Abstract
Background
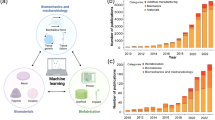
Four-dimensional (4D) printing is an advancement of three-dimensional (3D) printing technology, which allows a static 3D-printed structure to transform its shape with time, via self-folding/unfolding, in the presence of external stimuli, such as temperature, light, pH, water, mechanical stress, and magnetic field. According to the reports, this technology has tremendous potential in the area of biomedical engineering.
Objective
To review the recent research and application breakthroughs of 4D printing in various biomedical fields, such as tissue engineering, wound healing, drug delivery, soft robotics, and other medical devices.
Materials and Methods
A thorough literature search has been performed regarding the 4D printing technology and its application in biomedical engineering using the “Web of Science” browser.
Results
After reviewing all the available literature related to the biomedical application of 4D printing, it can be summarized that 4D printing technology has remarkably diverse biomedical application potential.
Conclusion
4D printing technology revolutionizes research in the healthcare sector, such as tissue engineering, self-assembling human-scale biomaterials, and organ printing. However, the mainstream use of 4D printing, along with its translatability, is dependent on further intensive research and advancements.
Lay Summary
The goal of 4D printing is to synthesize smart shape-changing products using conventional 3D printing instruments. The fourth dimension in 4D printing is “time.” Post printing, the 3D-printed construct can be transformed into the shape of interest by exposure to specific stimuli. 4D printing is a rapidly growing technology and has been explored in different fields including biomedical engineering. This review documented a detailed discussion regarding the application of 4D printing technology in the area of biomedical engineering.
Future Work
One of the major obstacles in 4D printing technology is cellular survival in the presence of a stimulus. The stimulus should require to be well optimized in order to support cellular survival. Other problems are passive and unreliable actuation, deficiency in regulating intermediary states of deformation and restriction in the availability of material. In future work, more effective techniques or optimized methodologies for governing the application of stimuli are required to make the technique flawless in this particular field.








Similar content being viewed by others
References
Hu H, Tian H, Li X, Shao J, Ding Y, Liu H, An N. Biomimetic mushroom-shaped microfibers for dry adhesives by electrically induced polymer deformation. ACS Appl Mater Interfaces. 2014;6:14167–73. https://doi.org/10.1021/am503493u.
Li X, Tian H, Ding Y, Shao J, Wei Y. Electrically templated dewetting of a UV-curable prepolymer film for the fabrication of a concave microlens array with well-defined curvature. ACS Appl Mater Interfaces. 2013;5:9975–82. https://doi.org/10.1021/am402043u.
Li X, Tian H, Shao J, Ding Y, Chen X, Wang Li, Bingheng Lu. Decreasing the saturated contact angle in electrowetting-on-dielectrics by controlling the charge trapping at liquid-solid interfaces. Adv Func Mater. 2016;26:2994–3002. https://doi.org/10.1002/adfm.201504705.
Yang Q, Gao B, Feng Xu. Recent advances in 4D bioprinting. Biotechnol J. 2020;15:1–10. https://doi.org/10.1002/biot.201900086.
Li X, Shang J, Wang Z. Assembly automation intelligent materials : a review of applications in 4D printing article information : to cite this document : About Emerald www.emeraldinsight.com Emerald is a global publisher linking research and practice to the benefit of society. Assembly Autom. 2016.
Neuss S, Blomenkamp I, Stainforth R, Boltersdorf D, Jansen M, Butz N, Perez-bouza A, Knu R. Biomaterials The use of a shape-memory poly ( e -caprolactone ) dimethacrylate network as a tissue engineering scaffold. Biomaterials. 2009;30:1697–705. https://doi.org/10.1016/j.biomaterials.2008.12.027.
Cui J, Kratz K, Heuchel M, Hiebl B, Lendlein A. Mechanically active scaffolds from composites. 2011. https://doi.org/10.1002/pat.1733.
Li Y, Rios O, Keum JK, Chen J, Kessler MR. Photoresponsive liquid crystalline epoxy networks with shape memory behavior and dynamic ester bonds. ACS Appl Mater Interfaces. 2016;8:15750–7. https://doi.org/10.1021/acsami.6b04374.
Tabata O, Hirasawa H, Aoki S, Yoshida R, Kokufuta E. Ciliary motion actuator using self-oscillating gel. IEEE. 2002;00:405–8. https://doi.org/10.1109/memsys.2001.906562.
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Erratum: correction: Autonomic healing of polymer composites. Nature. 2002;415:817–817. https://doi.org/10.1038/415817a.
Kalista SJ, Ward TC. Thermal characteristics of the self-healing response in poly(ethylene-co-methacrylic acid) copolymers. J R Soc Interface. 2007;4:405–11. https://doi.org/10.1098/rsif.2006.0169.
Kalista SJ, Ward TC, Oyetunji Z. Self-healing of poly(ethylene-co-methacrylic acid) copolymers following projectile puncture. Mech Adv Mater Struct. 2007;14:391–7. https://doi.org/10.1080/15376490701298819.
Tibbits S. The emergence of “4D printing”. 2013.
Li Y-c, Zhang YS, Akpek A, Shin SR, Khademhosseini A. 4D bioprinting : the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2017;9:1–16. https://doi.org/10.1088/1758-5090/9/1/012001 (IOP Publishing).
Tamay DG, Usal TD, Alagoz AS, Yucel D. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol. 2019;7:1–22. https://doi.org/10.3389/fbioe.2019.00164.
Morouço P, Lattanzi W, Alves N. Four-dimensional bioprinting as a new era for tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2017;5:1–3. https://doi.org/10.3389/fbioe.2017.00061.
Miao S, Castro N, Nowicki M, Xia L, Cui H, Zhou X, Zhu W, et al. 4D printing of polymeric materials for tissue and organ regeneration. Biochem Pharmacol. 2017; 1–15. https://doi.org/10.1016/j.mattod.2017.06.005. (Elsevier Ltd).
Tibbits S. 4D printing: multi-material shape change. Archit Des. 2014;84:116–21.
Miao S, Zhu W, Castro NJ, Leng J, Zhang LG. Four-dimensional printing hierarchy scaffolds with highly biocompatible smart polymers for tissue engineering applications. Tissue Eng - Part C. 2016;22:952–63. https://doi.org/10.1089/ten.tec.2015.0542.
Kokkinis D, Schaffner M, Studart AR. Multimaterial magnetically assisted 3D printing of composite materials. Nat Commun. 2015; 6. https://doi.org/10.1038/ncomms9643.
Qin Z, Compton BG, Lewis JA, Buehler MJ. Structural optimization of 3D-printed synthetic spider webs for high strength. Nat Commun. 2015;6:1–7. https://doi.org/10.1038/ncomms8038 (Nature Publishing Group).
Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, Wei Y, Chen S. Bio-inspired detoxification using 3d-printed hydrogel nanocomposites. Nat Commun. 2014;5:1–9. https://doi.org/10.1038/ncomms4774 (Nature Publishing Group).
