Abstract
Inclisiran (Leqvio®; Novartis) is a first-in-class, cholesterol-lowering small interfering RNA (siRNA) conjugated to triantennary N-acetylgalactosamine carbohydrates (GalNAc). Inclisiran received its first approval in December 2020 in the EU for use in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet. It is intended for use in combination with a statin or a statin with other lipid-lowering therapies in patients unable to reach low-density lipoprotein cholesterol goals with the maximum tolerated statin dose. In patients who are statin-intolerant or for whom a statin is contraindicated, inclisiran can be used alone or in combination with other lipid-lowering therapies. Inclisiran is administered as a twice-yearly subcutaneous injection. This article summarizes the milestones in the development of inclisiran leading to this first approval for primary hypercholesterolaemia or mixed dyslipidaemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.13550015. |
An siRNA-based therapeutic is being developed by Novartis for the treatment of hypercholesterolaemia and mixed dyslipidaemia |
Received its first approval on 9 December 2020 in the EU |
Approved for use in primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia |
1 Introduction
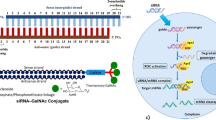
Inclisiran (Leqvio®), a first-in-class small interfering RNA (siRNA)-based therapeutic, is being developed by Novartis for the treatment of hypercholesterolaemia. Synthetic siRNAs engage the endogenous RNA interference (RNAi) pathway to prevent the expression of select genes [1]. Inclisiran is a long-acting synthetic siRNA targeting proprotein convertase subtilsin/kexin type 9 (PCSK9) mRNA and conjugated to triantennary N-acetylgalactosamine carbohydrates (GalNAc) [2]. This GalNAc platform allows for precise, targeted uptake of the drug by hepatocytes [2, 3]. PCSK9, a protein predominantly expressed in the liver, is a promising therapeutic target in the management of hypercholesterolaemia due to its role in lipid metabolism and the regulation of plasma cholesterol levels [2].
Inclisiran was approved in the EU on 9 December 2020 for use in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet [3,4,5]. It is intended for use in combination with a statin or a statin with other lipid-lowering therapies in patients unable to reach low-density lipoprotein cholesterol goals with the maximum tolerated statin dose. In patients who are statin-intolerant or for whom a statin is contraindicated, inclisiran can be used alone or in combination with other lipid-lowering therapies [3,4,5]. The EU approval was based on the results of the ORION clinical development program, in which inclisiran was demonstrated to be effective in reducing LDL-C in patients with elevated LDL-C despite receiving maximally tolerated statin therapy [5,6,7]. Inclisiran is available as a solution for subcutaneous injection in a pre-filled syringe [3]. Each syringe contains inclisiran sodium equivalent to inclisiran 284 mg in a 1.5 mL solution. The recommended dose is inclisiran 284 mg given subcutaneously as a single injection on day 1, day 90 and every 6 months thereafter [3].

Key milestones in the development of subcutaneous inclisiran for use in hypercholesterolaemia and mixed dyslipidaemia. CHMP Committee for Medicinal Products for Human Use, HoFH homozygous familial hypercholesterolaemia
A New Drug Application (NDA) for inclisiran in patients with atherosclerotic cardiovascular disease (ASCVD) and familial hypercholesterolaemia was submitted to the US FDA in December 2019 [8]. The approval process has, however, been delayed due to coronavirus disease 2019 (COVID-19)-related travel restrictions [9].
Ongoing multinational ORION clinical trials are evaluating the long-term safety and efficacy of inclisiran, as well as its use in adults with homozygous familial hypercholesterolaemia and adolescents with familial hypercholesterolaemia (heterozygous and homozygous).
