Abstract
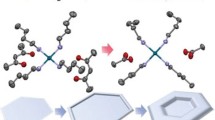
A novel one-pot approach to synthesize the tiara-like Pd(II) thiolate complex compound, [Pd(SCH3)2]6 was developed. In this strategy, dimethyl sulfoxide (DMSO) was used as a thiolate source instead of methyl mercaptan (CH3SH). DMSO was first decomposed into CH3SH and formaldehyde (HCHO); then, the in situ as-formed CH3SH molecules reacted with palladium acetate, and formed [Pd(SCH3)2]6. By tuning the reaction condition, the morphology of the [Pd(SCH3)2]6 assemblies can change from microprism to nanosphere. The characterization of the pyrolysis product demonstrated that these two kinds of [Pd(SCH3)2]6 assemblies with different shapes could further decompose into palladium or palladium sulfides through different pyrolysis conditions.
中文摘要
本文以醋酸钯为钯源, 二甲基亚砜为硫源, 在乙二醇和醋酸存在的条件下通过一步法制备了一种六核钯–甲硫醇团簇化合物 [Pd(SCH3)2]6. 其具有特征的类花冠形结构. 对其反应机制进行了探讨, 首先二甲基亚砜分解生成甲硫醇和甲醛, 钯与甲硫醇反应原位生 成钯–甲硫醇团簇. 这些生成的团簇分子进一步组装成微米尺寸大小的棱柱. 通过向反应体系中引入一种表面活性剂, 产物的形貌从微 米棱柱转变为纳米球. 350°C下, [Pd(SCH3)2]6的微米棱柱在空气中分解得到金属钯单质. 对其热解产物进行电镜表征, 发现其在保持原 有棱柱形貌的基础上形成了孔道结构. 在不同的热解条件下可以得到钯或硫化钯热解产物.
Similar content being viewed by others
References
Alemany P, Hoffmann R. Toroidal nickel thiolates: structure and bonding. J Am Chem Soc, 1993, 115: 8290–8297
Krebs B, Henkel G. Transition-metal thiolates: from molecular fragments of sulfidic solids to models for active centers in biomolecules. Angew Chem Int Ed, 1991, 30: 769–788
Dance IG. The structural chemistry of metal thiolate complexes. Polyhedron, 1986, 5: 1037–1104
Desireddy A, Conn BE, Guo J, et al. Ultrastable silver nanoparticles. Nature, 2013, 501: 399–402
Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science, 2007, 318: 430–433
Hsu IJ, Hsieh CH, Ke SC, et al. New members of a class of iron-thiolate-nitrosyl compounds: trinuclear iron-thiolate-nitrosyl complexes containing Fe3S6 core. J Am Chem Soc, 2007, 129: 1151–1159
Eichhofer A, Andrushko V, Bodenstein T, Fink K. Trinuclear early/late-transition-metal thiolate complexes. Eur J Inorg Chem, 2014, 3510–3520
Jian FF, Jiao K, Li Y, Zhao PS, Lu LD. [Ni6(SCH2CH2OH)12]: a double crown [12]metallacrown-6 nickel(II) cluster. Angew Chem Int Ed, 2003, 42: 5722–5724
Woodward P, Dahl LF, Abel EW, Crosse BC. A new type of cyclic transition metal complex, [Ni(SC2H5)2]6. J Am Chem Soc, 1965, 87: 5251–5253
Zhu M, Zhou S, Yao C, Liao L, Wu Z. Reduction-resistant and reduction-catalytic double-crown nickel nanoclusters. Nanoscale, 2014, 6: 14195–14199
Yang HY, Wang Y, Yan JZ, et al. Structural evolution of atomically precise thiolated bimetallic [Au12+n Cu32(SR)30+n ]4-(n=0, 2, 4, 6) nanoclusters. J Am Chem Soc, 2014, 136: 7197–7200
Melzer MM, Mossin S, Cardenas AJP, et al. A copper(II) thiolate from reductive cleavage of an S-nitrosothiol. Inorg Chem, 2012, 51: 8658–8660
Ibrahim MM, Seebacher J, Steinfeld G, Vahrenkamp H. Tris(thloimidazolyl) borate-zinc-thiolate complexes for the modeling of biological thiolate alkylations. Inorg Chem, 2005, 44: 8531–8538
Ananikov VP, Orlov NV, Zalesskiy SS, et al. Catalytic adaptive recognition of thiol (SH) and selenol (SeH) groups toward synthesis of functionalized vinyl monomers. J Am Chem Soc, 2012, 134: 6637–6649
Chen J, Liu L, Weng L, et al. Synthesis and properties evolution of a family of tiara-like phenylethanethiolated palladium nanoclusters. Sci Rep, 2015, 5: 16628
Anson CE, Eichhöfer A, Issac I, et al. Synthesis and crystal structures of the ligand-stabilized silver chalcogenide clusters [Ag154Se77(dppxy)18], [Ag320(StBu)60S130(dppp)12], [Ag352S128(StC5H11)96], and [Ag490S188(StC5H11)114]. Angew Chem Int Ed, 2008, 47: 1326–1331
