Abstract
Objective
We aimed to estimate the cost-effectiveness, burden of disease and budget impact of inclisiran added to standard-of-care lipid-lowering therapy in the real-world secondary cardiovascular prevention population in Switzerland.
Methods
An open-cohort Markov model captured event risks by sex, age and low-density lipoprotein cholesterol based on epidemiological and real-world data. Low-density lipoprotein cholesterol reduction with add-on inclisiran was based on trial results and translated to meta-analysis-based relative risks of cardiovascular events. Unit costs for 2018 were based on publicly available sources, adopting a Swiss healthcare system perspective. Price assumptions of Swiss francs (CHF) 500 and CHF 3,000 per dose of inclisiran were evaluated, combined with uptake assumptions for burden of disease and budget impact. The assessment of cost-effectiveness used a discount rate of 3% per year. We performed deterministic and probabilistic sensitivity analyses, and extensive scenario analyses.
Results
Patients treated with inclisiran gained a 0.291 qualityadjusted life-year at an incremental cost per QALY gained of CHF 21,107/228,040 (life-long time horizon, discount rate 3%) under the lower/higher price. Inclisiran prevented 1025 cardiovascular deaths, 3425 acute coronary syndrome episodes, and 1961 strokes in 48,823 patients ever treated during 10 years; the 5-year budget impact was CHF 49.3/573.4 million under the lower/higher price. Estimates were sensitive to calibration targets and treatment eligibility; burden of disease/budget impact results also to uptake. Limitations included uncertainties about model assumptions and the size and characteristics of the population modelled.
Conclusions
Inclisiran may be cost-effective at a willingness to pay of CHF 30,000 if priced at CHF 500; a threshold upwards of CHF 250,000 will be required if priced at CHF 3000. Inclisiran could enable important reductions in cardiovascular burden particularly under broader eligibility with a budget impact range from moderate to high depending on price.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conventional lipid-lowering therapy may fail to reduce low-density lipoprotein cholesterol levels to target, leaving patients at risk of cardiovascular morbidity despite maximally tolerated dosing. |
We developed a dynamic open-cohort model structure that enables, in one coherent framework, estimation of cost-effectiveness, burden of disease and budget impact under real-world assumptions. |
Inclisiran added to standard-of-care lipid-lowering therapy in secondary cardiovascular prevention patients may be cost-effective from the perspective of the Swiss healthcare system at a willingness-to-pay threshold of Swiss francs (CHF) 30,000 if priced at CHF 500 per dose; a willingness to pay upwards of CHF 250,000 would be required if inclisiran was priced at CHF 3000. |
Inclisiran could enable important reductions in cardiovascular burden at the population level, particularly under broader eligibility with a budget impact range from modest to high, depending on price. |
1 Introduction
Prevention and management of cardiovascular disease (CVD) are a key public health priority in Switzerland. In 2017 alone, there were over 21,000 CVD-related deaths (31% of all deaths) [1] and nearly 50,000 CVD-related hospitalisations of which over 22,000 were due to acute coronary syndrome (ACS) and about 25,000 due to stroke [2]. These conditions jointly accounted for nearly 16% of the total healthcare expenditures [3]. Clinical guidelines on CVD concentrate strongly on risk factors; lowering low-density lipoprotein cholesterol (LDL-C) with statins or statins in combination with ezetimibe are among the primary strategies [4,5,6]. While these therapies are effective [7, 8], multiple factors contribute to nearly 30% of patients stopping statins within the first year [9,10,11,12,13]. Among the very high and high cardiovascular risk patients, over 80% fail to achieve the guideline-recommended LDL-C target [14].
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) entered the arena of lipid-lowering drugs several years ago [15]. The PCSK9i available on the market, evolocumab and alirocumab, are human monoclonal antibodies. Their high clinical efficacy and favourable safety profile come at a high cost compared to statins that are largely available as generics [15,16,17]. Under the current reimbursement of PCSK9i in Switzerland, their use is restricted to the most at-risk patients and requires initiation by a specialist and a prior cost authorisation [18]. Reimbursement eligibility for secondary prevention requires an LDL-C above 2.6 mmol/L, leaving many patients without therapeutic options.
Inclisiran is a first-in-class, small-interfering ribonucleic acid molecule inhibiting PCSK9 protein synthesis in liver cells, administered as a subcutaneous injection. It received marketing approval in the European Union [19] and Switzerland [20] based on the ORION clinical trials that showed strong LDL-C lowering and provided a good, albeit not final, understanding of the efficacy and safety of the drug [21]. The need for additional LDL-C lowering not met in many patients raises the question of whether, compared to current PCSK9i policies, broader access is warranted for inclisiran. In England, the National Institute for Health and Care Excellence (NICE) has recently recommended the use of inclisiran in patients with prior CVD events and LDL-C ≥2.6 mmol/L, implying such a broadening of access [22]. Related decision making requires evidence on the likely cost-effectiveness, impact on burden of disease and budget impact. The classical clinical trial-based approach to the cost-effectiveness analysis may not fully reflect the use of the new therapy in the real world. Heterogeneity in patient, clinical management and health system characteristics limits the transferability of trial evidence between settings and from trials to policy [23]. Drawing on a primary care database, we characterise the real-world secondary cardiovascular prevention population in Switzerland and estimate the likely impact of inclisiran in these patients using a newly developed decision-analytic model.