Visser J, Melchels FPW, Jeon JE, Van Bussel EM, Kimpton LS, Byrne HM, Dhert WJA, Dalton PD, Hutmacher DW, Malda J. Three-dimensionally printed microfibres. Nat Commun. 2015; 1–10. https://doi.org/10.1038/ncomms7933. (Nature Publishing Group).
Gao B, Yang Q, Zhao X, Jin G, Ma Y, Xu F. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016;34:746–56. https://doi.org/10.1016/j.tibtech.2016.03.004 (Elsevier Ltd).
Murphy SV, Atala A. review 3D bioprinting of tissues and organs. Nat Publ Group. 2014;32:773–85. https://doi.org/10.1038/nbt.2958 (Nature Publishing Group).
Ursan I, Chiu L, Pierce A. Three-dimensional drug printing : a structured review. J Am Pharm Assoc. 2013;53:136–44. https://doi.org/10.1331/JAPhA.2013.12217.
Stanton MM, Samitier J, Sánchez S. Bioprinting of 3D hydrogels. Lab on a Chip. Royal Society of Chemistry; 2015. https://doi.org/10.1039/C5LC90069G.
Khoo ZX, Ee J, Teoh M, Liu Y, Chua CK. 3D printing of smart materials : a review on recent progresses in 4D printing. Virtual Phys Prototyping. 2015; 10. https://doi.org/10.1080/17452759.2015.1097054. (Taylor & Francis: 103–122).
Hendrikson WJ, Rouwkema J, Clementi F, Van Blitterswijk CA, Farè S, Moroni L. Towards 4D printed scaffolds for tissue engineering: exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication. 2017; 9. https://doi.org/10.1088/1758-5090/aa8114.
Miao S, Cui H, Nowicki M, Lee SJ, Almeida J, Zhou X, Zhu W, et al. Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation. Biofabrication. 2018; 10. https://doi.org/10.1088/1758-5090/aabe0b.
Zhang M, Vora A, Han W, Wojtecki RJ, Maune H, Le ABA, Thompson LE, et al. Dual-responsive hydrogels for direct-write 3D printing. Macromolecules. 2015;48:6482–8. https://doi.org/10.1021/acs.macromol.5b01550.
Hu YY, Huang WM. Elastic and elastic-plastic analysis of multilayer thin films: closed-form solutions. J Appl Phys. 2004;96:4154–60. https://doi.org/10.1063/1.1786339.
Morrison RJ, Hollister SJ, Niedner MF, Mahani MG, Park AH, Mehta DK, Ohye RG, Green GE. Erratum: mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients (Science Translational Medicine (2015) 7 (287er4)). Sci Transl Med. 2015;7:1–12. https://doi.org/10.1126/scitranslmed.aac4749.
Javaid M, Haleem A. 4D printing applications in medical field: a brief review. Clin Epidemiol Glob Health. 2019; 7. https://doi.org/10.1016/j.cegh.2018.09.007. (INDIACLEN: 317–321).
Zarek M, Layani M, Cooperstein I, Sachyani E, Cohn D, Magdassi S. 3D printing of shape memory polymers for flexible electronic devices. Adv Mater. 2016;28:4449–54. https://doi.org/10.1002/adma.201503132.
Aguilar MR, San Román J. Introduction to smart polymers and their applications. Smart Polymers Appl. 2014; 1–11. https://doi.org/10.1533/9780857097026.1.
Li YJ, Zhang FH, Liu YJ, Leng JS. 4D printed shape memory polymers and their structures for biomedical applications. Sci China Technol Sci. 2020;63:545–60. https://doi.org/10.1007/s11431-019-1494-0.
Zhao W, Liu L, Zhang F, Leng J, Liu Y. Shape memory polymers and their composites in biomedical applications. Mater Sci Eng C. 2019;97:864–83. https://doi.org/10.1016/j.msec.2018.12.054 (Elsevier B.V.).
Gladman S, Elisabetta A, Matsumoto A, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mater. 2016;15:413–8. https://doi.org/10.1038/nmat4544.
Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm Res. 2017;34:427–37. https://doi.org/10.1007/s11095-016-2073-3 (Pharmaceutical Research).
Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C. 2018;83:195–201. https://doi.org/10.1016/j.msec.2017.09.002 (Elsevier).
Stratesteffen H, Köpf M, Kreimendahl F, Blaeser A, Jockenhoevel S, Fischer H. GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication. 2017; 9. https://doi.org/10.1088/1758-5090/aa857c.
Wei H, Zhang Q, Yao Y, Liu L, Liu Y, Leng J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl Mater Interfaces. 2017;9:876–83. https://doi.org/10.1021/acsami.6b12824.
Shinoda H, Azukizawa S, Maeda K, Tsumori F. Bio-mimic motion of 3D-printed gel structures dispersed with magnetic particles. J Electrochem Soc. 2019;166:B3235–9. https://doi.org/10.1149/2.0361909jes.
Martin JJ, Fiore BE, Erb RM. Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat Commun. 2015;6:1–7. https://doi.org/10.1038/ncomms9641 (Nature Publishing Group).
Wu J, Yuan C, Ding Z, Isakov M, Mao Y, Wang T, Dunn ML, Jerry Qi H. Multi-shape active composites by 3D printing of digital shape memory polymers. Sci Rep. 2016;6:1–11. https://doi.org/10.1038/srep24224 (Nature Publishing Group).
Ren L, Li B, Song Z, Liu Q, Ren Lei, Zhou X. Bioinspired fiber-regulated composite with tunable permanent shape and shape memory properties via 3d magnetic printing. Composites Part B: Eng. 2019;164:458–66. https://doi.org/10.1016/j.compositesb.2019.01.061 (Elsevier).
Kolesky DB, Truby RL, Sydney Gladman A, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–30. https://doi.org/10.1002/adma.201305506.
Hua D, Zhang X, Ji Z, Yan C, Bo Yu, Li Y, Wang X, Zhou F. 3D printing of shape changing composites for constructing flexible paper-based photothermal bilayer actuators. J Mater Chem C. 2018;6:2123–31. https://doi.org/10.1039/c7tc05710e.
Yang H, Leow WR, Wang T, Wang J, Jiancan Yu, He Ke, Qi D, Wan C, Chen X. 3D printed photoresponsive devices based on shape memory composites. Adv Mater. 2017;29:1–7. https://doi.org/10.1002/adma.201701627.
Gupta MK, Meng F, Johnson BN, Kong YL, Tian L, Yeh YW, Masters N, Singamaneni S, McAlpine MC. 3D printed programmable release capsules. Nano Lett. 2015;15:5321–9. https://doi.org/10.1021/acs.nanolett.5b01688.
Zhang B, Zhang W, Zhang Z, Zhang YF, Hingorani H, Liu Z, Liu J, Ge Qi. Self-healing four-dimensional printing with an ultraviolet curable double-network shape memory polymer system. ACS Appl Mater Interfaces. 2019;11:10328–36. https://doi.org/10.1021/acsami.9b00359.