Chakraborty I, Govindarajan A, Erusappan J, et al. The superstable 25 kDa monolayer protected silver nanoparticle: measurements and interpretation as an icosahedral Ag152(SCH2CH2Ph)60 cluster. Nano Lett, 2012, 12: 5861–5866
Albrecht C, Schwieger S, Bruhn C, et al. Alkylthio bridged 44 cve triangular platinum clusters: synthesis, oxidation, degradation, ligand substitution, and quantum chemical calculations. J Am Chem Soc, 2007, 129: 4551–4566
Heaven MW, Dass A, White PS, Holt KM, Murray RW. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18]. J Am Chem Soc, 2008, 130: 3754–3755
Chong H, Li P, Wang S, et al. Au25 clusters as electron-transfer catalysts induced the intramolecular cascade reaction of 2-nitrobenzonitrile. Sci Rep, 2013, 3: 3214
Li G, Zeng C, Jin R. Thermally robust Au99(SPh)42 nanoclusters for chemoselective hydrogenation of nitrobenzaldehyde derivatives in water. J Am Chem Soc, 2014, 136: 3673–3679
Li G, Lei Z, Wang QM. Luminescent molecular Ag-S nanocluster [Ag62S13(SBut)32](BF4)4. J Am Chem Soc, 2010, 132: 17678–17679
Udaya Bhaskara Rao T, Pradeep T. Luminescent Ag7 and Ag8 clusters by interfacial synthesis. Angew Chem Int Ed, 2010, 49: 3925–3929
Zhu M, Aikens CM, Hendrich MP, et al. Reversible switching of magnetism in thiolate-protected Au25 superatoms. J Am Chem Soc, 2009, 131: 2490–2492
Antonello S, Perera NV, Ruzzi M, Gascón JA, Maran F. Interplay of charge state, lability, and magnetism in the molecule-like Au25(SR)18 cluster. J Am Chem Soc, 2013, 135: 15585–15594
Wu ZN, Li YC, Liu JL, et al. Colloidal self-assembly of catalytic copper nanoclusters into ultrathin ribbons. Angew Chem Int Ed, 2014, 53: 12196–12200
Wu ZN, Dong CW, Li YC, et al. Self-assembly of Au-15 into single-cluster-thick sheets at the interface of two miscible high-boiling solvents. Angew Chem Int Ed, 2013, 52: 9952–9955
Li L, Wang Q. Spontaneous self-assembly of silver nanoparticles into lamellar structured silver nanoleaves. ACS Nano, 2013, 7: 3053–3060
Liu Y, Wu ZN, Zhang H. Deriving the colloidal synthesis of crystalline nanosheets to create self-assembly monolayers of nanoclusters. Adv Colloid Interface Sci, 2014, 207: 347–360
Jia XF, Li J, Wang EK. Supramolecular self-assembly of morphology-dependent luminescent Ag nanoclusters. Chem Commun, 2014, 50: 9565–9568
Beletskaya IP, Cheprakov AV. The heck reaction as a sharpening stone of palladium catalysis. Chem Rev, 2000, 100: 3009–3066
Jiang B, Song S, Wang J, et al. Nitrogen-doped graphene supported Pd@PdO core-shell clusters for C-C coupling reactions. Nano Res, 2014, 7: 1280–1290
Wang Z, Chen W, Han Z, et al. Pd embedded in porous carbon (Pd@ CMK-3) as an active catalyst for Suzuki reactions: accelerating mass transfer to enhance the reaction rate. Nano Res, 2014, 7: 1254–1262
Long R, Wu D, Li Y, et al. Enhancing the catalytic efficiency of the Heck coupling reaction by forming 5 nm Pd octahedrons using kinetic control. Nano Res, 2015, 8: 2115–2123
Li L, Zhou C, Zhao H, Wang R. Spatial control of palladium nanoparticles in flexible click-based porous organic polymers for hydrogenation of olefins and nitrobenzene. Nano Res, 2015, 8: 709–721
Xu B, Yang H, Zhou G, Wang X. Strong metal-support interaction in size-controlled monodisperse palladium-hematite nano-heterostructures during a liquid-solid heterogeneous catalysis. Sci China Mater, 2014, 57: 34–41
Gao D, Zhou H, Wang J, et al. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J Am Chem Soc, 2015, 4288–4291
Huang H, Bao S, Chen Q, et al. Novel hydrogen storage properties of palladium nanocrystals activated by a pentagonal cyclic twinned structure. Nano Res, 2015, 8: 2698–2705
Huang XQ, Tang SH, Mu XL, et al. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nanotechnol, 2011, 6: 28–32
