Abstract
Background
Inclisiran is a novel, cholesterol-lowering therapy, with a long duration of effect, administered every 6 months (subcutaneously by a healthcare professional). In the ORION-10 trial in US patients with atherosclerotic cardiovascular disease (ASCVD) in addition to maximum tolerated statins, with or without ezetimibe, inclisiran demonstrated statistically significant reductions in low-density lipoprotein cholesterol (LDL-C) of up to 51%. This is the first peer-reviewed publication to investigate the price at which inclisiran is cost effective in the US.
Objective
The aim of this study was to determine the maximum price at which inclisiran is cost effective in addition to standard of care, in US patients with ASCVD, versus standard of care alone, at different willingness-to-pay thresholds.
Design, Setting and Participants
A lifetime Markov model from the US health system perspective, including 15 health states, was used to evaluate the cost effectiveness of inclisiran. The following states were separated by time from a previous cardiovascular event (0–1 years, 1–2 years, 2+ years [‘stable’]): initial, unstable angina, myocardial infarction, and stroke. Additional states included revascularization and death (cardiovascular or non-cardiovascular causes). Baseline risk of cardivoascular events were from US database sources or published literature. Reductions in LDL-C from inclisiran were from the ORION-10 trial. LDL-C reduction was used to adjust baseline risk of cardiovascular events, based on established relationships between 1 mmol/L reduction in LDL-C and decreases in cardiovascular events, from the Cholesterol Treatment Trialists studies. The population included adults with a history of ASCVD, and LDL-C ≥ 70 mg/dL, despite maximum tolerated doses of statin therapy.
Interventions
Inclisiran as an adjunct to standard of care, compared with standard of care alone.
Main Outcomes and Measures
The threshold price of inclisiran.
Results
Inclisiran as an adjunct to standard of care resulted in threshold annual inclisiran prices of $6383, $9973, and $13,563 at willingness-to-pay thresholds of $50,000, $100,000, and $150,000 per quality-adjusted life-year, respectively. Probabilistic sensitivity analysis showed that at a threshold of $100,000 per QALY, inclisiran had a 100% probability of being cost effective, with an annual price below $9000. At the publicly available price of $3250 per dose, inclisiran was found to have an incremental cost-effectiveness ratio just above the $50,000 per QALY threshold, of $51,686.
Conclusions and Relevance
This study identified the price at which inclisiran is cost effective for the US health system, at generally accepted willingness-to-pay thresholds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The price at which inclisiran is cost effective adjunctive to standard of care (SoC) versus SoC alone in US adults with atherosclerotic cardiovascular disease and elevated low-density lipoprotein cholesterol ≥70 mg/dL, despite maximum tolerated doses of statins (with or without ezetimibe), was investigated at willingness-to-pay thresholds of $50,000, $100,000 and $150,000 per quality-adjusted life-year (QALY). |
Inclisiran was found to be cost effective at the evaluated thresholds, at a price ranging from $6383 to $13,563 per year ($3191 to $6782 per dose). |
At the publicly available price of $3250 per dose, inclisiran was found to have an incremental cost-effectiveness ratio just above the $50,000 per QALY threshold, of $51,686. |
1 Introduction
Cardiovascular disease (CVD) is the leading cause of death in most racial and ethnic groups within the US, predominantly due to ischemic heart disease and stroke [1]. Atherosclerotic CVD (ASCVD) is the most common subtype of CVD [2], accounting for over 600,000 hospitalizations due to acute myocardial infarction (MI) and 24,000 due to unstable angina (UA) in 2014 [3].
Low-density lipoprotein cholesterol (LDL-C) is both a biomarker of increased risk and a causal factor in the pathophysiology of ASCVD [4]. People with raised cholesterol (predominantly high LDL-C) are at elevated risk of cardiovascular (CV) events leading to hospitalization or death [5]. Trials of statin therapy and other LDL-C-lowering interventions, have consistently shown a 22% relative risk reduction in the risk of CV events per 1 mmol/L (38.67 mg/dL) reduction in LDL-C [4], although the magnitude of effect can be influenced by duration of therapy, with at least 1–2 years of treatment needed to achieve these LDL-C reductions [6]. Thus, lowering LDL-C represents a considerable opportunity to improve overall morbidity and mortality.