2 Methods
We developed a dynamic open-cohort Markov model [24] suitable to consistently perform cost-effectiveness, burden of disease and budget impact analyses for real-world populations (Electronic Supplementary Material [ESM]). Outcomes included non-fatal and fatal cardiovascular events, death from other causes, life-years, quality-adjusted life-years (QALYs), costs in total and by category, and incremental cost-effectiveness ratios (ICERs). Costs were assessed from the Swiss statutory health insurance perspective. In the base-case and uncertainty analyses, lifelong, 10-year, and 5-year time horizons were adopted for cost-effectiveness, burden of disease, and budget impact, respectively. In the assessment of cost-effectiveness, costs and effects were discounted by 3%.
We defined the information needs for the model and evaluated potentially relevant Swiss and international data sources, determined based on the prior knowledge and experience of the research team and considering sources accepted by NICE in relevant technology appraisals [22, 25]. Model inputs characterising population size and numbers of CVD events in Switzerland were drawn from the Global Burden of Disease project [26], World Health Organization Mortality Database [27], and Swiss national statistics [2, 28] (see Tables 2 and 3 of the ESM). Patient characteristics came from a database of routine medical data by Swiss primary care physicians (Family medicine research using Electronic medical records (FIRE)) [29]. Transition probabilities from the British Clinical Practice Research Datalink [22] were adjusted to reflect Swiss event occurrence and LDL-C levels. The LDL-C changes achieved with inclisiran were based on the ORION-10 trial [31] and the relationship between LDL-C and event risks on a published meta-analysis [8]. Health-state utilities were based on published UK and Swiss data [32, 33] and unit costs on published Swiss studies and national sources [18, 34,35,36,37,38,39]. With the future public price of inclisiran in Switzerland yet unknown, inclisiran cost assumptions were based on two hypothetical price points: reflecting, at the lower end, the yearly treatment cost of ezetimibe (Ezetrol®) resulting from the public list price at the launch, Swiss Francs (CHF) 971 [40], and at the upper end, the yearly cost resulting from the public list price of the PCSK9i monoclonal antibodies currently marketed in Switzerland, CHF 6067 [18]. Market uptake assumptions were provided by the manufacturer of inclisiran. Further details are provided below; base-case parameter values and distributional assumptions are presented in Table 1.
2.1 Population and Medical Strategies
The primary population of interest was defined as Swiss patients aged 40 years or above with a prior ischaemic cardiac or cerebrovascular event (secondary prevention population). In scenario analyses, we also approximated an alternative wider population of interest including very high-risk patients without a prior event, as defined by current European guidelines (very high-risk population) [6]. In the absence of data on LDL-C levels of untreated Swiss patients, the inclisiran strategy assumed eligibility for inclisiran treatment (284 mg/1.5 mL at days 0 and 90, then every half year) as an add-on for patients with LDL-C ≥1.8 mmol/L under any standard-of-care lipid-lowering treatment (SOC LLT). Alternative SOC LLT requirements and thresholds of ≥1.4 mmol/L (including all patients not reaching the current European treatment target [6]) and ≥2.6 mmol/L (reflecting the current Swiss reimbursement limitation for PCSK9i [18]) were considered in scenario analyses. The comparator strategy was current SOC LLT as observed in FIRE [29] (see Results and the ESM).
2.2 Model Structure
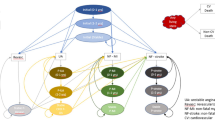
Inspired by Nghiem et al. [41], the model is a Markov cohort model with a 1-year cycle length that distinguishes 88 sub-cohorts characterised by age, sex and LDL-C group (<1.4 mmol/L, ≥1.4 to <1.8 mmol/L, ≥1.8 to <2.6 mmol/L, ≥2.6 mmol/L). Each sub-cohort is assigned its average age at entry, average LDL-C level and distribution of SOC LLT. Within each sub-cohort and as a function of these characteristics, patients transition through a series of CVD-related health states (see Fig. 1). The distribution of patients between health states does not reflect fractions of the sub-cohort but absolute patient numbers. The sub-cohorts are combined to the total modelled population using summation nodes.
