors, such as exposure to biomass fuel for cooking, respiratory infections, asthma, abnormal lung development, accelerated lung aging, and genetic risk factors, are other causes underlying the development of COPD. Also noted by Celli et al., the natural history of COPD differs according to these etiological factors. Therefore, failing to recognize the unique way each factor may influence the development and progression of COPD results in missed opportunities for research, prevention, and effective treatment. Second, structural lung abnormalities detectable by computed tomography and other technology, as well as symptoms such as cough and sputum, can be indicative of COPD even in the absence of airflow limitation (referred to as “pre-COPD”) [4], and PRISm (Preserved Ratio Impaired Spirometry) identifies patients with normal FEV1/FVC ratio but abnormal spirometry (FEV1 or FVC < 80% of predicted). A definition of COPD that requires the presence of airflow obstruction will fail to capture and ultimately fail to address early-stage COPD. Based on these considerations, and citing Celli et al. [4], GOLD’s 2023 report expands the definition of COPD to include several causal agents and stages:
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction [2].
This evolving definition no longer states that COPD is a preventable and treatable disease, although it is stated elsewhere in the 2023 Report that “COPD is a common, preventable, and treatable disease, but extensive under- and mis-diagnosis leads to patients receiving no treatment or incorrect treatment.” This definition also no longer includes the role of comorbidities, although it is stated elsewhere in the report that comorbidities, including cardiovascular disease, skeletal muscle dysfunction, metabolic syndrome, osteoporosis, depression, anxiety, and lung cancer, occur frequently in patients with COPD, and that “these comorbidities should be actively sought, and treated appropriately when present, because they influence health status, hospitalization and mortality independently of the severity of airflow obstruction due to COPD” [2].
Definition of COPD Exacerbation
As noted in GOLD’s 2023 report [2], exacerbations are important events in the management of COPD because they negatively impact health status, rates of hospitalization and readmission, and disease progression. In their 2022 Report, GOLD defined COPD exacerbation as an acute worsening of respiratory symptoms that results in additional therapy [7]. The revised definition in the 2023 Report further specifies the symptoms, the time frame, and the likely causes of COPD exacerbation:
An exacerbation of COPD (ECOPD) is defined as an event characterized by increased dyspnea and/or cough and sputum that worsens in < 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by infection, pollution, or other insult to the airways [2].
The 2023 Report also emphasizes that other acute events, particularly decompensated heart failure, pneumonia, and pulmonary embolism, may mimic or aggravate an ECOPD. Thus, the worsening of dyspnea without the classic characteristics of ECOPD may be due to conditions other than ECOPD [2]. The revised definition of ECOPD and further clarification of potential confounding and/or contributing factors can help guide the assessment of ECOPD severity and aid in effective treatment.
Inhaled Pharmacotherapeutics for COPD
Association of Inhaled Pharmacological Therapy with COPD Mortality
Several nonpharmacological interventions, such as smoking cessation, long-term oxygen therapy, and lung volume reduction surgery, have long been shown to reduce mortality in patients with COPD [8]. Until recently, however, pharmacological therapy was based solely on the control of COPD symptoms, with no proven benefit regarding survival. Beginning in 2020, GOLD Reports considered data emerging from clinical trials showing triple combinations of a long-acting beta agonist/long-acting muscarinic antagonist/inhaled corticosteroids (LABA/LAMA/ICS), in comparison with LAMA, LABA/LAMA or LABA/ICS, reduced mortality in study populations that were enriched for increased respiratory symptoms and a prior history of frequent and/or severe exacerbations [9,10,11].
By 2021, more definitive data from clinical trials emerged showing reductions in mortality with triple therapy. The Informing the Pathway of COPD Treatment (IMPACT) trial [12] and the Efficacy and Safety of Triple Therapy in Obstructive Lung Disease (ETHOS) [13] trial were each 52-week phase III, randomized, double-blind, parallel-group, multicenter trials that included 10,355 (IMPACT) and 8509 (ETHOS) patients with moderate to very severe COPD and a history of exacerbations. Triple therapy versus a LABA/LAMA combination showed relative risks (RR) and 95% confidence intervals (CI) of 0.72 (0.53–0.99) in IMPACT and 0.51 (0.33–0.80) in ETHOS, suggesting reductions in mortality of 28% and 49%, respectively, with reasonable statistical confidence that the observed reductions in mortality were not due to chance. The randomized protocols, the large sample sizes, and the consistency of the trials’ findings also provide some assurance that the observed reductions in mortality were not due to bias. The GOLD 2023 Report highlights the latter results in a new table of evidence supporting a reduction in mortality with pharmacotherapy and nonpharmacotherapy in patients with COPD [2], which is reproduced here (with permission):

In 2021, a meta-analysis of data from 21,909 patients in the ETHOS, KRONOS, IMPACT, and TRILOGY studies compared triple LABA/LAMA/ICS versus either dual LABA/LAMA or LABA/ICS therapies administered at fixed-dose combination via the same inhaler device, the latter to avoid bias resulting from different inhaler devices being used in comparator arms [14]. That analysis showed patients on triple therapy had a statistically nonsignificant 29% reduction in mortality (RR 0.71, 95% CI 0.49–1.08) compared with those on LABA/LAMA, but had similar risk as those on LABA/ICS. Taking non-fatal endpoints into account, patients on triple therapy had better overall outcomes compared with those on LABA/LAMA or LABA/ICS, a result that was observed in patients with either low or high blood eosinophil (EOS) counts.