Statins represent first-line treatment for ASCVD. Poor adherence and lack of public trust in their safety continue to limit their prolonged and reliable use [7]. Observational data suggest as many as half of those who begin statin therapy discontinue within 1 year [8, 9]. Even when adherent, patients may not achieve recommended LDL-C thresholds [10]. Ezetimibe is recommended by the American College of Cardiology for the treatment of ASCVD in adults when statin therapy is contraindicated, patients are intolerant to statins, or LDL-C is not appropriately controlled with statin therapy [11]. Uptake of ezetimibe is poor; real-world data from the 2009–2016 National Health and Nutrition Examination Survey (NHANES) showed that only 4.2% of US adults aged ≥ 35 years with prior ASCVD and LDL-C ≥ 70 mg/dL receiving statin therapy were receiving ezetimibe [12]. Recently, monoclonal antibodies against proprotein convertase subtilisin-kexin type 9 (PCSK9) have been approved for use in the US in a secondary prevention setting for patients with familial hypercholesterolemia or ASCVD [13]. They are effective and generally well tolerated, but require highly specialized handling, frequent injections, and uncertainty remains regarding their cost effectiveness [14]. PCSK9 inhibitor use remains low [15], and only approximately 60% of patients remain on PCSK9 inhibitors 1 year after initiation [16].
Inclisiran is a novel, long-acting, subcutaneously delivered, synthetic short-interfering ribonucleic acid (siRNA) selectively targeting PCSK9 synthesis within the liver, thereby harnessing the natural mechanism of the RNA-induced silencing complex. The ORION clinical trial program included, amongst others, three phase III, randomized, multicenter, double-blind trials (ORION-9 [17], ORION-10, and ORION-11 [18]) and included 3660 mostly statin-treated patients with established CVD (ORION-10), were ASCVD risk-equivalent (ORION-11), or who had heterozygous familial hypercholesterolemia (HeFH; ORION-9). Participants received either 300 mg inclisiran sodium or placebo, subcutaneously on days 1, 90, 270 and 450, in addition to baseline statin therapy. At day 510 in ORION-10, inclisiran reduced LDL-C by 51.3% relative to baseline LDL-C, whereas placebo increased LDL-C by 1.0%, leading to a between-group difference of 52.3% (95% confidence interval [CI] − 55.7 to − 48.8; p < 0.001) [18]. In ORION-11, inclisiran reduced LDL-C at day 510 by 45.8% and placebo increased LDL-C by 4.0%, resulting in a between-group difference of 49.9% (95% CI − 53.1 to − 46.6; p < 0.001).
The dosing schedule of 6-monthly injection (with an additional dose at 3 months in year 1) provides an opportunity to achieve improved adherence compared with existing LDL-C-lowering therapies.
The objective of this analysis is to determine the maximum price at which inclisiran is cost effective in addition to standard of care (SoC) in a US ASCVD population, versus SoC alone, at different willingness-to-pay (WTP) thresholds.
2 Methods
The Institutional Review Boards of the University of California, San Francisco, and Columbia University approved the analyses of Optum Clinformatics® Data Mart data. All other data were sourced from publicly available data.
2.1 Population
The base-case modeled the cost effectiveness of inclisiran in US patients with ASCVD and LDL-C ≥ 70 mg/dL, despite maximum tolerated doses of statins (with or without ezetimibe) (ORION-10 population) [18]. Patient characteristics were based on ORION-10 (Table 1).
Model results are based on a weighted average population, meaning the ASCVD population was stratified into subpopulations representing events that constitute ASCVD, and further stratified by time from previous event. Each subpopulation passed through the model separately, with results weighted by the real-world distribution of these subpopulations to derive population estimates of cost effectiveness.