1.1 Company Agreements
In February 2013, The Medicines Company and Alnylam Pharmaceuticals, Inc., entered into an exclusive global alliance to develop, manufacture and commercialise Alnylam’s ALN-PCS RNAi therapeutics targeting PCSK9 (including inclisiran) for the treatment of hypercholesterolaemia [10]. Under the terms of this license and collaboration agreement, Alnylam would complete certain preclinical and phase I clinical studies. The Medicines Company would then be responsible for leading and funding from phase II onwards, including potential commercialization. An upfront cash payment would be made from The Medicines Company to Alnylam. Alnylam would also potentially receive development and commercial milestone payments, and will be eligible for royalties on global sales of ALN-PCS products [10]. In January 2020, Novartis added inclisiran to its cardiovascular portfolio, following the acquisition of The Medicines Company [8]. As a result of the acquisition, Novartis has obtained global rights to develop, manufacture, and commercialize inclisiran under a license and collaboration agreement with Alnylam Pharmaceuticals, Inc. [8].
2 Scientific Summary
2.1 Pharmacodynamics
Inclisiran is a double-stranded, chemically synthesised siRNA directed against PCSK9 mRNA and conjugated with triantennary GalNAc on the sense strand [1, 3]. Specific binding of the GalNAc ligand to asialoglycoprotein receptors (ASGPR) enables targeted uptake of inclisiran into hepatocytes [1, 3]. Following uptake into hepatocytes, the antisense strand of inclisiran (which specifically corresponds to human PCSK9 mRNA) is integrated into the RNA-induced silencing complex, directing the catalytic breakdown of PCSK9 mRNA and thus preventing PCSK9 protein translation [3, 11]. In vitro, inclisiran delivered into HeLa and Hep3B cells inhibited PCSK9 synthesis with half-maximal inhibitory concentrations in the picomolar range [11]. Inhibited PCSK9 synthesis increases the recycling and expression of LDL-C receptors on the hepatocyte cell surface, in turn increasing LDL-C uptake and reducing circulating LDL-C levels [3].
Following a single subcutaneous dose of inclisiran (25, 100, 300, 500 or 800 mg) in healthy volunteers, least-squares mean PCSK9 reductions from baseline to day 84 of 74.5%, 69.9% and 73.1% were observed with inclisiran doses of 300 mg, 500 mg and 800 mg (all p < 0.001 vs placebo) [12]. Least-squares mean LDL-C reductions of 36.7%, 50.0%, 50.6% and 43.4% were observed with inclisiran doses of 100 mg, 300 mg, 500 mg and 800 mg (all p < 0.05 vs placebo). At inclisiran doses of 300 mg and higher, PCSK9 and LDL-C reductions were maintained over 6 months [12].
2.2 Pharmacokinetics
Subcutaneous inclisiran exhibits approximately dose-proportional pharmacokinetics over the dose range of 24–756 mg following a single administration [3, 11]. Peak plasma concentration (Cmax) was reached ≈ 4 h after administration of the recommended dose (284 mg) and concentrations were undetectable by 48 h after administration [3]. After multiple doses, no accumulation was observed. The in vitro plasma protein binding of inclisiran is 87% at clinically relevant plasma concentrations. The apparent volume of distribution was ≈ 500 L after subcutaneous administration of a single 284 mg dose to healthy adults [3]. Preclinical data suggest that inclisiran is primarily localized in the liver following subcutaneous administration [3, 11]. Inclisiran is mainly metabolized by non-specific nucleases into inactive shorter nucleotides [3, 11]. Inclisiran is not a substrate of common drug transporters and is not expected to be a cytochrome P450 substrate [3]. In addition, inclisiran is neither an inducer nor inhibitor of cytochrome P450 enzymes or common drug transporters; clinically significant interactions between inclisiran and other medicinal products are not anticipated [3]. Drug interaction assessments showed no clinically meaningful interactions with atorvastatin or rosuvastatin [11]. Inclisiran has a terminal elimination half-life of ≈ 9 h, with 16% being renally cleared [3].