Joshi S, Rawat K, Karunakaran C, Rajamohan V, Mathew AT, Koziol K, Thakur VK, Balan ASS. 4D printing of materials for the future: opportunities and challenges. Appl Maters Today. 2020;18:100490. https://doi.org/10.1016/j.apmt.2019.100490 (Elsevier Ltd).
Bajpai A, Baigent A, Raghav S, Brádaigh C, Koutsos V, Radacsi N. 4D printing: materials, technologies, and future applications in the biomedical field. Sustainability (Switzerland). 2020;12:1–32. https://doi.org/10.3390/su122410628.
Andreasen GF, Hilleman TB. An evaluation of 55 cobalt substituted nitinol wire for use in orthodontics. J Am Dent Assoc (1939). 1971;82:1373–5. https://doi.org/10.14219/jada.archive.1971.0209.
Airoldi G, Riva G, Vanelli M. Superelasticity and shape memory effect in NiTi orthodontic wires. J Phys IV. 1995;05:C8-1205-C8-1210. https://doi.org/10.1051/jp4/1995581205.
Pyo Y, Kang M, Jang Jy, Park Y, Son YH, Choi MC, Ha Jw, Chang YW, Lee CS. Design of a shape memory composite(SMC) using 4D printing technology. Sensors Actuators A: Phys. 2018;283:187–95. https://doi.org/10.1016/j.sna.2018.08.049 (Elsevier B.V.).
Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, Fisher JP, Zhang LG. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:1–10. https://doi.org/10.1038/srep27226 (Nature Publishing Group).
Miao S, Zhu W, Castro NJ, Leng J, Zhang LG. Four-dimensional printing hierarchy scaffolds with highly biocompatible smart polymers for tissue engineering applications. Tissue Eng - Part C: Methods. 2016;22:952–63. https://doi.org/10.1089/ten.tec.2015.0542.
Hsiao SH, Hsu SH. Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-Laden bioprinting. ACS Appl Mater Interfaces. 2018;10:29273–87. https://doi.org/10.1021/acsami.8b08362.
Zarek M, Mansour N, Shapira S, Cohn D. 4D printing of shape memory-based personalized endoluminal medical devices. Macromol Rapid Commun. 2017;38:1–6. https://doi.org/10.1002/marc.201600628.
Zhao W, Zhang F, Leng J, Liu Y. Personalized 4D printing of bioinspired tracheal scaffold concept based on magnetic stimulated shape memory composites. Compos Sci Technol. 2019;184:107866. https://doi.org/10.1016/j.compscitech.2019.107866 (Elsevier Ltd).
Behl M, Razzaq MY, Lendlein A. Multifunctional shape-memory polymers. Adv Mater. 2010;22:3388–410. https://doi.org/10.1002/adma.200904447.
Chen S, Jinlian Hu, Zhuo H, Zhu Y. Two-way shape memory effect in polymer laminates. Mater Lett. 2008;62:4088–90. https://doi.org/10.1016/j.matlet.2008.05.073.
Basit A, L’Hostis G, Pac MJ, Durand B. Thermally activated composite with two-way and multi-shape memory effects. Materials. 2013;6:4031–45. https://doi.org/10.3390/ma6094031.
Pei Eujin, Loh Giselle Hsiang. Technological considerations for 4D printing: an overview. Progress Addit Manuf. 2018;3:95–107. https://doi.org/10.1007/s40964-018-0047-1 (Springer International Publishing).
Lui YS, Sow WT, Tan LP, Wu Y, Lai Y, Li H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019;92:19–36. https://doi.org/10.1016/j.actbio.2019.05.005 (Acta Materialia Inc.).
Hoogenboom R. Temperature-responsive polymers: properties, synthesis and applications. Smart Polymers and their Applications. Woodhead Publishing Limited; 2014. https://doi.org/10.1533/9780857097026.1.15.
Sun L, Huang WM, Ding Z, Zhao Y, Wang CC, Purnawali H, Tang C. Stimulus-responsive shape memory materials: a review. Mater Des. 2012;33:577–640. https://doi.org/10.1016/j.matdes.2011.04.065 (Elsevier Ltd).
Hasirci V, Hasirci N. Controlled release systems. In Biomedical Engineering - Applications, Basis and Communications; 2018; 6:9–18. https://doi.org/10.1007/978-1-4939-8856-3.
Senatov FS, Niaza KV, Yu Zadorozhnyy M, Maksimkin AV, Kaloshkin SD, Estrin YZ. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J Mech Behav Biomed Mater. 2016;57:139–48. https://doi.org/10.1016/j.jmbbm.2015.11.036 (Elsevier).
Thérien-Aubin H, Zi Liang Wu, Nie Z, Kumacheva E. Multiple shape transformations of composite hydrogel sheets. J Am Chem Soc. 2013;135:4834–9. https://doi.org/10.1021/ja400518c.
Dutta S, Cohn D. Temperature and pH responsive 3D printed scaffolds. J Mater Chem B. 2018; 6. https://doi.org/10.1039/x0xx00000x.
Malachowski K, Breger J, Kwag HR, Wang MO, Fisher JP, Selaru FM, Gracias DH. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew Chem - Int Ed. 2014;53:8045–9. https://doi.org/10.1002/anie.201311047.
Jochum FD, Theato P. Temperature- and light-responsive smart polymer materials. Chem Soc Rev. 2013;42:7468–83. https://doi.org/10.1039/c2cs35191a.
Cabane E, Zhang X, Langowska K, Palivan CG, Meier W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases. 2012;7:1–27. https://doi.org/10.1007/s13758-011-0009-3.
Cui J, Del Campo A. Photo-responsive polymers: properties, synthesis and applications. Smart Polymers and their Applications. Woodhead Publishing Limited; 2014. https://doi.org/10.1533/9780857097026.1.93.
Ercole F, Davis TP, Evans RA. Photo-responsive systems and biomaterials: photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym Chem. 2010;1:37–54. https://doi.org/10.1039/b9py00300b.
Zhao Z, Jiangtao Wu, Xiaoming Mu, Haosen Chen H, Qi J, Fang D. Origami by frontal photopolymerization. Sci Adv. 2017;3:1–8. https://doi.org/10.1126/sciadv.1602326.
Boyle BM, French TA, Pearson RM, McCarthy BG, Miyake GM. Structural Color for additive manufacturing: 3D-printed photonic crystals from block copolymers. ACS Nano. 2017;11:3052–8. https://doi.org/10.1021/acsnano.7b00032.
Van Oosten CL, Bastiaansen CWM, Broer DJ. Printed artificial cilia from liquid-crystal network actuators modularly driven by light. Nat Mater. 2009;8:677–82. https://doi.org/10.1038/nmat2487 (Nature Publishing Group).
Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–80. https://doi.org/10.1152/physrev.2000.80.2.649.
Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I Traditional factors. Tissue Eng. 2001;7:679–89. https://doi.org/10.1089/107632701753337645.
Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–41. https://doi.org/10.1016/j.biomaterials.2012.04.050 (Elsevier Ltd).
Ionov L. Hydrogel-based actuators: possibilities and limitations. Mater Today. 2014;17:494–503. https://doi.org/10.1016/j.mattod.2014.07.002 (Elsevier Ltd).
Villar G, Graham AD, Bayley H. A tissue-like printed material. Science. 2013;340:48–52. https://doi.org/10.1126/science.1229495.
Jamal M, Kadam SS, Xiao R, Jivan F, Onn TM, Fernandes R, Nguyen TD, Gracias DH. Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv Healthc Mater. 2013;2:1142–50. https://doi.org/10.1002/adhm.201200458.
Mulakkal MC, Trask RS, Ting VP, Seddon AM. Responsive cellulose-hydrogel composite ink for 4D printing. Mater Des. 2018;160:108–18. https://doi.org/10.1016/j.matdes.2018.09.009 (The Authors).
Lv C, Xia H, Shi Q, Wang G, Wang YS, Chen QD, Zhang YL, Liu LQ, Sun HB. Sensitively humidity-driven actuator based on photopolymerizable PEG-DA films. Adv Mater Interfaces. 2017; 4. https://doi.org/10.1002/admi.201601002.
Lei D, Yang Y, Liu Z, Chen S, Song B, Shen A, Yang B, et al. A general strategy of 3D printing thermosets for diverse applications. Mater Horizons. 2019;6:394–404. https://doi.org/10.1039/c8mh00937f (Royal Society of Chemistry).
Ramanujan RV. Magnetic particles for biomedical applications. In Biomedical Materials; 2009; 477–491. https://doi.org/10.1007/978-0-387-84872-3.
Mcbain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomed. 2008;3:169–80.
Lin G, Makarov D, Schmidt OG. Magnetic sensing platform technologies for biomedical applications. Lab on a Chip. Royal Society of Chemistry; 2017. https://doi.org/10.1039/C7LC00026J.
Zhu P, Yang W, Wang R, Gao S, Li Bo, Li Qi. Applications of polymer, composite, and coating materials 4D printing of complex structures with a fast response time to magnetic stimulus school of materials science and engineering. Appl Maerials Interface. 2018;10:36435–42. https://doi.org/10.1021/acsami.8b12853.
Boncheva M, Andreev SA, Mahadevan L, Winkleman A, Reichman DR, Prentiss MG, Whitesides S, Whitesides GM. Magnetic self-assembly of three-dimensional surfaces from planar sheets. PNAS. 2005;102:3924–9.
Singh N, Jenkins GJS, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1:1–15. https://doi.org/10.3402/nano.v1i0.5358.
Markides H, Rotherham M, El Haj AJ. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J Nanomater. 2012;2012:1–11. https://doi.org/10.1155/2012/614094.
Pretsch T. Review on the functional determinants and durability of shape memory polymers. Polymer. 2010;2:120–58. https://doi.org/10.3390/polym2030120.
Sun S, Titushkin I, Cho M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69:133–41. https://doi.org/10.1016/j.bioelechem.2005.11.007.
Sun S, Wise J, Cho M. Human fibroblast migration in three-dimensional collagen. Tissue Eng. 2004;10:1548–58.
Caig CDMC, Rajnicek ANNM, Song B, Zhao MIN. Controlling cell behavior electrically : current views and future potential. 2005; 943–978. https://doi.org/10.1152/physrev.00020.2004.
Dong S-L, Han Lu, Cai-xia Du, Wang X-y, Li L-H, Wei Y. 3D printing of aniline tetramer-grafted- polyethylenimine and pluronic F127 composites for electroactive scaffolds. Macromol Rapid Commun. 2017;201600551:1–7. https://doi.org/10.1002/marc.201600551.
Cvetkovic C, Raman R, Chan V, Williams BJ, Tolish M, Bajaj P. Three-dimensionally printed biological machines powered by skeletal muscle. PNAS. 2014;111:10125–30. https://doi.org/10.1073/pnas.1401577111.
Reyes-Ortega F. pH-responsive polymers: properties, synthesis and applications. Smart Polymers and their Applications. Woodhead Publishing Limited; 2014. https://doi.org/10.1533/9780857097026.1.45.
Bagherifam S, Skjeldal FM, Griffiths G, Mælandsmo GM, Engebråten O, Nyström Bo, Hasirci V, Hasirci N. PH-responsive nano carriers for doxorubicin delivery. Pharm Res. 2015;32:1249–63. https://doi.org/10.1007/s11095-014-1530-0.
Ashfaq UA, Riaz M, Yasmeen E, Yousaf M. Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit Rev Ther Drug Carrier Syst. 2017;34:317–53. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2017017845.
Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–7. https://doi.org/10.1021/js980337n.
Makhlof A, Tozuka Y, Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharm Sci. 2011;42:445–51. https://doi.org/10.1016/j.ejps.2010.12.007 (Elsevier B.V.).
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:1–19. https://doi.org/10.1038/s41392-017-0004-3.
Dai S, Ravi P, Tam KC. pH-responsive polymers: Synthesis, properties and applications. Soft Matter. 2008;4:435–49. https://doi.org/10.1039/b714741d.
Arizaga A, Ibarz G, Piñol R. Stimuli-responsive poly(4-vinyl pyridine) hydrogel nanoparticles: synthesis by nanoprecipitation and swelling behavior. J Colloid Interface Sci. 2010;348:668–72. https://doi.org/10.1016/j.jcis.2010.05.051 (Elsevier Inc.).
Kocak G, Tuncer C, Bütün V. pH-responsive polymers. Polymer Chem. 2017;8:144–76. https://doi.org/10.1039/c6py01872f (Royal Society of Chemistry).
Li Y, Zhi X, Lin J, You X, Yuan J. Preparation and characterization of DOX loaded keratin nanoparticles for pH / GSH dual responsive release. Mater Sci Eng C. 2017;73:189–97. https://doi.org/10.1016/j.msec.2016.12.067 (Elsevier B.V.).
Anandhakumar S, Krishnamoorthy G, Ramkumar KM, Raichur AM. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method : an effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Mater Sci Eng C. 2017;70:378–85. https://doi.org/10.1016/j.msec.2016.09.003 (Elsevier B.V.).
Villanueva ME, Cuestas ML, Pérez CJ, Campo V, Orto D, Copello GJ. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J Colloid Interface Sci. 2019;536:372–80. https://doi.org/10.1016/j.jcis.2018.10.067.
Larush L, Kaner I, Fluksman A, Tamsut A, Pawar AA. 3D printing of responsive hydrogels for drug-delivery systems. 2017; 1: 219–229.
Mirani B, Pagan E, Currie B, Siddiqui MA, Hosseinzadeh R. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. 2017; 1700718: 1–15. https://doi.org/10.1002/adhm.201700718.