2.2 Model Structure
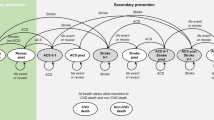
A lifetime Markov model was developed, including 15 mutually exclusive states (Fig. 1). Patients entered the model in any of three initial states, based on time from previous ASCVD event (0–1; 1–2; 2+ years) [19]. Three non-fatal CV (NF-CV) event states were included, i.e. UA, MI, and stroke, stratified based on time post-event (0–1; 1–2; 2+ years). Other states included revascularization and death (CV- or non-CV-related). Cycle length was 1 year, with half-cycle correction implemented based on the life-table method [20].
The model allowed annual transitions based on risks of CV events (fatal and NF) and non-CV death. Following movement to a post NF-CV event state, patients remained at risk of subsequent events (fatal or NF). Patients only moved health states when a ‘worse’ event occurred, to avoid illogical outcomes leading to quality-of-life improvement (events in order of severity are stroke, MI, UA, revascularization). Less severe events were captured as acute events, with one-off quality-adjusted life-year (QALY) loss and costs applied.
Costs and QALYs were discounted at 3% annually [21]. The model assumed the payer perspective, considering all direct health care costs, and the cost of inclisiran without any potential rebates/discounts (see electronic supplementary material [ESM] e1 for more model structure details).
2.3 Baseline Risk of Cardiovascular Events
US real-world data from Optum Clinformatics® Data Mart (a retrospective cohort study of patients with ASCVD from 2015 to 2020) informed baseline NF-CV event probabilities (see ESM e2a) [22]. The distribution across the various subpopulations from the Optum data (Table 1) was used to calculate weighted average model results. Each subpopulation entered the model in the relevant health state, e.g. 100% of the stroke 0–1 population would start in the initial state (year 0–1).
Fatal event rates were taken from published literature, as mortality was not recorded in the Optum database (Table 1 and ESM e2b).
Baseline risk of cardiovascular events required adjustment because they represented a population whose age and LDL-C level (eTable 1 in the ESM) did not match the modeled population (see ESM e2c/e2d).
Non-CV mortality was estimated from the Centers for Disease Control and Prevention [23]. CV mortality was identified using the CVD International Classification for Diseases, Tenth Revision (ICD-10) code as per the American Heart Association definition [24]. The proportion of non-CV-related deaths was applied to the latest US life-table data (2017). This was calculated separately for men and women, and weighted by the modeled gender split.
2.4 Treatment Strategies
The comparator was SoC, represented by the ORION-10 placebo arm. The distribution of SoC treatments were from ORION-10, split into eight mutually exclusive categories; those taking or not taking ezetimibe were split into those not taking statins or those taking low/moderate/high-intensity statins. Baseline LDL-C associated with each stratification was from ORION-10, allowing calculation of an overall SoC weighted average LDL-C, to enable LDL-C to be recalculated each cycle as people discontinue treatments.
Inclisiran was modeled adjunctive to SoC. LDL-C reduction with inclisiran was from ORION-10, based on the mean difference in LDL-C reduction between arms at day 510 (52.3%) [18]. This LDL-C reduction was applied to the baseline (SoC) LDL-C to determine the LDL-C level on inclisiran.
A link has been demonstrated between exposure time and treatment effect, with observed rate ratios per mmol/L reduction in LDL-C being smaller in the first year of treatment [25]. An adjustment was applied to reflect this (see ESM e3).
Patients who discontinued statins reverted to the LDL-C of patients receiving no statins at baseline in ORION-10, likewise for ezetimibe. Statin and ezetimibe discontinuation rates were assumed to be 23% per year [26]. A rate ratio of 0.5 versus statin discontinuation was applied to determine inclisiran discontinuation (see ESM e4) [27]. A discontinuation cap was included to prevent patients remaining on treatment falling indefinitely to zero; this was 20% for statins and inclisiran and 5% for ezetimibe (Campbell C, unpublished data, January 2022).