In a dedicated renal impairment study, patients with mild [creatinine clearance (CLCR) 60–89 mL/min], moderate (CLCR 30–59 mL/min) and severe (CLCR 15–29 mL/min) renal impairment showed ≈ 2.3-, 2.0- and 3.3-fold increases in inclisiran Cmax and ≈ 1.6-, 1.8- and 2.3-fold increases in inclisiran area under the concentration–time curve (AUC) relative to patients with normal renal function [3]. LDL-C reductions were comparable across renal function groups. No dose adjustments are required in patients with mild, moderate or severe renal impairment or end-stage renal disease, although haemodialysis should not be performed for at least 72 h after administration [3]. In a dedicated hepatic impairment study, patients with mild (Child–Pugh class A) and moderate (Child–Pugh class B) hepatic impairment showed ≈ 1.1- and 2.1-fold increases in inclisiran Cmax and ≈ 1.3- and 2.0-fold increases in AUC relative to patients with normal hepatic function. LCL-C reductions were similar between patients with mild hepatic impairment and those with normal function, while both baseline PCSK9 levels and LDL-C reductions were lower in patients with moderate hepatic impairment. In patients with mild to moderate hepatic impairment, no dose adjustments are required [3]. The use of inclisiran in patients with severe hepatic impairment (Child–Pugh class C) has not been evaluated and should therefore be approached with caution [3].
Features and properties of inclisiran
Alternative names | Leqvio; ALN-60212; ALN-PCSsc; KJX-839; PCSK9si |
Class | Amino sugars, antihyperlipidaemics, drug conjugates, siRNA |
Mechanism of Action | RNA interference mechanism directs the breakdown of mRNA for PCSK9 |
Route of Administration | Subcutaneous |
Pharmacodynamics | siRNA conjugate; inhibits PCSK9 synthesis in hepatocytes and reduces LDL-C levels in circulation |
Pharmacokinetics | Dose-proportional pharmacokinetics; peak plasma concentration reached ≈ 4 h after administration |
Common adverse reactions | Adverse reactions at the injection site (including injection site reaction, injection site pain, injection site erythema, injection site rash) |
ATC codes | |
WHO ATC code | C10A-X16 (Inclisiran) |
EphMRA ATC code | C10A9 (All other cholesterol/triglyceride regulators) |
Chemical Name | RNA, (Am-sp-(2′-deoxy-2′-fluoro)C-sp-Am-(2′-deoxy-2′-fluoro)A-(2′-deoxy-2′-fluoro)A-(2′-deoxy-2′-fluoro)A-Gm-(2′-deoxy-2′-fluoro)C-Am-(2′-deoxy-2′-fluoro)A-Am-(2′-deoxy-2′-fluoro)A-Cm-(2′-deoxy-2′-fluoro)A-Gm-(2′-deoxy-2′-fluoro)G-Um-(2′-deoxy-2′-fluoro)C-Um-Am-Gm-sp-Am-sp-Am), complex with RNA (Cm-sp-Um-sp-Am-Gm-Am–Cm-(2′-deoxy-2′-fluoro)C-Um-(2′-deoxy-2′-fluoro)G-Um-dT-Um-Um-Gm-Cm-Um-Um–Um-Um-Gm-Um) 3′-(((2S,4R)-1-(29-((2-(acetylamino)-2-deoxy-beta-D-galactopyranosyl)oxy)-14,14-bis((3-((3-((5-((2-(acetylamino)-2-deoxy-beta-D-galactopyranosyl)oxy)-1-oxopentyl)amino)propyl)amino)-3-oxopropoxy)methyl)-1,12,19,25-tetraoxo-16-oxa-13,20,24-triazanonacos-1-yl)-4-hydroxy-2-pyrrolidinyl)methyl hydrogen phosphate) (1:1) |
2.3 Therapeutic Trials
Inclisiran effectively reduced LDL-C levels in patients with ASCVD or an ASCVD risk equivalent (type 2 diabetes, familial hypercholesterolaemia, or a ≥ 20% 10-year risk of a cardiovascular event as assessed by Framingham Risk Score for Cardiovascular Disease or equivalent) in the randomized, double-blind, placebo-controlled, multinational phase III ORION-11 trial (NCT03400800) [7]. ORION-11 was conducted in the Czech Republic, Germany, Hungary, Poland, South Africa, Ukraine and the United Kingdom. The trial enrolled patients with elevated LDL-C levels despite receiving maximally tolerated statin therapy with or without additional lipid-lowering treatment. Enrolled patients were randomized to inclisiran free acid 284 mg (corresponding to inclisiran sodium 300 mg; n = 810) or placebo (n = 807) administered as a 1.5 mL subcutaneous injection on day 1, day 90 and every 6 months thereafter over a total of 540 days. The percentage change