Markov health state structure. Health states were defined as follows: “Very high risk prim” was used for very high risk patients who have not yet had a prior ischaemic cardiac or cerebrovascular event; “Revasc post” was used for very high risk patients who have not yet had a prior ischaemic cardiac or cerebrovascular event but had already undergone a cardiac revascularization (revasc) procedure that was not an immediate short-term treatment of an acute coronary syndrome (ACS) episode; “ACS 0–1” represented the first year after an ACS (i.e. unstable angina [UA] or myocardial infarction [MI]) event; “ACS post” represented subsequent years after an ACS (i.e. UA or MI) event; “Stroke 0–1” represented the first year after an acute cerebrovascular (i.e. ischaemic stroke) event; “Stroke post” represented subsequent years after an acute cerebrovascular (i.e. ischaemic stroke) event; “Stroke post and ACS 0–1” represented the first year after an ACS (i.e. UA or MI) event in patients who have already had at least one acute cerebrovascular (i.e. ischaemic stroke) event; “Stroke 0–1 and ACS post” represented the first year after an acute cerebrovascular (i.e. ischaemic stroke) event in patients who have already had at least one ACS (i.e. UA or MI) event; “Stroke post and ACS post” represented subsequent years (i.e. not the first year) after the last ACS or acute cerebrovascular event, in patients who have already had both types of events. “CVD death” and “Non-CVD death” are absorbing states entered at patient death due to either cardiovascular disease (CVD) or other causes. Health states “Very high risk prim” and “Revasc post” are not used for the modelling of the secondary prevention population, only for the very high risk population modelled in scenario analyses. “Revasc post” implies the patient has had a cardiac revascularization procedure that was not for the immediate short-term treatment of an ACS event. Further details on health state and event definitions are provided in the ESM
The model distinguishes prevalent patients forming part of the population of interest at model start (the treatment uptake of these patients can be spread over several years) and incident patients. Incident patients can enter the model in each cycle, in appropriate health states, with tunnel states allowing correct tracking of patient age. These functionalities are used for burden of disease and budget impact analyses, i.e. in these analyses, new-incident patients enter the model in each cycle. In contrast, cost-effectiveness analyses only consider prevalent patients and cycle 1 incident patients and assume full treatment uptake and immediate treatment start for eligible patients. To achieve a manageable reduction in real-world complexity, additional assumptions were required (ESM). Technical details on the implementation of the model in TreeAge software [42] are also provided in the ESM.
2.3 Epidemiological Data
The size of the prevalent secondary prevention population was approximated by multiplying the prevalence of ischaemic heart disease and ischaemic stroke by age and sex from the Global Burden of Disease project [26] with population counts by the Swiss Federal Statistical Office [28]. The size of the incident population by age and sex, defined here as patients who survived a first-time ischaemic heart disease or ischaemic stroke event in the reference year, was estimated from the Swiss statistics of inpatient episodes (MedStat) [2]. The size of the incident population was projected forward for 5 years and 10 years using the average annual growth rate of the incident secondary prevention population calculated from the Global Burden of Disease project [26].
The results of these calculations together with the LDL-C distribution from FIRE [14] determined the person numbers entering the sub-cohorts of the model. FIRE also provided the average LDL-C within each sex-age-LDL-C sub-cohort, the proportion receiving any SOC LLT, and the types of drugs under SOC LLT. For further details on the data sources, case definitions and secondary prevention population characteristics, see the ESM.
2.4 Event Risks and Clinical Effectiveness
Transition probabilities in the comparator strategy were based on values generated by the manufacturer of inclisiran using data from the Clinical Practice Research Datalink [22]. We adjusted these to the LDL-C levels of each of the 88 sub-cohorts using probability-rate-probability conversions and assuming a log-linear relationship between LDL-C change and event rates [22, 25]. Rate ratios per 1-mmol/L LDL-C change were based on the 2019 meta-analysis by the Cholesterol Treatment Trialists Collaboration [8]. Additional factors based on MedStat [2] were applied to ensure a plausible distribution of event risks across age groups, separately by sex, without affecting the overall event occurrence in the modelled population. The model was further calibrated to the expected event numbers in the Swiss secondary prevention population according to MedStat [2] for non-fatal events and the World Health Organization Mortality database for deaths [27] (see ESM for details and examples).
The impact of inclisiran was modelled based on its impact on LDL-C observed in the ORION-10 trial [31]. ORION-10 was preferred on grounds of similarity of the trial population with our secondary prevention population. Transition probabilities were adjusted based on the induced absolute LDL-C difference, by applying the same log-linear relationship as above. Implied were the assumptions that the relationship between LDL-C reduction and CVD event occurrence reported by Cholesterol Treatment Trialists holds for inclisiran, and that the effectiveness of inclisiran does not change over time. For further details, see the ESM.
2.5 Resource Use and Unit Costs
We considered the direct costs of non-fatal unstable angina/myocardial infarction and stroke events, fatal CVD events, revascularisation, background treatment with statins and ezetimibe, and costs of inclisiran including drug administration, as detailed in Table 1. Literature-based event cost-estimates covered drugs, diagnosis, in-patient and outpatient treatments, maintenance and follow-up care including for long-term sequelae. They were time adjusted using the increase in Swiss healthcare expenditure per capita [43]. The two hypothetical assumptions on the price per dose of inclisiran were CHF 500 (lower price, ezetimibe based) and CHF 3000 (higher price, PCSK9i monoclonal antibody based), to reflect twice-yearly maintenance dosing. All costs were expressed in 2018 CHF, the latest year for which consistent unit costs could be generated.
2.6 Utilities
Health-state utility values for the Swiss population without a prior CVD event were estimated based on age-specific and sex-specific Swiss utility values for the general population [32], which were separately calculated for each sub-cohort and updated in each model cycle. These were adjusted with a scaling factor from a UK study by Ara and Brazier [33] (ESM). Utility multipliers for the initial health states and subsequent events were also taken from Ara and Brazier [33]. As adverse events related to inclisiran were well balanced between the study arms [31], these were not considered in the analysis. Adverse events associated with SOC LLT were similarly excluded.