Issues Related to Inhaled Drug Delivery in Patients with COPD
GOLD’s 2017 Report first introduced the perennial section “Issues related to inhaled delivery,” which lists basic types of inhalation devices, provides guidance regarding the choice of inhaler device, and discusses the potential for their suboptimal use, which is considerable [15]. Key points made in this section include the fact that errors in device use are common and are mainly related to inspiratory flow (such as peak inspiratory flow rate), inhalation duration, coordination, dose preparation, exhalation maneuver prior to inhalation, and a breath-hold following inhalation. The 2017 and subsequent reports emphasize that the choice of inhalation device should involve a decision-making process based on the patient’s abilities, goals, and preferences; that inhaler device use training and education should be ongoing; and that clinicians should routinely check that patients continue to use their chosen device correctly. The reports also note the fact that patients may have physical and/or cognitive limitations that will reduce or preclude the effectiveness of education and training (we discuss this issue further in the next section).
The 2023 GOLD Report presents several updates to this section, including a greater emphasis on what is required from patients for each inhalation device and what clinicians can do to ensure proper device use [2]. The trend over recent GOLD reports of increasing attention to inhaler device choice is evident in the 2023 Report, which states that strength and dexterity are needed to actuate pressurized metered-dose inhalers (pMDIs), load dry powder inhalers (DPIs), and prime breath-actuated inhalers (BAIs). Tremor may result in shaking the device and loss of the dose. Whereas previous GOLD reports cite age as a risk factor for suboptimal inhaler use, the 2023 Report suggests that this association is confounded by cognitive impairment or reduced manual dexterity [2]. Overall, per the report, patients and clinicians should consider:
-
device size and portability
-
the number of steps required to prepare the device for use
-
the force needed to load or actuate the device
-
the inhalation maneuver required to use the device effectively
-
the time it takes the device to deliver drug(s)
-
the patient’s inspiratory flow, flow acceleration, and inhaled volume
-
the time and effort required for cleaning and maintenance of a device
-
the potential benefits of “smart” inhalers with sensors that can identify problems in real time and provide objective data on adherence and technique
Comorbidities, such as arthritis, osteoporosis, Parkinson’s disease, cognitive, memory or learning disabilities, neuromuscular weakness, heart disease, and extreme obesity, may limit the patient’s ability to effectively use any handheld inhalation device, particularly if their comorbidities are in advanced stages (discussed further in the next section). One may also consider whether a caregiver is involved and, if so, to what extent the caregiver is trained and available to help the patients with inhaler device use. Because there is no universal algorithm for weighting the many factors that can influence inhaler device choice, GOLD emphasizes here again that the decision should be made jointly by the patient and prescriber, with consideration of both objective and subjective input.
GOLD’s 2023 report re-emphasizes several of these key messages in the section “Ability to use delivery systems correctly” and in a table found in the section “Choice of inhaler device.” This table first appears in the GOLD 2023 Report, and is reproduced here (with permission):

Hand-Held Inhalers versus Nebulizers
Most patients with stable COPD are prescribed maintenance therapy via inhaler, due to the perceived convenience of inhalers compared with nebulizers. As we have discussed in detail elsewhere [1, 16], until recently, nebulizers were not generally recommended for maintenance therapy for COPD. For example, until 2010, GOLD Reports stated that “Nebulizers are not recommended for regular treatment because they are more expensive and require appropriate maintenance.” In 2010, GOLD no longer stated that nebulizers were not recommended for patients with stable COPD, but remained cautious regarding their use. Since the 2017 GOLD report, caveats regarding nebulizer use are no longer presented. Nonetheless, the 2023 GOLD Report still states that “there is no evidence for the superiority of nebulized therapy over hand-held devices in patients who are able to use these devices properly.” [2]. GOLD notes, however, that patients included in clinical trials are exceptionally trained and monitored for correct inhaler use and, therefore, may not be reflective of routine clinical practice.