in LDL-C level from baseline to day 510 was − 45.8% in the inclisiran group versus 4.0% in the placebo group [between-group difference (BGD) − 49.9%; 95% CI − 53.1 to − 46.6; p < 0.001], while the time-adjusted change in LDL-C level from baseline after day 90 and up to day 540 was − 45.8% in the inclisiran group versus 3.4% in the placebo group (BGD − 49.2%; 95% CI − 51.6 to − 46.8; p < 0.001) [co-primary endpoints]. With respect to key secondary endpoints, inclisiran significantly (p < 0.001 vs placebo) improved both absolute change in LDL-C level at day 510 and time-adjusted absolute change in LDL-C level from day 90 to day 540 [7]. Benefits of inclisiran over placebo for LDL-C change were observed irrespective of background lipid lowering therapy or PCSK9 levels [13]. In an prespecified exploratory analysis, cardiovascular events (cardiac death or any signs/symptoms of cardiac arrest, non-fatal myocardial infarction and/or stroke) occurred in 7.8% of inclisiran recipients versus 10.3% of placebo recipients (risk ratio 0.8; 95% CI 0.6–1.0) [14]; cardiovascular outcomes will be definitively assessed in the ORION-4 trial (NCT03705234).
Inclisiran was also effective in reducing LDL-C levels in patients with ASCVD in the randomized, double-blind, placebo-controlled, phase III ORION-10 trial (NCT03399370) conducted in the USA [7]. Patients eligible for enrolment had elevated LDL-C levels despite receiving maximally tolerated statin therapy with or without additional lipid-lowering treatment. They were randomized to inclisiran 284 mg (n = 781) or placebo (n = 780) administered as a 1.5 mL subcutaneous injection on day 1, day 90 and every 6 months thereafter over a total of 540 days. Inclisiran significantly improved percentage change in LDL-C level from baseline to day 510 relative to placebo (− 51.3% vs 1.0%; BGD − 52.3%; 95% CI − 55.7 to − 48.8; p < 0.001) and the time-adjusted change in LDL-C level from baseline after day 90 and up to day 540 relative to placebo (− 51.3% vs 2.5%; BGD − 53.8%; 95% CI − 56.2 to − 51.3; p < 0.001) [co-primary endpoints]. Absolute change in LDL-C level at day 510 and time-adjusted absolute change in LDL-C level from day 90 to day 540 were also significantly improved (p < 0.001) with inclisiran versus placebo (key secondary endpoints) [7]. Benefits of inclisiran over placebo for LDL-C lowering were observed across subgroups of patients with type 2 diabetes, metabolic syndrome and neither (p < 0.001 vs placebo in each subgroup) [15].
Inclisiran reduced LDL-C levels to a greater degree than placebo in adults with heterozygous familial hypercholesterolemia in the randomized, double-blind, placebo-controlled, multinational phase III ORION-9 trial (NCT03397121) [6]. For enrolment in ORION-9, patients had elevated LDL-C levels despite receiving the maximally tolerated dose of a statin with or without ezetimibe. They were randomized to receive inclisiran 284 mg (n = 242) or placebo (n = 240) administered as a 1.5 mL subcutaneous injection on day 1, day 90 and every 6 months thereafter over a total of 540 days. Inclisiran significantly improved percentage change in LDL-C level from baseline to day 510 (− 39.7% vs 8.2% with placebo; BGD − 47.9%; 95% CI − 53.5 to − 42.3; p < 0.001) and the time-averaged percentage change in LDL-C level between day 90 and day 540 (− 38.1% vs 6.2%; BGD − 44.3%; 95% CI − 48.5 to − 40.1; p < 0.001) [primary endpoints]. Mean absolute change from baseline in LDL-C level at day 510, time-averaged observed difference in LDL-C levels between day 90 and day 540, percentage change in PCSK9 level at day 510, mean absolute change in PCSK9 level at day 510, and time-averaged observed difference in PCSK9 levels between day 90 and day 540 were also significantly improved (p < 0.001) with inclisiran versus placebo (key secondary endpoints). Reductions in LDL-C levels with inclisiran versus placebo were observed across all familial hypercholesterolemia genotypes [6].