2.7 Inclisiran Uptake
While the cost-effectiveness analyses assumed a full uptake of inclisiran in eligible patients, the burden of disease and budget impact analyses required assumptions on uptake in the real world. As a starting point, the manufacturer of inclisiran provided an exemplary assumption based on its most recent launch in the area of CVD: the worldwide average uptake of sacubitril/valsartan (Entresto®) ranged from about 10% to 36% during the first 5 years after the launch. Because of a different formulation and because only a fraction of secondary prevention patients would qualify for inclisiran treatment, we selected assumptions such that about 10% of this population would ever be treated during 5-year and 10-year model time horizons. For the prevalent patient group, this led to uptake assumptions of 13% and 22% in the LDL-C ≥1.8 mmol/L to <2.6 mmol/L and LDL-C ≥2.6 mmol/L groups, respectively, equally spread over 5 years. The uptake in incident patients was assumed to increase over the first 5 years to 24% and 30% in the aforementioned LDL-C groups. Uptake after 5 years was assumed to remain stable; see ESM for details.
2.8 Validation
Model validation addressed face validation, internal validation, cross-validation, and external validation [44]. The validation steps showed satisfactory results. As a single exception, the model may moderately overestimate life expectancy. This was identified to be a consequence of the necessary calibration to plausible fatal CVD event numbers in the Swiss secondary prevention population, which has conservative implications for the cost-effectiveness of inclisiran.
2.9 Uncertainty Analyses
Uncertainty analyses in the cost-effectiveness part included univariate deterministic and multivariate probabilistic sensitivity analyses with 1000 iterations. Ranges of variation in the univariate deterministic sensitivity analysis were based on upper and lower 95% confidence limits. Where not available, parameter values (e.g. those representing unit costs) were varied by ± 30%. In the case of utilities and utility multipliers, the difference from 1 was varied by ± 30%. The probabilistic sensitivity analysis used distributions reflecting these ranges of variation (lognormal for rate ratios and normal for all other parameters to ensure consistency with results of the deterministic analysis). Scenario analyses assessed the impact of varying assumptions on SOC LLT and LDL-C requirements for inclisiran treatment eligibility, inclisiran uptake and effect, cardiovascular event costs and discount rate. We also tested alternative approaches to the consideration of incident patients, including an open-cohort approach as used for the burden of disease and budget impact parts. The uncertainty in the occurrence of clinical events in the comparator strategy was solely addressed in scenario analyses, given multiple transition probabilities and a strong influence of calibration. Other estimated characteristics of the Swiss secondary prevention population were not varied. Additional scenario analyses were used to approximate results for the very high-risk population. For the burden of disease and budget impact analyses, a suitable subset of the scenario analyses performed in the cost-effectiveness part was implemented. We followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [45].
3 Results
The size of the Swiss secondary prevention population was estimated at 302,738 patients (as of 2018). The number of incident patients was 17,024 and increased slightly in subsequent years (ESM). The average age of secondary prevention patients was 71 years, over 60% of these patients were male. Based on FIRE, the prevalence of diabetes mellitus was 27% [29]. The average LDL-C under SOC LLT was 2.3 mmol/L. Patients with LDL-C ≥1.8 mmol/L accounted for about 80% of the prevalent and incident cohorts (239,214 and 13,442 patients, respectively). In this sub-population, LDL-C averaged 2.7 mmol/L. With respect to background SOC LLT, 69% of patients were taking statins, of which more than half (63%) received high-intensity statins, and 15% were taking ezetimibe. For details, see the ESM.
3.1 Cost-Effectiveness
Adding inclisiran to SOC LLT in eligible patients increased per-person life expectancy in the secondary prevention population by 0.199 years and yielded an additional 0.159 QALYs (based on gains of 0.364 years and 0.291 QALYs in those actually treated with inclisiran). The incremental cost was CHF 3354/36,233 per person under the lower/higher price assumption respectively (Table 2). The resulting ICERs were CHF 21,107/228,040 per QALY gained under the lower/higher price.
In the univariate sensitivity analysis (Fig. 2), parameters related to costs of clinical events led to proportionally greater changes in ICER under the lower inclisiran price assumption, whereas parameters related to utilities were more impactful under the higher price. The impacts of inclisiran on LDL-C and background utility were in the top five most impactful parameters. Across inputs and ranges assessed, ICERs remained bounded within a relatively narrow range around the main result of ± CHF 5000 under the lower price and ±CHF 20,000 under the higher price.
Univariate sensitivity analysis of cost-effectiveness results by inclisiran price per dose. Panel A presents results of the univariate sensitivity analysis under inclisiran price per dose = Swiss francs (CHF) 500. Panel B presents results of the univariate sensitivity analysis under inclisiran price per dose = CHF 3000. The length of the bar indicates the resulting incremental cost-effectiveness ratio (ICER) when the respective parameter is set to its lower (lighter shade) and upper (darker shade) bound values (see text for ranges); the diagram is centred on the base-case ICER, i.e. CHF 21,107/228,040 under the lower/higher inclisiran price assumption. Results in tabular format are reported in the ESM. ACS acute coronary syndrome, CV cardiovascular, CVD cardiovascular disease, LDL-C low-density lipoprotein cholesterol, MI myocardial infarction, UA unstable angina
In scenario analyses (Tables 23–24 of the ESM), ICERs were most sensitive to calibration targets for non-fatal events (scenarios 15–18). Particularly large changes were observed when calibration targets for non-fatal and fatal events were varied jointly (scenario 18). Scenarios exploring alternative eligibility criteria, uptake, and effectiveness of inclisiran resulted in at most a ± 20% change over the base case (scenarios 1–5). Alternative assumptions on the target population (i.e. secondary prevention population [base case] vs very high-risk population), baseline utilities, and age-adjustment of transition probabilities had a similar impact (scenarios 9, 10, 19). Other features related to the real-world use of inclisiran including persistence and maximum age at treatment start (scenarios 6–8) had only a limited impact on the predicted cost-effectiveness. Alternative approaches to the consideration of incident patients were not influential (scenarios 21 and 22).