Nadgorny M, Xiao Z, Chen C, Connal LA. Three-dimensional printing of pH-responsive and functional polymers on an a ff ordable desktop printer. Appl Maerials Interface. 2016;8:28946–54. https://doi.org/10.1021/acsami.6b07388.
Huang L, Jiang R, Jingjun Wu, Song J, Bai H, Li B, Zhao Q, Xie T. Ultrafast digital printing toward 4D shape changing materials. Adv Mater. 2017;29:1–6. https://doi.org/10.1002/adma.201605390.
Kang Y, Walish JJ, Gorishnyy T, Thomas EL. Broad-wavelength-range chemically tunable block-copolymer photonic gels. Nat Mater. 2007;6:957–60. https://doi.org/10.1038/nmat2032.
Isobe T, Takahashi H, Ueki S, Takagi J, Saito Y. Activity-independent cell adhesion to tissue-type transglutaminase is mediated by α4β1 integrin. Eur J Cell Biol. 1999;78:876–83. https://doi.org/10.1016/S0171-9335(99)80089-2.
Brownlee M, Cerami A. A glucose-controlled insulin-delivery system : semisynthetic insulin bound to lectin. Science. 2016;206:1190–1.
Galuska SP, Geyer R, Gerardy-Schahn R, Mühlenhoff M, Geyer H. Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo. J Biol Chem. 2008;283:17–28. https://doi.org/10.1074/jbc.M707024200.
Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjug Chem. 2005;16:1133–9. https://doi.org/10.1021/bc0501303.
Wang X, Qin XH, Chengzhi Hu, Terzopoulou A, Chen XZ, Huang TY, Maniura-Weber K, Pané S, Nelson BJ. 3D printed enzymatically biodegradable soft helical microswimmers. Adv Func Mater. 2018;28:1–8. https://doi.org/10.1002/adfm.201804107.
Ceylan H, Ceren Yasa I, Yasa O, Tabak AF, Giltinan J, Sitti M. 3D-printed biodegradable microswimmer for drug delivery and targeted cell labeling. bioRxiv. 2018, 379024. https://doi.org/10.1101/379024.
Song KH, Highley CB, Rouff A, Burdick JA. Complex 3D-printed microchannels within cell-degradable hydrogels. Adv Func Mater. 2018;28:1–10. https://doi.org/10.1002/adfm.201801331.
Zelzer M, Todd SJ, Hirst AR, McDonald TO, Ulijn RV. Enzyme responsive materials: design strategies and future developments. Biomater Sci. 2013;1:11–39. https://doi.org/10.1039/c2bm00041e.
Yeh H-C, Brown TT, Maruthur N. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus. Ann Intern Med. 2013;157:336–47.
Tamborlane WV, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–76. https://doi.org/10.1016/s0084-3741(09)79371-0.
Adams A, Malkoc A, La Belle JT. The development of a glucose dehydrogenase 3D-printed glucose sensor: a proof-of-concept study. J Diabetes Sci Technol. 2018;12:176–82. https://doi.org/10.1177/1932296817715272.
Song E, da Costa TH, Choi JW. A chemiresistive glucose sensor fabricated by inkjet printing. Microsyst Technol. 2017;23:3505–11. https://doi.org/10.1007/s00542-016-3160-4 (Springer Berlin Heidelberg).
Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem. 2012;124:2166–70. https://doi.org/10.1002/ange.201106252.
Podual K, Doyle FJ, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J Control Release. 2000;67:9–17. https://doi.org/10.1016/S0168-3659(00)00195-4.
Song J, Millman JR. Economic 3D-printing approach for transplantation of human stem cell-derived β-like cells. Biofabrication. 2017;9:1–14. https://doi.org/10.1088/1758-5090/9/1/015002 (IOP Publishing).
Marchand Marion, Monnot Catherine, Muller Laurent, Germain Stéphane. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol. 2019;89:147–56. https://doi.org/10.1016/j.semcdb.2018.08.007 (Elsevier).
Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJA, Groll J, Hutmacher DW. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–28. https://doi.org/10.1002/adma.201302042.
Qu F, Guilak F, Mauck RL. Cell migration: implications for repair and regeneration in joint disease. Nat Rev Rheumatol. 2019;15:167–79. https://doi.org/10.1038/s41584-018-0151-0 (Springer US).
Morouço PG. The usefulness of direct digital manufacturing for biomedical applications. In Intensification of biobased processes, ed. Andrzej Górak and Andrzej Stankiewicz, 478–487. The Royal Society of Chemistry; 2018.
Poomathi N, Singh S, Prakash C, Subramanian A, Sahay R, Cinappan A, Ramakrishna S. 3D printing in tissue engineering: a state of the art review of technologies and biomaterials. Rapid Prototyp J. 2020;26:1313–34. https://doi.org/10.1108/RPJ-08-2018-0217.
An J, Chua CK, Mironov V. A perspective on 4D bioprinting. Int J Bioprint. 2016;2:3–5. https://doi.org/10.18063/IJB.2016.01.003.
Ghizal R, Fatima GR, Srivastava S. Smart polymers and their applications. Int J Eng Technol Manag Appl Sci. 2014;2:104–15. https://doi.org/10.1016/b978-0-85709-695-1.50018-2.
Ikegami T, Maehara Y. 3D printing of the liver in living donor liver transplantation. Nat Rev Gastroenterol Hepatol. 2013;10:697–8. https://doi.org/10.1038/nrgastro.2013.195 (Nature Publishing Group).
Batteux C, Haidar MA, Bonnet D. 3D-printed models for surgical planning in complex congenital heart diseases: a systematic review. Front Pediatr. 2019;7:1–19. https://doi.org/10.3389/fped.2019.00023.
Zhang F, Wen N, Wang L, Bai Y, Leng J. Design of 4D printed shape-changing tracheal stent and remote controlling actuation. Int J Smart Nano Mater. 2021;12:375–89. https://doi.org/10.1080/19475411.2021.1974972 (Taylor & Francis).
Ge Q, Dunn CK, Qi HJ, Dunn ML. Active origami by 4D printing. Smart Mater Struct. 2014; 23. https://doi.org/10.1088/0964-1726/23/9/094007.
Kirillova A, Maxson R, Stoychev G, Gomillion CT, Ionov L. 4D biofabrication using shape-morphing hydrogels. Adv Mater. 2017;29:1–8. https://doi.org/10.1002/adma.201703443.
Muehlenfeld C, Roberts SA. 3D / 4D printing in additive manufacturing : process engineering and novel excipients. In 3D and 4D printing in biomedical applications: process engineering and additive manufacturing, ed. Mohammed Maniruzzaman, 1st ed., 1–24. Wiley-VCHVerlag GmbH& Co; 2019.
Champeau M, Heinze DA, Viana TN, Rodrigues E, de Souza A, Chinellato C, Titotto S. 4D printing of hydrogels: a review. Adv Func Mater. 2020;30:1–22. https://doi.org/10.1002/adfm.201910606.