Adverse events were excluded, as the ORION program showed inclisiran had a similar adverse event profile to placebo [18]. The exception was injection-site reactions, which clinical experts suggested would not be associated with significant cost or health-related quality of life (HRQoL) impact, as these were found to be mild and did not persist in the ORION trials [18]. This is consistent with other CV risk reduction models [19]. However, a disutility associated with having injections themselves, has been included, as literature showed that treatment modality can have an effect on health state utility [28].
2.5 Costs and Utilities
For SoC, the average cost of 10 mg and 20 mg simvastatin represented both low- and medium-intensity statins, while 40 mg atorvastatin represented high-intensity statins. A weighted average cost of SoC per year was calculated, given the distribution of ORION-10 patients using each SoC component (Table 1).
All patients with ASCVD, regardless of therapy type, should receive, at minimum, one outpatient appointment per year (not included in the analysis [11]). Therefore, with two doses per year, one outpatient appointment and two injection procedures were costed annually. An additional appointment and injection procedure cost were included in year 1 to account for the additional dose in the first year. Zero administration costs for SoC were assumed.
The costs for UA, MI, stroke, revascularization events [29], and CV death [30] were from published literature and are inflated to 2021 US dollars using the medical consumer price index (Table 1) [31]. Acute event costs were applied as one-off costs to acute events less severe than the patient’s current health state.
Population age-adjusted utilities for patients with no history of CVD were based on the regression from Bhatt et al., [30] which analyzed patient-level data from the alirocumab ODYSSEY study and identified baseline age-adjusted utilities (controlling for age, sex, baseline EQ-5D-3L utility, CV history, and diabetes at baseline). Utility multipliers to represent baseline subpopulations were obtained from previous cost-effectiveness models in this disease area [32,33,34], and applied to population age-adjusted utilities.
Disutilities from CV events (Table 1) were applied for all health states in all years, except for revascularization, where disutility was assumed non-permanent and only applied to the cycle where the event occurred [30]. For subsequent CV events, for the same or worse event, the patient transitioned to the more severe state and the disutility from the second event was applied as the patient proceeded through the model; for a less severe event, a one-off short-term disutility was applied [30].
An annual disutility associated with having an injection has been included, i.e. − 0.00150 [35], based on a study investigating utilities associated with attributes of migraine prevention treatments, including route of administration (see ESM e5).
2.6 Outcomes
The primary outcome was the price at which inclisiran is cost effective over the lifetime analytic horizon, at WTP thresholds of $50,000, $100,000, and $150,000 per QALY [36].
An incremental cost-effectiveness ratio (ICER) is based on the difference in costs between treatments divided by the difference in QALYs (to give the cost per additional QALY) [Eq. 1].
A threshold analysis rearranges this equation and finds the total incremental cost (by varying the cost of inclisiran) that would lead to an ICER at a particular threshold (Eq. 2).
2.7 Sensitivity Analyses
One-way sensitivity analysis was undertaken, varying parameters within their 95% CI, or ± 15%, where this was not available.
Several prespecified scenario analyses were also performed (Table 2), including a scenario that uses the public list price of inclisiran [37].
Joint parameter uncertainty was explored through probabilistic sensitivity analysis (PSA), where all parameters were assigned distributions and varied jointly.
Table 1 presents the range and type of distribution utilized for each input parameter. A total of 1000 Monte Carlo simulations were recorded as results had stabilized by this point, with variation of < 0.01% between simulations. PSA was undertaken at various annual prices of inclisiran. Cost-effectiveness acceptability curves (CEAC) were generated, with the proportions of simulations where inclisiran is optimal (at each WTP threshold) plotted as a function of the price of inclisiran.
The model was constructed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). Statistical analyses of event rate data were undertaken using SAS (see the ESM for model validation details [Sect. e6]).