In the probabilistic sensitivity analysis, the 2.5th and 97.5th ICER percentiles were CHF 14,557 and CHF 28,497 per QALY gained under the lower price assumption and CHF 195,042 and CHF 278,316 under the higher price assumption. Figure 3 presents a cost-effectiveness scatterplot and cost-effectiveness acceptability curves. The probability inclisiran is cost-effective if priced at CHF 500 per dose was estimated at 99% under a willingness-to-pay (WTP) threshold of CHF 30,000 per QALY gained. If priced at CHF 3000, the probability of cost-effectiveness was <1% up to a WTP of CHF 200,000, and 97% at a WTP of CHF 250,000 per QALY gained.
Probabilistic sensitivity analysis-based cost-effectiveness plane and cost-effectiveness acceptability curves from 10,000 iterations by inclisiran price per dose. Panel A shows the cost and quality-adjusted life year (QALY) differences per person treated with inclisiran. Dashed lines represent thresholds of Swiss francs (CHF) 50,000, 100,000, 200,000, and 300,000 per QALY gained. The population size was 319,742 and the percentage treated was 0.54%. Panel B shows the corresponding cost-effectiveness acceptability curves for inclisiran price per dose = CHF 500. Panel C shows the corresponding cost-effectiveness acceptability curves for inclisiran price per dose = CHF 3000
3.2 Burden of Disease
Under the base-case eligibility and uptake assumptions, about 10% of the secondary prevention population would be treated with inclisiran over 10 years (Table 3). The greatest relative reduction in the number of events due to inclisiran was estimated for revascularisations and non-fatal ACS (about 4%), followed by stroke and CVD deaths (2–3%). With 788 deaths averted, all-cause mortality was least impacted by inclisiran because of competing risks (<0.1% reduction relative to the comparator strategy). Population gains in life expectancy and QALYs were both less than 0.1%, translating to 0.064 life-years and 0.058 QALYs gained per person relative to the comparator strategy.
The burden of disease estimates were most sensitive to assumptions that varied the number of patients treated (i.e. uptake, treatment eligibility; see Table 25 of the ESM). Scenarios assuming full uptake (i.e. inclisiran administered in all secondary prevention patients meeting the set LDL-C threshold and SOC LLT requirement) resulted in an over five-fold increase in the number of eligible patients with proportionate reductions in burden. Restricting treatment eligibility to patients taking high-intensity statins and ezetimibe resulted in the lowest impact in all outcomes (531 non-fatal ACS, 141 CV deaths averted, and 416 QALYs gained over 10 years). Similarly, introducing an age cut-off for starting inclisiran treatment, while fairly marginal when considering changes to the predicted ICER, reduced deaths avoided and QALYs gained by about 30%. Calibration targets for cardiovascular events remained a sensitive parameter.
3.3 Budget Impact
Under the base-case treatment eligibility and uptake assumptions, 33,398 patients would be treated with inclisiran over 5 years (Table 4). The net budget impact of the new therapy would be CHF 49.3/573.4 million under the lower/higher inclisiran price, increasing the current cost of CVD management in this population by about 0.4/4%. Cost reductions achieved through reduced CVD morbidity enabled by inclisiran would offset 55%/10% of the lower price/higher price inclisiran costs, respectively.
Aside from the price of inclisiran, budget impact estimates were most sensitive to assumptions on treatment eligibility (Tables 26–27 of the ESM). Restricting inclisiran eligibility to patients already treated with high-intensity statins led to a 45% decrease in the budget impact (CHF 67.7 million). Restricting eligibility to those treated with high-intensity statins and ezetimibe reduced the budget impact further (CHF 21.2 million). Increasing the LDL-C threshold eligibility to ≥2.6 mmol/L reduced the budget impact by 56% (to CHF 52.8 million). Scenarios unrelated to treatment eligibility and price resulted in an at most 5% change in the budget impact.
4 Discussion
We modelled the likely impacts of adding inclisiran to SOC LLT in Swiss secondary cardiovascular prevention patients with LDL-C ≥1.8 mmol/L. The new therapy was estimated to enable an additional 0.291 QALYs per person treated at an ICER of CHF 21,107/228,040 per QALY gained under an assumed price of CHF 500/3000 per dose of inclisiran. The estimated ICERs were fairly robust in the deterministic sensitivity analysis. Scenario analyses provided broader ICER ranges reflecting uncertainty about the size and characteristics of the target population. Changes in calibration targets, reflecting substantial uncertainty around true event rates in the target population, were particularly influential. Features related to the real-world use of inclisiran including persistence and maximum age at treatment start had only a limited impact on the predicted cost-effectiveness. In the very high-risk prevention patients, the benefits and the value for money were broadly comparable to the base-case estimates. Under base-case eligibility and uptake assumptions, inclisiran was shown to lead to important reductions in CVD mortality and morbidity. The budget impact in the first 5 years was 0.4% or 4% of the current cardiovascular treatment costs in the target population, depending on price.