Malekmohammadi S, Aminabad NS, Sabzi A, Zarebkohan A, Razavi M, Vosough M, Bodaghi M, Maleki H. Smart and biomimetic 3d and 4d printed composite hydrogels: opportunities for different biomedical applications. Biomedicines. 2021;9:1–46. https://doi.org/10.3390/biomedicines9111537.
Le Fer G, Becker ML. 4D printing of resorbable complex shape-memory poly(propylene fumarate) star scaffolds. ACS Appl Mater Interfaces. 2020;12:22444–52. https://doi.org/10.1021/acsami.0c01444.
Costa L, Silva-Correia J, Oliveira JM, Reis RL. Gellan gum-based hydrogels for osteochondral repair. In Advances in Experimental Medicine and Biology; 2018; 281–304. https://doi.org/10.1007/978-3-319-76711-6_13.
Cui H, Miao S, Esworthy T, Lee S-J, Zhou X, Yun S, Webster TJ, Harris BT, Zhang LG. HHS public access. Nano Res. 2019;12:1381–8. https://doi.org/10.1007/s12274-019-2340-9.A.
Miao S, Cui H, Nowicki M, Xia L, Zhou X, Lee SJ, Zhu W, Sarkar K, Zhang Z, Zhang LG. Stereolithographic 4D bioprinting of multiresponsive architectures for neural engineering. Adv Biosyst. 2018;2:1–10. https://doi.org/10.1002/adbi.201800101.
Fang JH, Hsu HH, Hsu RS, Peng CK, Lu YJ, Chen YY, Chen SY, Hu SH. 4D printing of stretchable nanocookie@conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting. NPG Asia Mater. 2020;12:1–16. https://doi.org/10.1038/s41427-020-00244-1 (Springer US).
Miao S, Nowicki M, Cui H, Lee SJ, Zhou X, Mills DK, Zhang LG. 4D anisotropic skeletal muscle tissue constructs fabricated by staircase effect strategy. Biofabrication. 2019; 11. https://doi.org/10.1088/1758-5090/ab1d07. (IOP Publishing).
Constante G, Apsite I, Alkhamis H, Dulle M, Schwarzer M, Caspari A, Synytska A, Salehi S, Ionov L. 4D biofabrication using a combination of 3D printing and melt-electrowriting of shape-morphing polymers. ACS Appl Mater Interfaces. 2021;13:12767–76. https://doi.org/10.1021/acsami.0c18608.
Uribe-Gomez J, Posada-Murcia A, Shukla A, Ergin M, Constante G, Apsite I, Martin D, et al. Shape-morphing fibrous hydrogel/elastomer bilayers fabricated by a combination of 3D printing and melt electrowriting for muscle tissue regeneration. ACS Appl Bio Mater. 2021;4:1720–30. https://doi.org/10.1021/acsabm.0c01495.
Cui H, Liu C, Esworthy T, Huang Y, Yu ZX, Zhou X, San H, et al. 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci Adv. 2020;6:eabb5067. https://doi.org/10.1126/sciadv.abb5067.
Wang Y, Cui H, Wang Y, Chengyao Xu, Esworthy TJ, Hann SY, Boehm M, Shen YL, Mei D, Zhang LG. 4D printed cardiac construct with aligned myofibers and adjustable curvature for myocardial regeneration. ACS Appl Mater Interfaces. 2021;13:12746–58. https://doi.org/10.1021/acsami.0c17610.
Lin C, Liu L, Liu Y, Leng J. 4D printing of bioinspired absorbable left atrial appendage occluders: a proof-of-concept study. ACS Appl Mater Interfaces. 2021;13:12668–78. https://doi.org/10.1021/acsami.0c17192.
Pedron S, Van Lierop S, Horstman P, Penterman R, Broer DJ, Peeters E. Stimuli responsive delivery vehicles for cardiac microtissue transplantation. Adv Func Mater. 2011;21:1624–30. https://doi.org/10.1002/adfm.201002708.
Lee YB, Jeon O, Lee SJ, Ding A, Wells D, Alsberg E. Inuction of four-dimensional spatiotemporal geometric transformations in high cell.pdf. Adv Funct Mater. 2021;31:2010104.
Ding A, Lee SJ, Ayyagari S, Tang R, Huynh CT, Alsberg E. 4D biofabrication via instantly generated graded hydrogel scaffolds. Bioactive Materials. 2022;7:324–32. https://doi.org/10.1016/j.bioactmat.2021.05.021 (KeAi Communications Co., Ltd).
Jen WY, Ser Jeng U, Hsu SH. Biodegradable water-based polyurethane shape memory elastomers for bone tissue engineering. ACS Biomater Sci Eng. 2018;4:1397–406. https://doi.org/10.1021/acsbiomaterials.8b00091.
Wang C, Yue H, Liu J, Zhao Q, He Z, Li K, Bingheng Lu, et al. Advanced reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of irregular shapes. Biofabrication. 2020;12: 045025. https://doi.org/10.1088/1758-5090/abab5b.
You D, Chen G, Liu C, Ye X, Wang S, Dong M, Sun M, et al. Printing of multi-responsive membrane for accelerated in vivo bone healing via remote.pdf. Adv Funct Mater. 2021;31:2103920.
Devillard CD, Mandon CA, Lambert SA, Blum LJ, Marquette CA. Bioinspired multi-activities 4D printing objects a new approach toward complex tissue engineering. Biotechnol J. 2018;13:1800098.
Zhang J, Zhao S, Zhu M, Zhu Y, Zhang Y, Liu Z, Zhang C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J Mater Chem B. 2014;2:7583–95. https://doi.org/10.1039/c4tb01063a (Royal Society of Chemistry).
D’Amora U, Russo T, Gloria A, Rivieccio V, D’Antò V, Negri G, Ambrosio L, De Santis R. 3D additive-manufactured nanocomposite magnetic scaffolds: effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioactive Mater. 2017;2:138–45. https://doi.org/10.1016/j.bioactmat.2017.04.003.
Betsch M, Cristian C, Lin YY, Blaeser A, Schöneberg J, Vogt M, Buhl EM, Fischer H, Campos DFD. Incorporating 4D into bioprinting: real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv Healthc Mater. 2018;7:1–9. https://doi.org/10.1002/adhm.201800894.
Kirillova A, Maxson R, Stoychev G, Gomillion CT, Ionov L. 4D biofabrication using shape-morphing hydrogels. Adv Mater. 2017;29:1703443.
Zhang C, Cai D, Liao P, Su JW, Deng H, Vardhanabhuti B, Ulery BD, Chen SY, Lin J. 4D Printing of shape-memory polymeric scaffolds for adaptive biomedical implantation. Acta Biomater. 2021;122:101–10. https://doi.org/10.1016/j.actbio.2020.12.042 (Elsevier Ltd).