3 Results
3.1 Base-Case Analysis
Of the ASCVD patients analyzed, around 90% were taking statins and 10% were taking ezetimibe. Average LDL-C was 104.97 mg/dL and average age was 66 years, with 45% having diabetes, and 31% were female. Independently of the inclisiran price modeled, inclisiran as an adjunct to SoC produces an additional 0.43 QALYs and incremental costs (excluding the cost of inclisiran) of $104,471. The annual cost of inclisiran at each WTP threshold of $50,000, $100,000, and $150,000 per QALY was found to be $6383, $9973, and $13,563, respectively (price per dose of $3191, $4987, and $6782, respectively), leading to incremental costs of $21,498, $42,990, and $64,482, respectively. Table 3 presents the base-case analysis results in the weighted average ASCVD population.
3.2 Sensitivity Analyses
The parameters with the most impact on the ICER at each threshold price were the rate ratios used for the relationship between LDL-C reduction and reduction in CV events, and the efficacy of inclisiran. The tornado diagrams at each threshold are shown in eFig. 4, eFig. 5, and eFig. 6 in the ESM.
PSA results as a function of the price of inclisiran are presented in Fig. 2. At each WTP threshold, inclisiran had 100% probability of being cost effective if the annual price is below $6000, $9000, or $12,000, respectively. Cost-effectiveness planes at each threshold are available in the ESM (e7b).
The scenarios (see eTable 4 in the ESM) that resulted in more favorable threshold prices for inclisiran than the base-case resulted when inclisiran efficacy was increased. The scenarios that resulted in less favorable threshold prices for inclisiran than the base-case were assuming the minimum proportion remaining on any of the treatments was 40%, having no discontinuation, and having alternative health state costs.
The scenario where the publicly available price of inclisiran was used led to an ICER just above the $50,000 per QALY threshold, of $51,686.
4 Discussion
This analysis assessed the threshold price at which inclisiran is cost effective in adults with a history of ASCVD and LDL-C ≥ 70 mg/dL, despite receiving maximum tolerated doses of statin therapy, with or without ezetimibe, at WTP thresholds of $50,000, $100,000, and $150,000 per QALY.
In the base-case, inclisiran was cost effective at annual prices of $6383, $9973, and $13,563 at WTP thresholds of $50,000, $100,000, and $150,000 per QALY, respectively, and was found to be just over the $50,000 threshold at the publicly available price of inclisiran. Cost effectiveness was driven by the relationship between LDL-C and CV events and the effectiveness of inclisiran.
Although the threshold prices have been identified in this analysis, in reality, payers will pay less than the list price, as the patients will have an estimated 20% average co-payment, meaning the required threshold prices to make inclisiran cost effective at the three WTP thresholds will be 20% higher than those identified here. Results are also expected to be more cost effective in higher risk groups, such as those with familial hypercholesterolemia.
4.1 Strengths and Limitations
The strengths of this analysis include the use of real-world estimates of baseline event rates, which greatly improved generalizability to a US population. Although the analysis was considered representative of NF event probabilities in a US ASCVD population, it did not inform the probability of CV death, where additional literature was sourced.
The principal limitation of this analysis was the reliance on predicted event risk reduction based on observed reductions in LDL-C. The total number of CV events observed in the ORION program were underpowered to draw meaningful conclusions regarding the benefits of inclisiran on CV outcomes [38]. Although a recognized limitation, the relationship applied from the 2019 Cholesterol Treatment Trialists (CTT) analysis represents the largest study to investigate the relationship between LDL-C reduction and event risk reduction [39]. Previous CTT versions have been used in the economic evaluation of inclisiran in other countries [38] and other therapies in this disease area [19]. This uncertainty will ultimately be addressed with the publication of the results of ORION-4, inclisiran’s CV outcomes trial.
The model utilized the results of a meta-analysis for the percentage reduction in LDL-C from inclisiran versus placebo. Treatment effects from ORION-10 were based on intention-to-treat outcomes and therefore implicitly contain the effects of discontinuation observed in the individual clinical trials. As the model also includes discontinuation as an input, then the treatment effect for inclisiran can be considered conservative.