The latter is an important caveat, given that inhalers and nebulizers have been widely considered to be equally effective when patients were trained to use their inhalers appropriately. However, poor inhaler technique compromises symptom relief in up to 94% of patients with COPD [17], and even extensive training may not mitigate patients’ misuse of inhalers. In a survey conducted by Hanania and colleagues [18], 79% of patients with COPD reported at least one physical or cognitive impairment that could limit their ability to correctly manipulate an inhaler device, including arthritis, poor eyesight, poor hearing, memory problems, tremor, difficulty with fine motor activities, depression, or anxiety, and more than half of the respondents had multiple limitations. Therefore, even assuming inhaler–nebulizer equivalence with perfect use, most patients with COPD may not achieve optimal benefits from inhalers due to comorbid physical and cognitive limitations that cannot be improved by device training alone. This may explain why recent investigations, especially those that include patient perceptions as an outcome measure, suggest nebulizers may provide more satisfactory symptom relief for many patients [16].
Although many patients in need of maintenance therapy for chronic lung disease can use pMDIs (with or without a spacer) or DPIs, certain patients will most likely benefit from drug administration by nebulizer [19, 20]. These include:
-
Patients with cognitive impairment, e.g., Alzheimer’s or other forms of dementia, intellectual disability, or altered consciousness, that precludes effective use of handheld inhalers;
-
Patients with impaired manual dexterity due to arthritis, Parkinsonism, or stroke;
-
Patients who have severe pain or muscle weakness due to neuromuscular disease;
-
Patients who are unable to use pMDIs or DPIs in an optimal manner despite adequate instruction and training, such as those patients who are generally debilitated after hospitalization or by chronic illness and are unable to coordinate their breathing with a pMDI, or they cannot generate adequate inspiratory flow for effective aerosol delivery from a DPI;
-
Patients with inadequate symptom relief with appropriate use of pMDIs/DPIs;
-
Patients who do not comply with the use of pMDIs and DPIs or who prefer nebulizers;
-
Patients who need respiratory medications that are not available in pMDI or DPI formulations; in the United States, for example, some inhaled bronchodilators, antibiotics, mucolytics, and prostaglandins are not available in hand-held inhalers;
-
Patients who are unable to afford therapy with pMDIs or DPIs.
Nebulizers are not free of potential problems with their use. For example, basic nebulizer inhalation technique, such as sitting in an upright position during therapy, may not be practiced by all patients [21]. Other potential disadvantages of nebulizers are their relatively poor efficiency, high residual volume of 0.5–1.5 mL, and a significant amount of aerosol wasted during exhalation with continuous operation. Limited access to accessories, the use of damaged parts, and patients engaging in self-repairs are other problems associated with nebulizer use [21]. Another limitation of nebulizers, which has now been resolved, was the lack of availability of LAMAs in solution. The approval of glycopyrrolate (Lonhala, Sunovion) in 2018 and revefenacin (Yupelri, Mylan/Theravance) in 2019 has overcome this limitation.
Adherence to Inhaled COPD Medications
The 2023 GOLD Report introduced a new section regarding adherence to COPD medications, noting that adherence to inhaled COPD medications is generally low (< 50%), despite its clear importance for symptom and exacerbation control, reducing healthcare use and costs, and improved survival and quality of life [2].
A variety of factors may contribute to poor adherence (Table 1) [22, 23], and patients with COPD experience as high as 50–80% non-adherence with prescribed inhaled therapy [22, 24]. Measures of adherence such as patient self-reports or canister weighing tend to overestimate adherence, thereby potentially masking a cause of poor treatment outcome [25, 26]. Relatively new to the market, “smart inhalers” collect real-time and objective data on inhaler use that reliably assess non-adherence [27], but several issues must be resolved (e.g., issues with cybersecurity and privacy, inconsistent insurance reimbursement for remote monitoring using digital inhalers, and difficulty for some patients with the technology and use of digital platforms) before their use can be implemented in routine clinical practice [28].
Interventions to improve adherence with inhaled therapy can have a positive impact on the clinical outcomes of patients with COPD. Achieving good (> 80%) adherence has been associated with less frequent exacerbations, hospitalization, and emergency room (ER) visits, improvements in lung function and quality of life, and lowered economic and societal burdens from COPD [24, 29, 30]. However, as GOLD’s 2023 Report notes, further research on medication adherence in COPD is needed to gain insight into the effectiveness of different self-management education and health behavior change strategies [2].