To date, only one published study by Kam and colleagues [46] considered the economic properties of inclisiran in a wider population currently not eligible for PCSK9i. The authors developed a Markov model populated with UK-based transition probabilities that described a narrow set of health states (myocardial infarction, revascularisation, CVD, and non-CVD deaths) in a population modelled after the ORION-10 trial [31]. From the perspective of the Australian health system and at an assumed annual inclisiran cost of AUD 6334 (similar to the higher price evaluated in our base-case analysis), the authors estimated an ICER slightly over AUD 125,000 per QALY gained, more favourable compared with our finding for the higher price. Differences are expected given different approaches to modelling (based on a single cohort aged 66 years in Kam et al. versus a population with a widespread age range ≥40 years and an average age of 71 years in our analysis). In addition, Swiss secondary prevention patients appeared somewhat healthier, displaying lower LDL-C levels, a lower incidence of diabetes, and, as a consequence, facing relatively lower cardiovascular risk which translated to relatively lower gains from inclisiran. Our findings are still broadly consistent with those of Kam et al., showing better value of inclisiran in populations with higher LDL-C.
The present analysis is subject to limitations. Our key challenge was in identifying the size and structure of the Swiss secondary prevention population and the occurrence of events in these patients. To derive the relevant inputs, Swiss sources were combined with international databases covering data from Switzerland and other industrial countries. In the absence of suitable Swiss data, we used starting transition probabilities derived from the British Clinical Practice Research Datalink database [22], as also used in the NICE Single Technology Appraisal of inclisiran, which were subsequently adjusted to the age and LDL-C characteristics of our population of interest. This implied a separate calculation for each sub-cohort and in each model cycle, hindering variation in the standard sensitivity analysis. However, a potential lack of applicability was mitigated by introducing calibration factors that scaled the model outputs in the comparator strategy to the number of annual non-fatal and fatal cardiovascular events realistically expected in the Swiss secondary prevention population. These calibration factors were extensively varied in scenario analyses. We also used UK-based utility multipliers for cardiovascular events [33] and factors to convert utilities in the general population to the non-CVD population [33]. These were, however, applied to general population utility estimates for Switzerland [32], minimising potential bias.
Unavoidable inconsistencies in case definitions, methods of data generation, and populations covered across the data sources were also addressed in the uncertainty analyses, by comparing different approaches to the derivation of parameters and evaluating alternative assumptions on parameter values. Generally, middle-of-the-road and conservative estimates were preferred over extreme values. To avoid additional layers of technical complexity, the presented results assumed the characteristics of the Swiss secondary prevention population were estimated correctly. Given uptake assumptions, the time horizon for the burden of disease analyses covered an initial period of dynamic development of the numbers of persons treated and relative stabilisation thereafter. Additional scenarios assumed immediate full treatment uptake of all eligible patients to facilitate interpretation. Because of a current lack of real-world adherence and persistence data for inclisiran, we assumed full adherence, and reduced persistence only in some cost-effectiveness scenarios. Research into these topics may be warranted after the introduction of inclisiran into the market. Given the low use of the currently available PCSK9i antibodies in the Swiss secondary prevention population (0.8% according to [27]), we did not consider the impact of these drugs in our analyses.
One major assumption of the model was that the meta-analysis-based relationship between LDL-C reduction and CVD event occurrence would hold for inclisiran. This was supported by review results from Ference et al. [5] that indicated the impact of lipid-lowering therapies on clinical outcomes is independent of the mechanism of action. Moreover, constrained by the data limited to within-trial observations of inclisiran-treated patients (1.4 years in ORION studies), we assumed that there would be no change in the efficacy of inclisiran over time. Several trials are in progress to directly quantify the impact of inclisiran on cardiovascular events and mortality allowing for a longer follow-up [47, 48]; the results, once available, may be used to update our analysis. Noteworthy, similar assumptions were accepted in the NICE appraisal of inclisiran in light of the potential benefits of this new therapy, further strengthening the policy relevance of the modelled evidence presented here.
Compared with conventional approaches, our innovative dynamic open-cohort model supports the generation of highly consistent cost-effectiveness, burden of disease, and budget impact predictions at cohort and population levels. Heterogeneity in population features relevant to the risk of cardiovascular events (i.e. age, sex, LDL-C, SOC LLT, diabetes) is easily accommodated, facilitating applications to other countries or populations. Moreover, the flexibility of the modelling framework and the data collated support further evaluations of health interventions other than inclisiran in patients at risk of CVD, including primary prevention patients in Swiss and other settings. Performing the cost-effectiveness part with an open-cohort instead of a closed-cohort approach was not influential in the present case but might induce substantial ICER differences for other intervention types, for example treatments with high initial costs and no or very low subsequent costs. Policy-relevant scenarios with respect to adherence, longer term efficacy, uptake and pricing scenarios can easily be implemented to inform reimbursement and budgeting discussions.