4.2 Comparison with Published Data
A cost-effectiveness analysis of inclisiran plus statins versus statins alone from an Australian healthcare perspective (Markov model, based on ORION-10) reported an ICER of AU$125,732 ($87,325.35) [40] per QALY, using an inclisiran price of AU$6334 ($4399). Differences in results may be explained by lower baseline event rates from the Australian analysis (based on the 2010 CTT publication [41], in which only 41% of patients had ASCVD) and a slightly different model structure (e.g. the exclusion of stroke).
The US Institute for Clinical and Economic Review (US-ICER) also assessed the comparative clinical effectiveness and value of inclisiran and found a cost per QALY of $157,000 [42]. Key differences in the US-ICER’s methodology include slightly different health states, including a composite acute coronary syndrome and stroke health state; different population and baseline LDL-C; no discontinuation was included; and different sources for the relationships between 1 mmol/L reduction in LDL-C and reduction in CV events (using the 2010 CTT analysis [41], although an updated 2019 version was available [39]). Specifically, the US-ICER derived baseline LDL-C by identifying population characteristics from NHANES for patients with LDL-C ≥ 70 mg/dL and taking statins. For those not taking ezetimibe (95.8%), baseline LDL-C was downwardly adjusted to reflect hypothetical ezetimibe use, and those with an LDL-C still ≥ 70 mg/dL were used to determine the average baseline LDL-C in the model (calculated as 88.8 mg/dL; by contrast, ORION-10 found a baseline LDL-C of 104.97 mg/dL). This methodology assumed inclisiran will be used by a population whose LDL-C remains ≥ 70 mg/dL after trying both statins and ezetimibe. This represents only a subpopulation of patients for whom inclisiran has been studied and does not reflect the current treatment pathway (as shown by the US-ICER NHANES analysis). It also assumes patients are fully compliant with ezetimibe. As a consequence of lower baseline LDL-C, and therefore lower baseline risks, the estimates produced by ICER are likely to be a more conservative estimate of the cost effectiveness of inclisiran (see ESM e8) and less likely to represent the cost-effective profile of inclisiran in clinical practice.
Cost-effectiveness analyses of PCSK9 inhibitors have demonstrated a variety of results, with ICERs ranging from $56,655 to $503,000 [30, 33, 34, 43, 44]. This may be attributed to factors such as a change in price of the interventions over time, different model structures, different approaches to outcomes (CV endpoints from trials or surrogate endpoints of LDL-C reduction being linked to CV outcomes), and different compositions of SoC (including inclusion or exclusion of ezetimibe).
5 Conclusions
Inclisiran as an adjunct to SoC provides a cost-effective use of US resources in US patients with ASCVD and elevated LDL-C, despite receiving maximum tolerated doses of statin therapy, when priced below $6383, $9973, and $13,563 per year at WTP thresholds of $50,000, $100,000, and $150,000 per QALY, respectively. A scenario with inclisiran at the publicly available price led to an ICER just above the $50,000 per QALY threshold, of $51,686.
References
Heron MP. Deaths: leading causes for 2013. Natl Vital Stat Rep. 2016;65(2):1–95.
Kaiser Permanente. Atherosclerotic Cardiovascular Disease (ASCVD) Primary Prevention Guideline. October 2020. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/ascvd-primary.pdf. Accessed 4 Feb 2021.
Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke Statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472.
Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the Cooper Center Longitudinal Study. Circulation. 2018;138(21):2315–25.
Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J. 2018;39(27):2540–5.
Fung V, Graetz I, Reed M, Jaffe MG. Patient-reported adherence to statin therapy, barriers to adherence, and perceptions of cardiovascular risk. PloS One. 2018;13(2):e0191817.
Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34.
Lahoz RS, D. Achouba, A. Electriwala, B. Ding, Y. Haeng Heo, J. Studer, R. Treatment patterns in atherosclerotic cardiovascular disease patients with hypercholesterolemia (ASCVD-H) and patients with familial hypercholesterolemia (FH), a retrospective database cohort study in the US. J Am Coll Cardiol. 2021;77(18 Suppl 1):1582.