Mashkina AV, Sakhaltueva LG. Gas-phase thiophene hydrogenation to tetrahydrothiophene over sulfide catalysts. Kinet Catal, 2002, 43: 107–114
Raybaud P, Hafner J, Kresse G, Toulhoat H. Ab initio density functional studies of transition-metal sulphides: II. electronic structure. J Phys Condens Matter, 1997, 9: 11107
Dey S, Jain VK. Platinum group metal chalcogenides. Platinum Met Rev, 2004, 48: 16–29
Bladon JJ, Lamola A, Lytle FW, et al. A palladium sulfide catalyst for electrolytic plating. J Electrochem Soc, 1996, 143: 1206–1213
Li X, Wen J, Low J, Fang Y, Yu J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci China Mater, 2014, 57: 70–100
Liu J, Zhao Y, Liu J, et al. From Cu2S nanocrystals to Cu doped CdS nanocrystals through cation exchange: controlled synthesis, optical properties and their p-type conductivity research. Sci China Mater, 2015, 58: 693–703
Yang ZQ, Smetana AB, Sorensen CM, Klabunde KJ. Synthesis and characterization of a new tiara Pd(II) thiolate complex, [Pd(SC12H25)2]6, and its solution-phase thermolysis to prepare nearly monodisperse palladium sulfide nanoparticles. Inorg Chem, 2007, 46: 2427–2431
Jose D, Jagirdar BR. Synthesis and characterization of Pd(0), PdS, and Pd@PdO core-shell nanoparticles by solventless thermolysis of a Pd-thiolate cluster. J Solid State Chem, 2010, 183: 2059–2067
Carlsen L, Egsgaard H, Harpp DN. Gas-phase thermolyses 4. Gasphase thermolyses of thietan 1-oxide and 1,2-oxathiolan 2-oxide-evidence for the intermediacy of 1,2-oxathiolan. J Chem Soc Perkin Trans 2, 1981, 1166–1170
Spek AL. Refcode AFACUG. Cambridge Crystallographic Database, 2007
Nobusada K, Yamaki T. Electronic properties of palladium-thiolate complexes with tiara-like structures. J Phys Chem A, 2004, 108: 1813–1817
Stash AI, Perepelkova TI, Noskov YG, Buslaeva TM, Romm IP. Palladium clusters Pd4(SEt)4(OAc)4 and Pd6(SEt)12: structure and properties. Russ J Coord Chem, 2001, 27: 585–590
Higgins JD, Suggs JW. Preparation, structure and spectroscopic studies of the palladium mercaptides Pd8(S-Npr)16 and Pd6(SNpr)12. Inorg Chim Acta, 1988, 145: 247–252
Stash AI, Levashova VV, Lebedev SA, et al. Palladium clusters Pd4(SR)4(OAc)4 and Pd6(SR)12 (R = Bu, Ph): structure and properties. Russ J Coord Chem, 2009, 35: 136–141
Schneider I, Horner M, Olendzki RN, Strahle J. Structure of cyclo-hexakis[bis-µ-(methoxycarbonylmethylthiolato)-palladium( II)], [Pd(SCH2COOCH3)2]6. Acta Crystallogr Sect C Cryst Struct Commun, 1993, 49: 2091–2093
Mahmudov KT, Hasanov XI, Maharramov AM, et al. A hexanuclear metallacrown palladium(II) cluster derived from 2-mercaptoethanol. Inorg Chem Commun, 2013, 29: 37–39
Author information
Authors and Affiliations
Corresponding authors
Additional information
Quanchen Feng received his MSc degree in chemistry from Beihang University in 2013. Currently, he is a PhD candidate in inorganic chemistry under the supervision of Prof. Yadong Li at Tsinghua University. His research interest is mainly focused on the simple synthesis of intermetallic nanomaterials and their applications as heterogeneous catalysts.
Jinpeng Li received his PhD degree in chemistry from Tohoku University in Japan in 2012. Currently, he is a postdoctoral researcher in the group of Prof. Yadong Li at Tsinghua University. His research focuses on the synthesis of metallic nanomaterial composite and their applications in heterogeneous catalysis.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Feng, Q., Wang, W., Cheong, WC. et al. Synthesis of palladium and palladium sulfide nanocrystals via thermolysis of a Pd–thiolate cluster. Sci. China Mater. 58, 936–943 (2015). https://doi.org/10.1007/s40843-015-0109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-015-0109-3