Initiation of Treatment
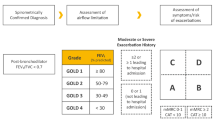
In 2017, the GOLD Reports began to address recommendations for initial treatment within specific GOLD Groups based on their level of dyspnea assessed by the modified Medical Research Council (mMRC) questionnaire and COPD assessment test (CAT): Group A (mMRC 0–1 or CAT < 10; lower number of exacerbations), Group B (mMRC ≥ 2 or CAT ≥ 10; lower number of exacerbations), Group C (mMRC 0–1 or CAT < 10; higher number of exacerbations), and Group D (mMRC ≥ 2 or CAT ≥ 10; higher number of exacerbations). In practice, few patients fall into Group C; if a patient’s exacerbation risk is relatively high, the mMRC and/or CAT are typically elevated. GOLD’s 2023 Report refines the ABCD model by combining Groups C and D into a new Group E. GOLD’s figure representing this new model of initial pharmacological treatment is reproduced here (with permission):

As per the figure, initial treatment for patients in Group A is a short- or long-term bronchodilator, whereas patients in Group B should receive a LABA/LAMA combination, preferably via single inhaler therapy. The current report also recommends that patients in Group E initially use a LABA/LAMA combination, with triple therapy a consideration if EOS ≥ 300/µL. These recommendations differ from those of GOLD’s previous (2022) Report [7], which only recommended a single long-acting bronchodilator for Groups B, C, and D, more specifically a LAMA for Groups C and D. The 2022 report did not recommend a LABA/LAMA combination unless symptoms were severe (e.g., CAT ≥ 20), with consideration of a LABA/ICS combination for Group D if eosinophil levels are ≥ 300/µL; in 2022, triple therapy was not mentioned as an option for initial treatment in any group. As of 2023, single therapy is only recommended for patients in Group A. Another change in 2023 is the discontinued recommendation of a LABA/ICS combination, which was previously an option for patients in Group D with EOS ≥ 300/µL. Thus, the 2023 GOLD Report presents an updated model for initial pharmacological treatment (Table 2).
Follow-Up Pharmacological Treatment
As with initial treatment, recommendations regarding follow-up treatment are based on symptoms of dyspnea and exacerbations, but do not depend on the patient’s GOLD group at initial diagnosis. Central to this model is an algorithm for changing treatments (escalation or de-escalation) when needed to improve therapeutic outcomes. Based on GOLD’s 2023 recommendations [2], a LABA/LAMA combination may be considered to improve dyspnea symptoms in patients using a single long-acting drug. If symptoms persist, consider switching inhaler devices or molecules, implementing or escalating nonpharmacological treatment(s), and/or investigating (and treating) other causes of dyspnea. In the 2022 GOLD Report [7], both LABA/ICS and triple therapy were options to control dyspnea; neither ICS combination is recommended to control symptoms in the 2023 Report. The GOLD 2023 figure showing recommendations for follow-up pharmacological treatment is reproduced here (with permission):

To control exacerbations in patients using a single long-acting drug, a LABA/LAMA combination may be considered if EOS < 300/µL; if EOS ≥ 300/µL, then escalate directly to triple therapy. If the patient is already using LABA/LAMA, recommended escalation is either to triple therapy if EOS ≥ 100/µL, or to roflumilast or azithromycin (the latter preferentially in former smokers) if EOS < 100/µL. Regarding de-escalation, patients on triple therapy, or those taking a LABA/ICS combination, who are experiencing side effects, may try to stop taking ICS; but if EOS ≥ 300/µL, this is more likely to be associated with further exacerbations. This follow-up pharmacological treatment model differs from that presented in the previous (2022) GOLD Report in how EOS levels guide escalation and de-escalation decisions [7]. Also, in the 2023 Report, dual therapy with LABA/ICS is no longer shown as an option for control of exacerbations [2]. Thus, as of 2023, treatment models for control of dyspnea and exacerbations, respectively, do not include options for dual therapy with a LABA/ICS combination, with triple therapy remaining only as a next-step escalation for patients with EOS ≥ 300/µL whose exacerbations are not well-controlled by a LABA, LAMA, or LABA/LAMA combination.
Conclusion
The ongoing refinement of GOLD guidelines and recommendations for treating patients with COPD and improving outcomes is exemplified in the significant changes and additions found in the GOLD 2023 Report. These include more detailed and pragmatic definitions of COPD and COPD exacerbation, expanded information on the consequences of pharmacological treatment and adherence to treatment on patient outcomes, practical guidance for improving adherence to pharmacological therapy, and refined models for initiating and following up with pharmacological treatment. Also of note, GOLD’s 2023 Report has expanded information on what is required from patients and clinicians to ensure proper device choice and use, crucial topics that have received steadily increasing attention from GOLD in recent years.