Karalis DG, Victor B, Ahedor L, Liu L. Use of lipid-lowering medications and the likelihood of achieving optimal LDL-cholesterol goals in coronary artery disease patients. Cholesterol. 2012;2012:861924.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–350.
Institute for Clinical and Economic Review. Bempedoic acid and inclisiran for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value. Final Report. 2 March 2021. Institute for Clinical and Economic Review; 2021
US FDA. Repatha (evolocumab) injection. US FDA; 2015.
National Institute for Health and Care Excellence. NICE Clinical Guideline (CG181): Cardiovascular disease: risk assessment and reduction, including lipid modification. National Institute for Health and Care Excellence; 2016.
Chamberlain AM, Gong Y, Shaw KM, et al. PCSK9 inhibitor use in the real world: data from the national patient centered research network. J Am Heart Assoc. 2019;8(9):e011246.
Rymer JA, Mues KE, Monda KL, et al. Use of low density lipoprotein lowering therapies before and after PCSK9 inhibitor initiation. J Am Heart Assoc. 2020;9(9):e014347.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
National Institute for Health and Care Excellence. Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia (TA393). National Institute for Health and Care Excellence; 2016.
Barendregt JJ. The half-cycle correction: banish rather than explain it. Med Decis Making. 2009;29(4):500–2.
Institute for Clinical and Economic Review. 2020–2023 value assessment framework. January 31, 2020. Updated February 3, 2022. https://icer.org/wp-content/uploads/2020/11/ICER_2020_2023_VAF_02032022.pdf. Accessed 15 Mar 2021.
Clinformatics® Data Mart. Eden Prairie, MN: Optum; updated 2020.
Centers for Disease Control and Prevention. Underlying Cause of Death, 1999–2020. 2021. https://wonder.cdc.gov/ucd-icd10.html. Accessed 13 Feb 2021.
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61.
Burke JP, Simpson RJ Jr, Paoli CJ, McPheeters JT, Gandra SR. Longitudinal treatment patterns among US patients with atherosclerotic cardiovascular disease or familial hypercholesterolemia initiating lipid-lowering pharmacotherapy. J Clin Lipidol. 2016;10(6):1470-1480.e1473.
Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–26.
Matza LS, Cong Z, Chung K, Stopeck A, Tonkin K, Brown J, Braun A, Van Brunt K, McDaniel K. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence. 2013;7:855–65. https://doi.org/10.2147/PPA.S44947
Fox KM, Wang L, Gandra SR, Quek RGW, Li L, Baser O. Clinical and economic burden associated with cardiovascular events among patients with hyperlipidemia: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16(1):13.
Bhatt DL, Briggs AH, Reed SD, et al. Cost-Effectiveness of alirocumab in patients with acute coronary syndromes: the odyssey outcomes trial. J Am Coll Cardiol. 2020;75(18):2297–308.
US Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): U.S. city average, by expenditure category. Updated February 10, 2022. https://www.bls.gov/news.release/cpi.t01.htm. Accessed 14 Mar 2021.
Kazi DS, Garber AM, Shah RU, et al. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann Intern Med. 2014;160(4):221–32.
Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743–53.
Kazi DS, Penko J, Coxson PG, Guzman D, Wei PC, Bibbins-Domingo K. Cost-effectiveness of alirocumab: a just-in-time analysis based on the ODYSSEY outcomes trial. Ann Intern Med. 2019;170(4):221–9.
Matza LS, Deger KA, Vo P, Maniyar F, Goadsby PJ. Health state utilities associated with attributes of migraine preventive treatments based on patient and general population preferences. Qual Life Res. 2019;28(9):2359–72.
Dubois RW. Cost–effectiveness thresholds in the USA: are they coming? Are they already here? J Compar Effect Res. 2016;5(1):9–12.