5 Conclusions
From the perspective of the Swiss healthcare system, inclisiran may be cost-effective in secondary cardiovascular prevention patients at a WTP threshold of CHF 30,000 per QALY gained if priced at CHF 500 per dose. A threshold upwards of CHF 250,000 would be required if inclisiran was priced at CHF 3000. Similar value for money was estimated for a broader population at very high risk of CVD events. Inclisiran could enable important reductions in cardiovascular burden particularly under broader eligibility with a budget impact range from modest to high depending on price and actual uptake. These findings should be interpreted considering the uncertainty around the size and characteristics of the Swiss secondary prevention population and the stated limitations.
Change history
30 August 2022
Missing Open Access funding information has been added in the Funding Note
07 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40273-022-01171-5
References
Swiss Federal Statistical Office. Cause of death statistics 2017. Cardiovascular diseases and cancer continue to be the leading causes of death in Switzerland. https://www.bfs.admin.ch/bfs/de/home/aktuell/medienmitteilungen.assetdetail.11227251.html. Accessed 15 Sep 2021.
Swiss Federal Statistical Office. Medical statistics of hospitals. https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/enquetes/ms.html. Accessed 15 Sep 2021.
Wieser S, Riguzzi M, Pletscher M, et al. How much does the treatment of each major disease cost? A decomposition of Swiss National Health Accounts. Eur J Health Econ. 2018;19:1149–61.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–37.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41:111–88.
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–69.
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–15.
De Vera MA, Bhole V, Burns LC, et al. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78:684–98.
Ofori-Asenso R, Ilomaki J, Tacey M, et al. Prevalence and incidence of statin use and 3-year adherence and discontinuation rates among older adults with demuentia. Am J Alzheimers Dis Other Demen. 2018;33:527–34.
Ofori-Asenso R, Ilomaki J, Tacey M, et al. Switching, discontinuation, and reeinitiation of statins among older adults. J Am Coll Cardiol. 2018;72:2675–7.
Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–34.
Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61.
Meier R, Rachamin Y, Rosemann T, et al. The impact of the 2019 European guideline for cardiovascular risk management: a cross-sectional study in general practice. J Clin Med. 2020;9:2140.
Whayne TF Jr. Defining the role of PCSK9 inhibitors in the treatment of hyperlipidemia. Am J Cardiovasc Drugs. 2016;16:83–92.
Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–87.
Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol. 2019;16:155–65.
Swiss Federal Office of Public Health. Spezialitätenliste. 2021. https://www.bag.admin.ch/bag/de/home/begriffe-a-z/spezialitaetenliste.html. Accessed 15 May 2021.
Lamb YN. Inclisiran: first approval. Drugs. 2021;81:389–95.
Swiss Agency for Therapeutic Products. Authorised human medicines with new active substances. 2021. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines.html. Accessed 15 Aug 2021.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382:1520–30.
NICE National Institute for Health and Care Excellence. Single technology appraisal. Inclisiran for treating primary hypercholesterolaemia or mixed dyslipidaemia [ID1647]. Committee papers. https://www.nice.org.uk/guidance/gid-ta10703. Accessed 15 Dec 2021.
Nordon C, Karcher H, Groenwold RH, et al. The “efficacy-effectiveness gap”: historical background and current conceptualization. Value Health. 2016;19:75–81.
Ethgen O, Standaert B. Population-versus cohort-based modelling approaches. Pharmacoeconomics. 2012;30:171–81.
NICE National Institute for Health and Care Excellence. Single technology appraisal. Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. Committee papers. www.nice.org.uk/guidance/ta393. Accessed 15 Dec 2021.
Institute for Health Metrics and Evaluation (IHME). Global health data exchange. http://ghdx.healthdata.org/gbd-results-tool. Accessed 15 Nov 2021.
World Health Organization (WHO). Mortality database. https://apps.who.int/healthinfo/statistics/mortality/whodpms/. Accessed 15 Nov 2021.
Swiss Federal Statistical Office. Population statistics. 2020. https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung/bevoelkerung.assetdetail.9486043.html. Accessed 15 Dec 2020.
Chmiel C, Bhend H, Senn O, et al. The FIRE project: a milestone for research in primary care in Switzerland. Swiss Med Wkly. 2011;140: w13142.
Morgan C, Durand A, Loh J, et al. Risk of major adverse cardiovascular events (MACE) in ASCVD, ASCVD-risk equivalent and FH patients with elevated low-density lipoprotein cholesterol (LDL-C). Internal report. Neuchâtel: Novartis; 2020.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–19.
Perneger TV, Combescure C, Courvoisier DS. General population reference values for the French version of the EuroQol EQ-5D health utility instrument. Value Health. 2010;13:631–5.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13:59–68.
Wieser S, Ruthemann I, De Boni S, et al. Cost of acute coronary syndrome in Switzerland in 2008. Swiss Med Wkly. 2012;142: w13655.
Pletscher M, Plessow R, Eichler K, et al. Cost-effectiveness of dabigatran for stroke prevention in atrial fibrillation in Switzerland. Swiss Med Wkly. 2013;143: w13732.
Moschetti K, Petersen SE, Pilz G, et al. Cost-minimization analysis of three decision strategies for cardiac revascularization: results of the “suspected CAD” cohort of the European Cardiovascular Magnetic Resonance Registry. J Cardiovasc Magn Reson. 2016;18:3.
