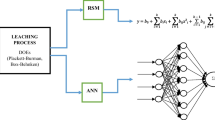
Abstract
The effects of operating conditions on copper, iron, and cobalt dissolution from sulfide ores in sulfuric acid–sodium chloride media are predicted using an artificial neural network (ANN) model. The artificial neural network model was developed, trained, and predicted using the feed-forward back-propagation (BP) algorithm. The sulfuric acid concentration, sodium chloride concentration, temperature, leaching time, and particle size were used as input variables to the model. A total of 204 sets of data generated from the leaching experiments were used to develop and train the model. To reach the network with a good agreement and highest generalizability and to reduce the error between the measured and predicted values, the neural networks with a various number of hidden layers (one to ten hidden layers) were investigated. In the regression analysis of the {5–10–3} architecture, the R2 values were 0.998, 0.997, and 0.997, while the MSE values were 0.111, 0,148, and 0.106 for the training, validation, and testing sets, respectively. The results showed that ANN has a high potential for predicting copper, cobalt, and iron recoveries. The increase in the number of hidden layers was found to improve the performance of the ANN model.
Graphical Abstract














Similar content being viewed by others
References
Reijnders L (2021) Is near-zero waste production of copper and its geochemically scarce companion elements feasible? Miner Process Extr Metall Rev 00(00):1–28. https://doi.org/10.1080/08827508.2021.1986706
Shengo ML, Kime M, Mambwe MP, Nyembo TK (2019) A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J Sustain Min. https://doi.org/10.1016/j.jsm.2019.08.001
Song S, Sun W, Wang L, Liu R, Han H (2019) Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J Environ Chem Eng 7(1):102777. https://doi.org/10.1016/j.jece.2018.11.022
Crundwell FK, Preez NB, Knights BDH (2020) Production of cobalt from copper-cobalt ores on the African copperbelt – an overview. Miner Eng 156(May):106450. https://doi.org/10.1016/j.mineng.2020.106450
Dehaine Q, Tijsseling LT, Glass HJ, Törmänen T, Butcher AR (2020) Geometallurgy of cobalt ores: a review. Miner Eng 160(September):2021. https://doi.org/10.1016/j.mineng.2020.106656
Dutrizac JE (1990) Elemental sulphur formation during the ferric chloride leaching of chalcopyrite. Hydrometallurgy 23:153–176
Tabelin CB et al (2021) Copper and critical metals production from porphyry ores and E-wastes: a review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour Conserv Recycl 170(April):105610. https://doi.org/10.1016/j.resconrec.2021.105610
Nkulu G, Gaydardzhiev S, Mwema E (2013) Statistical analysis of bioleaching copper, cobalt and nickel from polymetalic concentrate originating from Kamoya deposit in the Democratic Republic of Congo. Miner Eng 48:77–85. https://doi.org/10.1016/j.mineng.2012.10.007
Abdollahi H, Saneie R, Shafaei SZ, Mirmohammadi M, Mohammadzadeh A, Tuovinen OH (2021) Bioleaching of cobalt from magnetite-rich cobaltite-bearing ore. Hydrometallurgy 204(August):105727. https://doi.org/10.1016/j.hydromet.2021.105727
Bampole DL, Luis P, Mulaba-Bafubiandi AF (2019) Sustainable copper extraction from mixed chalcopyrite–chalcocite using biomass. Trans Nonferrous Met Soc China English Ed 29(10):2170–2182. https://doi.org/10.1016/S1003-6326(19)65123-X
Nkulu G, Gaydardzhiev S, Mwema E, Compere P (2015) SEM and EDS observations of carrollite bioleaching with a mixed culture of acidophilic bacteria. Miner Eng 75:70–76. https://doi.org/10.1016/j.mineng.2014.12.005
Zhong S, Li Y (2019) An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl. J Mater Res Technol 8(4):3487–3494. https://doi.org/10.1016/j.jmrt.2019.06.020
Toro N (2021) Leaching chalcocite in chloride media—a review. Minerals. https://doi.org/10.3390/min11111197
Behrad A, Nazari S, Darezereshki E (2022) Bioleaching of copper from chalcopyrite ore at higher NaCl concentrations. Miner Eng 175(June 2021):107281. https://doi.org/10.1016/j.mineng.2021.107281
Mwanat MH-M, Kasongo KB (2021) Cobalt dissolution from concentrate in sulfuric acid—ferrous sulfate system: process parameters optimization by response surface methodology (RSM). J Sustain Metall. https://doi.org/10.1007/s40831-021-00460-1
Quezada V, Roca A, Benavente O, Melo E (2021) The effects of sulphuric acid and sodium chloride agglomeration and curing on chalcopyrite leaching. Metals. https://doi.org/10.3390/met11060873
Skrobian M, Havlik T, Ukasik M (2005) Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution. Hydrometallurgy 77(1–2):109–114. https://doi.org/10.1016/j.hydromet.2004.10.015