Kuang X, Chen K, Dunn CK, Jiangtao Wu, Li VCF, Jerry Qi H. 3D printing of highly stretchable, shape-memory, and self-healing elastomer toward novel 4D printing. ACS Appl Mater Interfaces. 2018;10:7381–8. https://doi.org/10.1021/acsami.7b18265.
Kim SH, Seo YB, Yeon YK, Lee YJ, Park HS, Sultan MT, Lee JM, et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials. 2020;260:120281. https://doi.org/10.1016/j.biomaterials.2020.120281 (Elsevier Ltd).
Zarek M, Mansour N, Shapira S, Cohn D. 4D printing of shape memory-based personalized endoluminal medical devices. Macromol Rapid Commun. 2017;38:1600628.
Cui C, Kim DO, Pack MY, Han B, Han L, Sun Y, Han LH. 4D printing of self-folding and cell-encapsulating 3D microstructures as scaffolds for tissue-engineering applications. Biofabrication. 2020; 12. https://doi.org/10.1088/1758-5090/aba502.
Luo Y, Lin X, Chen B, Wei X. Cell-laden four-dimensional bioprinting using near- cell-laden four-dimensional bioprinting using near-infrared-triggered shape-morphing alginate / polydopamine bioinks. Biofabrication. 2019;11:045019 (IOP Publishing).
Ko H, Ratri MC, Kim K, Jung Y, Tae G, Shin K. Formulation of sugar/hydrogel inks for rapid thermal response 4D architectures with sugar-derived macropores. Sci Rep. 2020;10:1–10. https://doi.org/10.1038/s41598-020-64457-8.
Seo JW, Shin SR, Park YJ, Bae H. Hydrogel production platform with dynamic movement using photo-crosslinkable/temperature reversible chitosan polymer and stereolithography 4D printing technology. Tissue Eng Regen Med. 2020;17:423–31. https://doi.org/10.1007/s13770-020-00264-6 (Springer Singapore).
Singaravelu S, Ramanathan G, Sivagnanam UT. Dual-layered 3D nanofibrous matrix incorporated with dual drugs and their synergetic effect on accelerating wound healing through growth factor regulation. Mater Sci Eng C. 2017;76:37–49. https://doi.org/10.1016/j.msec.2017.02.148 (Elsevier B.V.).
Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep. 2019;9:1–15. https://doi.org/10.1038/s41598-018-38366-w (Springer US).
Montero FE, Rezende RA, da Silva JVL, Sabino MA. Development of a smart bioink for bioprinting applications. Front Mech Eng. 2019;5:1–12. https://doi.org/10.3389/fmech.2019.00056.
Smandri A, Nordin A, Hwei NM, Chin KY, Aziz IA, Fauzi MB. Natural 3D-printed bioinks for skin regeneration and wound healing: a systematic review. Polymers. 2020;12:1–19. https://doi.org/10.3390/polym12081782.
Xu W, Molino BZ, Cheng F, Molino PJ, Yue Z, Dandan Su, Wang X, Willför S, Chunlin Xu, Wallace GG. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl Mater Interfaces. 2019;11:8838–48. https://doi.org/10.1021/acsami.8b21268.
Osidak EO, Karalkin PA, Osidak MS, Parfenov VA, Sivogrivov DE, Pereira FDAS, Gryadunova AA, et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J Mater Sci - Mater Med. 2019;30:1–12. https://doi.org/10.1007/s10856-019-6233-y.
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, Heng BC, Bunpetch V, Zhang S, Ouyang H. A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep. 2017;7:1–12. https://doi.org/10.1038/s41598-017-04149-y (Springer US).
Nocera AD, Comín R, Salvatierra NA, Cid MP. Development of 3D printed fibrillar collagen scaffold for tissue engineering. Biomed Microdevices. 2018;20:1–13. https://doi.org/10.1007/s10544-018-0270-z (Biomedical Microdevices).
Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, Kim MB, Lee H, Kim JH, Cho DW. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. https://doi.org/10.1016/j.biomaterials.2018.03.040 (Elsevier Ltd).
Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3:511–29. https://doi.org/10.1089/wound.2012.0401.
Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 2018;36:907–22. https://doi.org/10.1016/j.tibtech.2018.04.004 (Elsevier Ltd).
Ribeiro MP, Espiga A, Silva D, Baptista P, Henriques J, Ferreira C, Silva JC, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17:817–24. https://doi.org/10.1111/j.1524-475X.2009.00538.x.
Domínguez-Robles J, Martin NK, Fong ML, Stewart SA, Irwin NJ, Rial-Hermida MI, Donnelly RF, Larrañeta E. Antioxidant pla composites containing lignin for 3D printing applications: a potential material for healthcare applications. Pharmaceutics. 2019;11:5–7. https://doi.org/10.3390/pharmaceutics11040165.
Ajalloueian F, Tavanai H, Hilborn J, Donzel-Gargand O, Leifer K, Wickham A, Arpanaei A. Emulsion electrospinning as an approach to fabricate PLGA/chitosan nanofibers for biomedical applications. BioMed Res Int. 2014; 2014. https://doi.org/10.1155/2014/475280.
Wischke C, Neffe AT, Steuer S, Lendlein A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J Control Release. 2009;138:243–50. https://doi.org/10.1016/j.jconrel.2009.05.027 (Elsevier B.V.).
Firth J, Gaisford S, Basit AW. A new dimension: 4D printing opportunities in pharmaceutics. In AAPS Advances in the Pharmaceutical Sciences Series; 2018; 31:153–162. https://doi.org/10.1007/978-3-319-90755-0_8.
Ghosh A, Li L, Xu L, Dash RP, Gupta N, Lam J, Jin Q, et al. Gastrointestinal-resident, shape-changing microdevices extend drug release in vivo. Sci Adv. 2020; 6. https://doi.org/10.1126/sciadv.abb4133.
Villar G, Heron AJ, Bayley H. Formation of droplet networks that function in aqueous environments. Nat Nanotechnol. 2011;6:803–8. https://doi.org/10.1038/nnano.2011.183 (Nature Publishing Group).
Stoychev G, Puretskiy N, Ionov L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter. 2011;7:3277–9. https://doi.org/10.1039/c1sm05109a.
Kokardekar RR, Shah VK, Mody HR. PNIPAM poly (N-isopropylacrylamide): a thermoresponsive “smart” polymer in novel drug delivery systems. Internet J Med Update. 2012;7:59–62.
Breger JC, Yoon C, Xiao R, Kwag HR, Wang MO, Fisher JP, Nguyen TD, Gracias DH. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl Mater Interfaces. 2015;7:3398–405. https://doi.org/10.1021/am508621s.
Li H, Go G, Ko SY, Park JO, Park S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater Struct. 2016;25:27001. https://doi.org/10.1088/0964-1726/25/2/027001 (IOP Publishing).
Azam A, Laflin KE, Jamal M, Fernandes R, Gracias DH. Self-folding micropatterned polymeric containers. Biomed Microdevice. 2011;13:51–8. https://doi.org/10.1007/s10544-010-9470-x.
