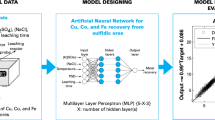
Abstract
Leaching is a complex solid–liquid reaction which has an important influence on the recovery efficiency of the spent lithium-ion batteries (LIBs). Therefore, it is of significant importance to utilize an appropriate technique to predict the effect of operating parameters on the optimized recovery rate. In the present study, a combined method of the artificial neural network (ANN) and particle swarm optimization algorithm (PSO) was used as a model to predict the leaching efficiency of cobalt from spent LIBs. To find the dependency of the leached percentage of cobalt on the operational parameters as model inputs, 42 repeatable numerous experiments are performed using H2SO4 in the presence of H2O2. It was found that the proposed model can be a useful technique in the demonstration of the nonlinear relationship between the leaching efficiency and the process parameters. The performance of PSO–ANN models was validated by statistical thresholds and compared with those of common ANN technique. Moreover, it was found that the pulp density of the leaching solution and the concentration of sulfuric acid were the most important reaction parameters of the spent LIBs recovery, respectively.











Similar content being viewed by others
References
Zhang X, Xie Y, Lin X et al (2013) An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J Mater Cycles Waste Manag 15:420–430
Zhang P, Yokoyama T, Itabashi O et al (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47:259–271
Dorella G, Mansur MB (2007) A study of the separation of cobalt from spent Li-ion battery residues. J Power Sources 170:210–215
Pant D, Dolker T (2017) Green and facile method for the recovery of spent lithium nickel manganese cobalt oxide (NMC) based lithium ion batteries. Waste Manag 60:689–695
Li L, Zhai L, Zhang X et al (2014) Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J Power Sources 262:380–385
Badawy SM, Nayl AA, El Khashab RA, El-Khateeb MA (2014) Cobalt separation from waste mobile phone batteries using selective precipitation and chelating resin. J Mater Cycles Waste Manag 16:739–746
Libraries T (2017) Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain Chem Eng 5:5224–5233
Honório KM, De Lima EF, Quiles MG et al (2010) Artificial neural networks and the study of the psychoactivity of cannabinoid compounds. Chem Biol Drug Design 75:632–640
Marini F, Bucci R, Magrì AL, Magrì AD (2008) Artificial neural networks in chemometrics: History, examples and perspectives. Microchem J 88:178–185
Taylor P, Kundu P, Debsarkar A et al (2014) Artificial neural network modelling in biological removal of organic carbon and nitrogen for the treatment of slaughterhouse wastewater in a batch reactor. Environ Technol 35:1296–1306
Khataee A, Fathinia M, Rad TS (2016) Kinetic modeling of nalidixic acid degradation by clinoptilolite nanorod-catalyzed ozonation process. RSC Adv 6:44371–44382
Thakur V, Ramesh A (2018) Analyzing composition and generation rates of biomedical waste in selected hospitals of Uttarakhand, India. J Mater Cycles Waste Manag 20:877–890
Galván IM, Zaldívar JM, Hernández H, Molga E (1996) The use of neural networks for fitting complex kinetic data. Comput Chem Eng 20:1451–1465
Normandin A, Grandjean BPA, Thibault J (1993) PVT data analysis using neural network models. Ind Eng Chem Res 32:970–975
Aldrich C, Deventer J, Reuteri MA (1994) The application of neural nets in the metallurgical industry. Miner Eng 7:793–809
Ijadpanah-Saravi H, Safari M, Noruzi-Masir B et al (2017) Intelligent tools to model photocatalytic degradation of beta-naphtol by titanium dioxide nanoparticles. J Chemom 31:e2907
Lazzús JA (2010) Prediction of flash point temperature of organic compounds using a hybrid method of group contribution + neural network + particle swarm optimization. Chin J Chem Eng 18:817–823
Momeni E, Armaghani DJ, Hajihassani M (2015) Prediction of uniaxial compressive strength of rock samples using hybrid particle swarm optimization-based artificial neural networks. Measurement 60:50–63