References
Terry PD, Dhand R. Inhalation therapy for stable COPD: 20 years of GOLD reports. Adv Ther. 2020;37:1812–28.
Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2023 Report). https://goldcopd.org/2023-gold-report-2/
Skully JL. What is a disease? EMBO Rep. 2004;5:650–3.
Celli B, Fabbri L, Criner G, Martinez FJ, Mannino D, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med. 2022;206:1317–25.
White F. Application of disease etiology and natural history to prevention in primary health care: a discourse. Med Princ Pract. 2020;29:501–13.
Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2001 Report).
Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2022 Report).
Mannino DM, Kiri VA. Changing the burden of COPD mortality. Int J COPD. 2006;1:219–33.
Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2020 Report).
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80.
Vestbo J, Fabbri L, Papi A, Petruzzelli S, Scuri M, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52:1801230. https://doi.org/10.1183/13993003.01230-2018.
Lipson DA, Crim C, Criner GJ, Day NC, Cransfield MT, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:1508–16.
Martinez FJ, Rabe KF, Ferguson GT, Wedzicha JA, Singh D, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203:553–64.
Calzetta L, Ritondo BL, de Marco P, Cazzola M, Rogliani P. Evaluating triple ICS/LABA/LAMA therapies for COPD patients: a network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies. Expert Rev Respir Med. 2021;15:143–52.
Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2017 Report).
Terry PD, Dhand R. Maintenance therapy with nebulizers in patients with stable COPD: need for reevaluation. Pulm Ther. 2020;6:177–92.
Usmani OS, Lavorini F, Marshall J, Dunlop WCN, Heron L, Farrington E, Dekhuijzen R. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. https://doi.org/10.1186/s12931-017-0710-y.
Hanania NA, Braman S, Adams SG, Adewuya R, Ari A, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5:111–23.
Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, et al. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9:58–72.
Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–72.
Alhaddad B, Smith FJ, Robertson T, Watman G, Taylor KM. Patients’ practices and experiences of using nebuliser therapy in the management of COPD at home. BMJ Open Respir Res. 2015;2:e000076. https://doi.org/10.1136/bmjresp-2014-000076.
Sulaiman I, Cushen B, Greene G, Seheult J, Seow D, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1333–43.
Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–8.
Makela MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes costs in patients with asthma and COPD. Respir Med. 2013;107:1481–90. https://doi.org/10.1016/j.rmed.2013.04.005.
Tommelein E, Mehuys E, Van Tongelen I, Brusselle G, Boussery K. Accuracy of the medication adherence report scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48:589–95.
Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1995;152:580–8.
Chan AHY, Pleasants RA, Dhand R, Tilly SL, Schworer SA, et al. Digital inhalers for asthma or chronic obstructive pulmonary disease: a scientific perspective. Pulm Ther. 2021;7:345–76.
Pleasants RA, Chan EH, Mosnaim G, Costello RW, Dhand R, et al. Integrating digital inhalers into clinical care of patients with asthma and chronic obstructive pulmonary disease. Respir Med. 2022;205:107038. https://doi.org/10.1016/j.rmed.2022.107038.
Van Boven JF, Chavannes NH, van der Molen T, Rutten-van Molken MPMH, Postma MJ, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108:103–13.
Zafari Z, Lynd LD, FitzGerald J, Sadatsafavi M. Economic and health effect of full adherence to controller therapy in adults with uncontrolled asthma: a simulation study. J Allergy Clin Immunol. 2014;134:908–15.
Acknowledgements
The authors thank Ms. Katie Langefeld, Program Director for GOLD, for her helpful comments on the manuscript and for providing several figures from GOLD’s 2023 Report.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Paul Terry and Rajiv Dhand conceived, designed, and drafted the manuscript.
Disclosures
Dr Paul Terry discloses no personal, financial, or commercial conflict of interest related to this manuscript. Dr Rajiv Dhand is on Advisory Boards of Astra-Zeneca, GSK, and Boehringer Ingelheim; has received honoraria from UpToDate and Mylan; and has received research support from Mylan/Theravance and Viatris.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by either of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Terry, P.D., Dhand, R. The 2023 GOLD Report: Updated Guidelines for Inhaled Pharmacological Therapy in Patients with Stable COPD. Pulm Ther 9, 345–357 (2023). https://doi.org/10.1007/s41030-023-00233-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00233-z