Inclisiran was associated with reductions in LDL-C and PCSK9 levels in adults with homozygous familial hypercholesterolaemia in the open-label, single-arm, multicenter phase II ORION-2 study (NCT02963311) [16]. This proof-of-concept pilot study enrolled patients with homozygous familial hypercholesterolaemia receiving maximally tolerated lipid-lowering therapy. Patients were administered inclisiran 284 mg (n = 4) as a 1.5 mL subcutaneous injection on day 1 and either day 90 or 104 if mean PCSK9 levels were not reduced from baseline by > 70% on day 60 or 90. Three patients achieved durable LDL-C reductions [− 11.7% to − 33.1% at day 90 (primary endpoint) and − 17.5% to − 37.0% at day 180], while the remaining patient showed no reduction. All patients showed robust, durable reductions in PCSK9 levels. These data were considered sufficient to justify a long-term phase III trial in patients with homozygous familial hypercholesterolaemia (ORION-5; NCT03851705) [16].
Inclisiran produced durable LDL-C reductions in patients with high cardiovascular risk and elevated LDL-C levels in the randomized, double-blind, placebo-controlled, multicentre phase II ORION-1 study (NCT02597127) [17]. This dose-finding study enrolled patients who had elevated LDL-C levels despite receiving the maximal possible dose of a statin with or without additional lipid-lowering therapy (n = 501 randomized). Patients received a single dose of inclisiran 200 mg, 300 mg, 500 mg or placebo, or two doses (days 1 and 90) of inclisiran 100 mg, 200 mg, 300 mg or placebo. Inclisiran was associated with dose-dependent reductions in LDL-C and PCSK9 levels. Least-squares mean reductions in LDL-C from baseline to day 180 were 28–42% after a single dose of inclisiran, while reductions of 36–53% were observed after two doses (p < 0.001 for all doses vs placebo) [primary endpoint] [17]. At days 180, 240 and 360, the greatest LDL-C reductions were observed in patients who received two doses of inclisiran 300 mg (53%, 47% and 31%, respectively) [17, 18]. In an interim analysis of data from ORION-3 (NCT03060577; n = 382), the open-label extension of ORION-1, patients treated with twice-a-year inclisiran sodium 300 mg achieved a 51% reduction in LDL-C levels through day 210 (p < 0.001; primary endpoint) and a time-averaged absolute LDL-C reduction of 59.4 mg/dL (p < 0.001); effects were independent of group assignment in ORION-1 [19].
Key clinical trials of inclisiran
Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
Inclisiran, PL | Adolescents with HeFH and elevated LDL-C | III | Not yet recruiting | Multinational | ORION-16; NCT04652726 | Novartis Pharmaceuticals |
Inclisiran, PL | Adolescents with HoFH and elevated LDL-C | III | Not yet recruiting | Multinational | ORION-13; NCT04659863 | Novartis Pharmaceuticals |
Inclisiran, PL | ASCVD or ASCVD risk equivalents and elevated LDL-C | III | Completed | Multinational | ORION-11; NCT03400800; MDCO-PCS-17-08 | The Medicines Company |
Inclisiran, PL | ASCVD and elevated LDL-C | III | Completed | USA | ORION-10; NCT03399370; MDCO-PCS-17-04 | The Medicines Company |
Inclisiran, PL | HeFH and elevated LDL-C | III | Completed | Multinational | ORION-9; NCT03397121; MDCO-PCS-17-03 | The Medicines Company |
Inclisiran | ASCVD, ASCVD risk equivalents, HeFH or HoFH and elevated LDL-C | III | Active, not recruiting | Multinational | ORION-8; NCT03814187; MDCO-PCS-17-05 | Novartis Pharmaceuticals |
Inclisiran, PL | HoFH and elevated LDL-C | III | Active, not recruiting | Multinational | ORION-5; NCT03851705; MDCO-PCS-17-02 | Novartis Pharmaceuticals |