IBM. Micromedex Red Book. Armonk: IBM Corporation
Kam N, Perera K, Zomer E, Liew D, Ademi Z. Inclisiran as adjunct lipid-lowering therapy for patients with cardiovascular disease: a cost-effectiveness analysis. Pharmacoeconomics. 2020;38(9):1007–20.
Armitage J, Baigent C, Barnes E, et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–15.
Organisation for Economic Co-operation and Development. Purchasing power parities (PPP) [indicator] 2020. https://doi.org/10.1787/067eb6ec-en. https://www.oecd-ilibrary.org/finance-and-investment/purchasing-power-parities-ppp/indicator/english_1290ee5a-en. Accessed 4 Feb 2021.
Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681.
Institute for Clinical and Economic Review. Bempedoic acid and inclisiran for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value. evidence report. January 22, 2021. https://icer.org/wp-content/uploads/2021/02/ICER_High-Cholesterol_Evidence-Report_012221.pdf. Accessed 16 Feb 2021.
Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(10):1069–78.
Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(7):691–5.
Cholesterol Treatment Trialists Collaborators, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590.
Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130(12):966–75.
Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291(2):195–201.
Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med. 2002;162(21):2405–10.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532-540.
Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24(6):796–800.
Centers for Medicare & Medicaid Services. Physician Fee Schedule Look-Up Tool. Updated January 1, 2022. https://www.cms.gov/medicare/physician-fee-schedule/search/overview. Accessed 19 Mar 2021.
Campbell C, Desai NR, Electricwala B, et al. The comparative efficacy of inclisiran, PCSK9 inhibiting monoclonal antibodies, and ezetimibe for the treatment of high cholesterol in adults with or at risk of ASCVD: a systematic literature review and network meta-analysis [poster 1552-060/60]. Presented at the Annual Scientific Sessions of the American College of Cardiology: 2–4 April 2022. Washington, DC
Acknowledgements
The authors would like to acknowledge the contributions made by Steve Kay, who contributed to the work reported in this manuscript but does not fulfil the authorship criteria. Steve Kay is an employee of Model Outcomes and contributed to statistical analyses.
Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Contributions
This manuscript has been developed and approved for submission by all authors. All authors have participated in the preparation of this manuscript to sufficiently meet all authorship criteria, and no person/s other than those listed meet the authorship criteria.
Corresponding author
Ethics declarations
Funding
This study was funded by Novartis Pharmaceuticals Corporation, USA
Conflicts of interest
Caresse Campbell, Batul Electricwala, and Joaquim Cristino are employees of Novartis Pharmaceuticals Corporation, USA, and shareholders of Novartis Pharmaceuticals Corporation stock. Nihar R. Desai is an employee of Yale University School of Medicine and works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay-for-performance programs. Nihar R. Desai also reports research grants and consulting for Amgen, Astra Zeneca, Boehringer Ingelheim, Cytokinetics, MyoKardia, Relypsa, Novartis, and scPharmaceuticals. Margaret Petrou, David Trueman, and Fionn Woodcock are employees of Source Health Economics, UK, the health economics and outcomes research (HEOR) company that conducted the cost-effectiveness analysis and provided writing services. Work by Source Health Economics was funded by Novartis. Inclisiran, sold under the brand name Leqvio®, has been developed by Novartis and is approved in Europe for lowering LDL-cholesterol levels in patients with hypercholesterolemia or mixed dyslipidemia. The efficacy and safety of inclisiran has been evaluated in the ORION clinical trial program; ORION clinical trials cited in this manuscript were developed and funded by Novartis. Caresse Campbell and Margaret Petrou had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Ethics approval
Not required for this study. Methods for the economic evaluation were evaluated against the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Desai, N.R., Campbell, C., Electricwala, B. et al. Cost Effectiveness of Inclisiran in Atherosclerotic Cardiovascular Patients with Elevated Low-Density Lipoprotein Cholesterol Despite Statin Use: A Threshold Analysis. Am J Cardiovasc Drugs 22, 545–556 (2022). https://doi.org/10.1007/s40256-022-00534-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00534-9