Wang Y, Miao Y, Zhang J, Wu JP, Kirk TB, Xu J, Ma Dong, Xue W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater Sci Eng C. 2018;84:44–51. https://doi.org/10.1016/j.msec.2017.11.025 (Elsevier).
Melocchi A, Inverardi N, Uboldi M, Baldi F, Maroni A, Pandini S, Briatico-Vangosa F, Zema L, Gazzaniga A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): design concept and 4D printing feasibility. Int J Pharm. 2019;559:299–311. https://doi.org/10.1016/j.ijpharm.2019.01.045 (Elsevier).
Han D, Morde RS, Mariani S, La Mattina AA, Vignali E, Yang C, Barillaro G, Lee H. 4D printing of a bioinspired microneedle array with backward-facing barbs for enhanced tissue adhesion. Adv Func Mater. 2020;30:1909197.
Wan X, Wei H, Zhang F, Liu Y, Leng J. 3D printing of shape memory poly d l-lactide-co-trimethylene carbonate by direct ink writing. J Appl Polym Sci. 2019;136:48177.
Kim D, Kim T, Lee YG. 4D printed bifurcated stents with kirigami-inspired structures. J Vis Exp. 2019;2019:1–9. https://doi.org/10.3791/59746.
Bodaghi M, Damanpack AR, Liao WH. Self-expanding/shrinking structures by 4D printing. Smart Mater Struct. 2016;25:105034. https://doi.org/10.1088/0964-1726/25/10/105034 (IOP Publishing).
Bon B, Silvia IC, Morselli D, Esposti MD, Fabbri P, De Maria C, Viligiardi TF, Morabito A, Giorgi G, Valentini L. Printable smart 3D architectures of regenerated silk on poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Mater Des. 2021;201:109492. https://doi.org/10.1016/j.matdes.2021.109492 (The Authors).
Zhang Y, Wang Q, Yi S, Lin Zi, Wang C, Chen Z, Jiang L. 4D printing of magnetoactive soft materials for on-demand magnetic actuation transformation. ACS Appl Mater Interfaces. 2021;13:4174–84. https://doi.org/10.1021/acsami.0c19280.
Liu J, Erol O, Pantula A, Liu W, Jiang Z, Kobayashi K, Chatterjee D, et al. Dual-gel 4D printing of bioinspired tubes. ACS Appl Mater Interfaces. 2019;11:8492–8. https://doi.org/10.1021/acsami.8b17218.
Chen D, Liu Q, Han Z, Zhang J, Song HL, Wang K, Song Z, et al. 4D Printing strain self-sensing and temperature self-sensing integrated sensor–actuator with bioinspired gradient gaps. Adv Sci. 2020;7:2000584. https://doi.org/10.1002/advs.202000584.
Guo J, Zhang R, Zhang L, Cao X. 4D printing of robust hydrogels consisted of agarose nanofibers and polyacrylamide. ACS Macro Lett. 2018;7:442–6. https://doi.org/10.1021/acsmacrolett.7b00957.
Langford T, Mohammed A, Essa K, Elshaer A, Hassanin H. 4D printing of origami structures for minimally invasive surgeries using functional scaffold. Appl Sci (Switzerland). 2021;11:1–13. https://doi.org/10.3390/app11010332.
Ji Z, Yan C, Bo Yu, Zhang X, Cai M, Jia X, Wang X, Zhou F. 3D printing of hydrogel architectures with complex and controllable shape deformation. Adv Mater Technol. 2019;4:1–8. https://doi.org/10.1002/admt.201800713.
Ploszajski AR, Jackson R, Ransley M, Miodownik M. 4D printing of magnetically functionalized chainmail for exoskeletal biomedical applications. MRS Adv. 2019;4:1361–6. https://doi.org/10.1557/adv.201.
Cheng CY, Xie H, Xu Zy, Li L, Jiang MN, Tang L, Yang KK, Wang YZ. 4D printing of shape memory aliphatic copolyester via UV-assisted FDM strategy for medical protective devices. Chem Eng J. 2020;396: 125242. https://doi.org/10.1016/j.cej.2020.125242.
Podstawczyk D, Nizioł M, Szymczyk-Ziółkowska P, Fiedot-Toboła M. Development of thermoinks for 4D direct printing of temperature-induced self-rolling hydrogel actuators. Adv Func Mater. 2021;31:2009664.
Nishiguchi A, Zhang H, Schweizerhof S, Schulte MF, Mourran A, Möller M. 4D printing of a light-driven soft actuator with programmed printing density. ACS Appl Mater Interfaces. 2020;12:12176–85. https://doi.org/10.1021/acsami.0c02781.
Song Z, Ren L, Zhao C, Liu H, Zhenglei Yu, Liu Q, Ren L. Biomimetic nonuniform, dual-stimuli self-morphing enabled by gradient four-dimensional printing. ACS Appl Mater Interfaces. 2020;12:6351–61. https://doi.org/10.1021/acsami.9b17577.
Hu Y, Wang Z, Jin D, Zhang C, Sun R, Li Z, Kai Hu, et al. Botanical-inspired 4D printing of hydrogel at the microscale. Adv Func Mater. 2020;30:1907377.
Narupai B, Smith PT, Nelson A. 4D printing of multi-stimuli responsive protein-based hydrogels for autonomous shape Transformations. Adv Func Mater. 2021;31:2011012.
Whitesides GM. Soft robotics. Angew Chem - Int Ed. 2018;57:4258–73. https://doi.org/10.1002/anie.201800907.
Polygerinos P, Wang Z, Galloway KC, Wood RJ, Walsh CJ. Soft robotic glove for combined assistance and at-home rehabilitation. Robot Autonomous Syst. 2015;73:135–43. https://doi.org/10.1016/j.robot.2014.08.014 (Elsevier B.V.).
Zolfagharian A, Kaynak A, Bodaghi M, Kouzani AZ, Gharaie S. Applied sciences control-based 4D printing : adaptive 4D-printed systems. 2020.
Ong CS, Nam L, Ong K, Krishnan A, Huang CY, Fukunishi T, Hibino N. 3D and 4D bioprinting of the myocardium: current approaches, challenges, and future prospects. BioMed Res Int. 2018; 2018. https://doi.org/10.1155/2018/6497242
Acknowledgements
The authors acknowledge the infrastructure provided by the Indian Institute of Technology Roorkee for their research activities in this field.
Author information
Authors and Affiliations
Contributions
Souvik Ghosh: literature review, writing—original draft, and review and editing; Siddhi Chaudhuri: writing—original draft; Partha Roy: validation and review; Debrupa Lahiri: conceptualization, validation, review and editing, supervision, and project administration.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, S., Chaudhuri, S., Roy, P. et al. 4D Printing in Biomedical Engineering: a State-of-the-Art Review of Technologies, Biomaterials, and Application. Regen. Eng. Transl. Med. 9, 339–365 (2023). https://doi.org/10.1007/s40883-022-00288-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00288-5