Inclisiran, PL | ASCVD | III | Recruiting | United Kingdom | ORION-4; NCT03705234 | University of Oxford |
Inclisiran, PL | High cardiovascular risk and elevated LDL-C | II | Not yet recruiting | Japan | NCT04666298 | Novartis Pharmaceuticals |
Inclisiran, evolocumab | ASCVD or ASCVD risk equivalents and elevated LDL-C | II | Active, not recruiting | Multinational | ORION-3; NCT03060577; MDCO-PCS-16-01 | Novartis Pharmaceuticals |
Inclisiran, SOC | HoFH and elevated LDL-C | II | Completed | Multinational | ORION-2; NCT02963311; MDCO-PCS-16-02 | The Medicines Company |
Inclisiran, placebo | ASCVD or ASCVD risk equivalents and elevated LDL-C | II | Completed | Multinational | ORION-1; NCT02597127; MDCO-PCS-15-01 | The Medicines Company |
2.4 Adverse Events
Inclisiran administered as a subcutaneous injection was generally safe and well tolerated as a lipid lowering therapy in clinical trials, showing a safety profile comparable to that of placebo [6, 7, 12, 16, 17, 20, 21]. In the pivotal phase III clinical trials (ORION-9, ORION-10 and ORION-11), adverse reactions at the injection site were the only adverse reactions associated with inclisiran (occurring in 8.2% of inclisiran recipients versus 1.8% of placebo recipients) [3]. In inclisiran recipients, the most frequently reported adverse reactions at the injection site were injection site reaction (3.1% of patients), injection site pain (2.2%), injection site erythema (1.6%) and injection site rash (0.7%). Adverse reactions at the injection site led to treatment discontinuation in 0.2% of inclisiran recipients (vs 0.0% of placebo recipients). All adverse reactions at the injection site were of mild or moderate severity and transient, resolving without sequelae [3].
Aside from injection site adverse events, the adverse event profile of inclisiran was well documented [22]. Serious adverse events occurred in 20.4% of inclisiran recipients compared with 23.0% placebo recipients in phase III trials, and there was no evidence of kidney, liver, muscle or platelet toxicity. Treatment-emergent adverse events led to treatment discontinuation in 2.5% of inclisiran recipients compared with 1.9% of placebo recipients [22]. There were no overall differences in safety between younger patients and older patients ≥ 65 or ≥ 75 years of age [3]. The safety profile of inclisiran in patients with impaired renal function was similar to that in patients with normal renal function in an analysis of data from ORION-1 and the phase I ORION-7 renal study (NCT03159416) [23].
During the pivotal clinical trials, 1.8% of patients tested positive for anti-inclisiran antibodies prior to dosing and 4.9% of patients tested positive over 18 months of inclisiran treatment; neither safety nor efficacy differed in any clinically significant manner based on the presence of anti-inclisiran antibodies [3].
2.5 Ongoing Clinical Trials
Ongoing phase III clinical trials evaluating inclisiran include the ORION-4 cardiovascular outcomes trial (NCT03705234; currently recruiting) in adults with ASCVD and the ORION-5 trial (NCT03851705) in adults with homozygous familial hypercholesterolaemia. ORION-8 (NCT03814187), an ongoing open-label extension study of the phase III lipid-lowering trials (ORION-5, ORION-9, ORION-10 and ORION-11), is currently assessing the long-term use of inclisiran in adults with high cardiovascular risk and elevated LDL-C. ORION-3 (NCT03060577), the open-label, active-comparator extension study of the phase II ORION-1 trial, is similarly assessing the long-term use of inclisiran in this patient population.