Schur NTS, Reinau D, Schenkglenks M, Meier CR. Helsana-Arzneimittelreport für die Schweiz 2020. Auswertungsergebnisse der Helsana Arzneimitteldaten aus den Jahren 2016 bis 2019. Basel, Switzerland: Helsana, 2020. https://www.helsana.ch/de/helsana-gruppe/medien-publikationen/mitteilungen/arzneimittelreport-2020.html. Accessed 15 Dec 2021.
FMH Swiss Medical Association. Ambulante Tarife Tarmed. Bern, Switzerland: FMH, 2020. https://www.fmh.ch/themen/ambulante-tarife/tarmed.cfm. Accessed 15 Sep 2021.
ELIGO. Tarmed-Taxpunktwerte-Liste. Tarmed-Taxunktwerte unter KVG, ab Anfang des Monats. 2018. https://eligo.ch/Tarmed-Taxpunktwerte.html. Accessed 15 Dec 2021.
Open Drug Database (ODDB). ODDB, 2021. https://ch.oddb.org/de/gcc/price_history/reg/56195/seq/01/pack/002. Accessed 15 Dec 2021.
Nghiem N, Wilson N, Blakely T. Technical background to the cardiovascular disease model used in the BODE programme. BODE3 Programme. University of Otago, Wellington, New Zealand, 2015. https://www.otago.ac.nz/wellington/otago070188.pdf. Accessed 15 Dec 2021.
TreeAge Healthcare®. Williamstown (MA): TreeAge Software, LLC. https://www.treeage.com/. Accessed 15 Oct 2021.
Federal Statistical Office. Statistic on the costs and financing of the health system. 2020. https://www.bfs.admin.ch/bfs/en/home/statistics/health/costs-financing.html. Accessed 15 Jun 2021.
Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–7. Value Health. 2012;15:843–50.
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS): explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–50.
Kam N, Perera K, Zomer E, et al. Inclisiran as adjunct lipid-lowering therapy for patients with cardiovascular disease: a cost-effectiveness analysis. Pharmacoeconomics. 2020;38:1007–20.
ClinicalTrials.gov. Identifier NCT03814187, trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C (ORION-8). Bethesda (MD): National Library of Medicine (US); 2021. https://clinicaltrials.gov/ct2/show/NCT03814187. Accessed 15 Sep 2021.
ClinicalTrials.gov. Identifier NCT03705234, a randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease (ORION-4). Bethesda (MD): National Library of Medicine (US); 2021. https://clinicaltrials.gov/ct2/show/NCT03705234. Accessed 15 Sep 2021.
Ludman P, British Cardiovascular Society. BCIS National Audit Adult Interventional Procedures 1st April 2018 to 31st March 2019. 2018.
Schulman-Marcus J, Feldman DN, Rao SV, et al. Characteristics of patients undergoing cardiac catheterization before noncardiac surgery: a report from the National Cardiovascular Data Registry CathPCI Registry. JAMA Intern Med. 2016;176:611–8.
Acknowledgements
We thank Patrick Schwab and Gian-Paolo Klinke from the Swiss Federal Statistical Office for providing aggregate data from the Swiss Medical Statistics of Hospitals, Dragana Radovanovic and André Höpli for providing aggregate data from the AMIS-plus registry, the FIRE study group of primary care physicians for providing electronic medical records data and Christina Tzogiou from Zurich University of Applied Sciences for supporting the collection of unit cost data. We thank Jonas Mueller from Novartis Pharma Schweiz AG for providing relevant information on inclisiran. The contributors are not in any way responsible for the content of this article. The responsibility for the content is with the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of Basel.
Conflicts of interest/competing interests
MS received research funding from Novartis via an employment institution and, unrelated to the work reported in the article, remuneration for participation in advisory boards from Amgen and Sandoz. RM received research funding from Novartis and Amgen via an employment institution unrelated to the work reported in the article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The parameters values used in the modelling study are included in the published article (and its supplementary information files) or are available from the corresponding author on reasonable request for non-commercial purposes, as is the model. Access to underlying data from the Swiss Federal Office of Statistics, the FIRE database and the Global Burden of Disease project is possible via the data owners, following their regulations. Where relevant, contact can be established with the corresponding author.
Code availability
The model was implemented in TreeAge and is available from the corresponding author (MS) on reasonable request for non-commercial purposes.
Author contributions
MS conceived and designed the study. MS developed and implemented the model in TreeAge, and performed the model validation. KG, PS and RM contributed to the study design and model development. MS, KG and PS performed the analysis. KG and PS collated data and derived model inputs. RM collated data and derived unit costs. YR and RM provided aggregate FIRE data on patient characteristics, treatments and events in secondary cardiovascular prevention patients. KG and MS drafted the manuscript. All authors reviewed the manuscript for important intellectual content and approved the final version.
Additional information
The original Online version of this article was revised: The first sentence of the Results section in the Abstract and the fourth sentence of the figure 2 legend have been incorrectly published.
The Original online version of this article was revised: The Open Access funding information was missed and published in the original version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Galactionova, K., Salari, P., Mattli, R. et al. Cost-Effectiveness, Burden of Disease and Budget Impact of Inclisiran: Dynamic Cohort Modelling of a Real-World Population with Cardiovascular Disease. PharmacoEconomics 40, 791–806 (2022). https://doi.org/10.1007/s40273-022-01152-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01152-8