Roh S-B, Oh S-K, Park E-K, Choi WZ (2017) Identification of black plastics realized with the aid of Raman spectroscopy and fuzzy radial basis function neural networks classifier. J Mater Cycles Waste Manag 19:1093–1105
Rao R, Sahu JN (2018) Modeling and optimization by particle swarm embedded neural network for adsorption of zinc (II) by palm kernel shell based activated carbon from aqueous environment. J Environ Manag 206:178–191
Xia B, Cui D, Sun Z et al (2018) State of charge estimation of lithium-ion batteries using optimized Levenberg–Marquardt wavelet neural network. Energy 153:694–705
Khajeh M, Kaykhaii M, Hossein S, Shakeri M (2014) Particle swarm optimization—artificial neural network modeling and optimization of leachable zinc from flour samples by miniaturized homogenous liquid–liquid microextraction. J Food Compos Anal 33:32–38
Ahmadi M-A, Ahmad Z, Phung LTK et al (2016) Estimation of water content of natural gases using particle swarm optimization method. Pet Sci Technol 34:595–600
Khajeh M, Dastafkan K (2014) Removal of molybdenum using silver nanoparticles from water samples: particle swarm optimization–artificial neural network. J Ind Eng Chem 20:3014–3018
Ghaedi M, Ghaedi AM, Ansari A et al (2014) Artificial neural network and particle swarm optimization for removal of methyl orange by Gold nanoparticles loaded on activated carbon and Tamarisk. Spectrochim Acta Part A Mol Biomol Spectrosc 132:639–654
Sheikhan M, Pardis R, Gharavian D (2013) State of charge neural computational models for high energy density batteries in electric vehicles. Neural Comput Appl 22:1171–1180
Rahman A, Anwar S, Izadian A (2016) Electrochemical model parameter identification of a lithium-ion battery using particle swarm optimization method. J Power Sources 307:86–97
Agarwal S, Tyagi I, Kumar V et al (2016) Kinetics and thermodynamics of methyl orange adsorption from aqueous solutions—artificial neural network-particle swarm optimization modeling. J Mol Liquid 218:354–362
Mansouri I, Shahri A, Zahedifar H (2016) A new algorithm in nonlinear analysis of structures using particle swarm optimization. IIUM Eng J 17:157–168
Wang W-Y, Yen CH, Lin J-L, Xu R-B (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manag 21(2):300–307
Jha MK, Kumari A, Jha AK et al (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag 33:1890–1897
Joo S, Shin D, Oh C et al (2016) Selective extraction of nickel from cobalt, manganese and lithium in pretreated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy 159:65–74
Gao W, Liu C, Cao H et al (2018) Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag 75:477–485
Ebrahimzade H, Khayati GR, Schaffie M (2018) Leaching kinetics of valuable metals from waste Li-ion batteries using neural network approach. J Mater Cycles Waste Manag 20:2117–2129
Grenman H, Salmi T, Murzin DY (2011) Solid–liquid reaction kinetics—experimental aspects and model development. Rev Chem Eng 27:53–77
Meshram P, Abhilash A, Pandey BD et al (2019) Extraction of metals from spent lithium ion batteries—role of acid, reductant and process intensification in recycling. Indian J Chem Technol 25:368–375
Touboul M, Le Samedi E, Sephar N et al (1993) Binary systems with Li2SO4 as one of the components. J Therm Anal Calorim 40:1151–1156
Meshram P, Pandey BD, Mankhand TR, Deveci H (2016) Acid baking of spent lithium ion batteries for selective recovery of major metals: a two-step process. J Ind Eng Chem 43:117–126
Takacova Z, Havlik T, Kukurugya F, Orac D (2016) Cobalt and lithium recovery from active mass of spent Li-ion batteries : theoretical and experimental approach. Hydrometallurgy 163:9–17
He LP, Sun SY, Song XF, Yu JG (2017) Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag 64:171–181
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebrahimzade, H., Khayati, G.R. & Schaffie, M. PSO–ANN-based prediction of cobalt leaching rate from waste lithium-ion batteries. J Mater Cycles Waste Manag 22, 228–239 (2020). https://doi.org/10.1007/s10163-019-00933-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-019-00933-2