The placebo-controlled, multinational, phase III ORION-13 (NCT04659863) and ORION-16 (NCT04652726) trials, which are not yet recruiting, will evaluate inclisiran in adolescents aged 12 to 17 years with homozygous familial hypercholesterolaemia and heterozygous familial hypercholesterolaemia, respectively, and elevated LDL-C on stable, standard of care background lipid-lowering therapy. Also yet to begin recruiting is a phase II trial (NCT04666298) that will compare inclisiran and placebo in Japanese patients with high cardiovascular risk and elevated LDL-C and a phase III trial that will evaluate the efficacy and safety of inclisiran in Asian patients with ASCVD or ASCVD high risk and elevated LDL-C (as an adjunct to diet and maximally tolerated statins with or without additional lipid-lowering therapy).
3 Current Status
Inclisiran received its first approval on 9 December 2020 for the treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia in the EU.
Change history
13 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40265-021-01529-7
References
Nair JK, Willoughby JLS, Chan A, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61.
German CA, Shapiro MD. Small interfering RNA therapeutic inclisiran: a new approach to targeting PCSK9. BioDrugs. 2020;34(1):1–9.
European Medicines Agency. Leqvio 284 mg solution for injection in pre-filled syringe: summary of product characteristics. 2021. http://www.ema.europa.eu/. Accessed 25 Jan 2021.
European Medicines Agency. Leqvio (inclisiran): an overview of Leqvio and why it is authorised in the EU. 2021. http://www.ema.europa.eu/. Accessed 25 Jan 2021.
Novartis. Novartis receives EU approval for Leqvio® (inclisiran), a first-in-class siRNA to lower cholesterol with two doses a year [media release]. 11 Dec 2020. http://www.novartis.com/.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Novartis. Novartis successfully completes acquisition of The Medicines Company, adding a potentially first-in-class, investigational cholesterol-lowering therapy inclisiran [media release]. 7 Jan 2020. http://www.novartis.com.
Pagliarulo N. FDA delays decision on Novartis cholesterol therapy [media release]. 21 Dec 2020. http://www.biopharmadive.com/.
The Medicines Company, Alnylam Pharmaceuticals. The Medicines Company and Alnylam form strategic alliance to develop and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia [media release]. 4 Feb 2013. http://www.themedicinescompany.com.
European Medicines Agency. Leqvio (inclisiran): CHMP public assessment report. 2021. http://www.ema.europa.eu/. Accessed 25 Jan 2021.
Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51.
Ray KK, Kallend D, Leiter L, et al. Effect of inclisiran on LDL-C reduction across different background lipid lowering treatments: analyses from ORION-11. J Am Coll Cardiol. 2020;75(11):2078.
Ray KK, Wright S, Kallend D, et al. Inclisiran and cardiovascular outcomes: analyses from ORION-11 [abstract no. 1423-191]. J Am Coll Cardiol. 2020;75(11):238.
Wright RS, Kallend D, Ray KK, et al. Evaluation of LDL-C reductions by siRNA treatment with inclisiran in patients with diabetes mellitus, metabolic syndrome or neither [abstract no. 1360-102]. J Am Coll Cardiol. 2020;75 (11 Suppl 1):2005.
Hovingh GK, Lepor NE, Kallend D, et al. Inclisiran durably lowers low-density lipoprotein cholesterol and proprotein convertase subtilisin/kexin type 9 expression in homozygous familial hypercholesterolemia: the ORION-2 pilot study. Circulation. 2020;141(22):1829–31.
Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40.
Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 2019;4(11):1067–75.
The Medicines Company. New long-term data show that twice-a-year dosing with inclisiran results in persistent lowering of LDL cholesterol with no material safety observations out to three years [media release]. 20 May 2019. http://www.themedicinescompany.com.
Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020. https://doi.org/10.1016/j.amjcard.2020.08.018.
Wang Y, Wang J, Wang S. Comparative effectiveness of inclisiran 100, 300, and 500 mg in a population with hyperlipidemia: a network meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. 2018;18(4):271–82.
Wright RS, Kallend D, Ray K, et al. ORION: a pooled analysis of phase III studies of inclisiran [conference presentation]. In: ACC (American College of Cardiology) Conference. 2020.
Wright RS, Collins MG, Stoekenbroek RM, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin Proc. 2020;95(1):77–89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lamb, Y.N. Inclisiran: First Approval. Drugs 81, 389–395 (2021). https://doi.org/10.1007/s40265-021-01473-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01473-6