Khoshkhoo M (2014) Chalcopyrite dissolution in sulphate-based leaching and bioleaching systems. Doctoral thesis, Luleå tekniska universitet
Kasongo KB, Mwanat MH, Malenga NE, Makhatha ME (2022) Kinetic study of copper and cobalt dissolution from sulfidic ores in sulphate—chloride Media. Min Metall Explor 0123456789:8–13. https://doi.org/10.1007/s42461-022-00671-4
Winand R (1991) Chloride hydrometallurgy. Hydrometallurgy 27(3):285–316
Carneiro MFC, Leão VA (2007) The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 87(3–4):73–82. https://doi.org/10.1016/j.hydromet.2007.01.005
Velásquez Yévenes L (2009) The kinetics of the dissolution of chalcopyrite in chloride media. Doctoral thesis, Murdoch University
Warren GW, Wadsworth ME, El-Raghy SM (1982) Passive and transpassive anodic behavior of chalcopyrite in acid solutions. J Electron Mater 21(1):571–579. https://doi.org/10.1007/BF02669170
Flores V, Keith B, Leiva C (2020) Using artificial intelligence techniques to improve the prediction of copper recovery by leaching. J Sens. https://doi.org/10.1155/2020/2454875Research
Mwanat MH-M, Kasongo KB, Muliangala MF, Kayembe MM, Kapiamba KF, Ngenda BR (2022) Simulation of simultaneous leaching of copper and cobalt minerals in acid—reductive media: sensitivity analysis and optimization. J Sustain Metall. https://doi.org/10.1007/s40831-022-00535-7
Leiva C, Flores V, Salgado F, Poblete D, Acuña C (2017) Applying softcomputing for copper recovery in leaching process. Sci Program. https://doi.org/10.1155/2017/6459582
Jorjani E, Chelgani SC, Mesroghli S (2008) Application of artificial neural networks to predict chemical desulfurization of Tabas coal. Fuel 87:2727–2734. https://doi.org/10.1016/j.fuel.2008.01.029
Jordan MI, Bishop CM (1996) Neural networks. ACM Comput Surv 28(1):73–75. https://doi.org/10.1145/234313.234348
Kasongo B, Monga J-J, Mwanat H (2021) Implementation of Artificial Neural network into the copper and cobalt leaching process. In: 2021 Southern African Universities Power Engineering Conference/Robotics and Mechatronics/Pattern Recognition Association of South Africa (SAUPEC/RobMech/PRASA), pp. 1–5. https://doi.org/10.1109/SAUPEC/RobMech/PRASA52254.2021.9377230
Aggarwal CC (2018) Neural networks and deep learning. Springer, 10, 978-3
Okwu MO, Tartibu LK (2020) Metaheuristic optimization: nature-inspired algorithms swarm and computational intelligence, theory and applications. Springer Nature
Akkurt S, Ozdemir S, Tayfur G, Akkurt S, Ozdemir S, Tayfur G (2016) Genetic algorithm—artificial neural network model for the prediction of germanium recovery from zinc plant residues. Miner Process Extr Metall. https://doi.org/10.1179/037195502766647048
Chelgani SC, Jorjani E (2009) Artificial neural network prediction of Al2O3 leaching recovery in the Bayer process—Jajarm alumina plant (Iran). Hydrometallurgy 97(1–2):105–110. https://doi.org/10.1016/j.hydromet.2009.01.008
Ebrahimzade H, Khayati GR, Schaffie M (2020) PSO–ANN-based prediction of cobalt leaching rate from waste lithium-ion batteries. J Mater Cycles Waste Manag 22(1):228–239. https://doi.org/10.1007/s10163-019-00933-2
Hoseinian FS, Abdollahzade A, Mohamadi SS, Hashemzadeh M (2017) Recovery prediction of copper oxide ore column leaching by hybrid neural genetic algorithm. Trans Nonferrous Met Soc China (English Edn.) 27(3):686–693. https://doi.org/10.1016/S1003-6326(17)60076-1
Ebrahimzade H, Reza G, Mahin K (2018) Leaching kinetics of valuable metals from waste Li-ion batteries using neural network approach. J Mater Cycles Waste Mange 20:10163
Kasongo KB, Mwanat HM (2021) Application of Taguchi method and artificial neural network model for the prediction of reductive leaching of cobalt(III) from oxidised low-grade ores. S Afr J Sci 117(5):1–8. https://doi.org/10.17159/SAJS.2021/8743
Abdollahi H, Noaparast M, Ziaedin S, Akcil A, Panda S (2019) Prediction and optimization studies for bioleaching of molybdenite concentrate using artificial neural networks and genetic algorithm. Miner Eng 130(March 2018):24–35. https://doi.org/10.1016/j.mineng.2018.10.008
Heaton J (2012) Introduction to the math of neural networks. Heaton Research, St. Louis
Al-Thyabat S (2008) On the optimization of froth flotation by the use of an artificial neural network. J China Univ Min Technol 18(3):418–426. https://doi.org/10.1016/S1006-1266(08)60087-5
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Zhongwei Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasongo, K.B., Mwanat, M.HM., Malenga, N.E. et al. Modeling and Analysis of Copper, Iron, and Cobalt Recovery in a Hybrid Sulfuric Acid–Sodium Chloride Media Using Artificial Neural Network. J. Sustain. Metall. 8, 2001–2014 (2022). https://doi.org/10.1007/s40831-022-00622-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00622-9