Abstract
Background
Numerous drugs have the potential to be affected by cytochrome P450 (CYP) 2B6-mediated drug–drug interactions (DDIs).
Objectives
In this work, we extend a static approach to the prediction of the extent of pharmacokinetics DDIs between substrates and inhibitors or inducers of CYP2B6.
Methods
This approach is based on the calculation of two parameters (the contribution ratio [CR], representing the fraction of dose of the substrate metabolized via this pathway and the inhibitory or inducing potency of the perpetrator [IR or IC, respectively]) calculated from the area under the concentration–time curve (AUC) ratios obtained in in-vivo DDI studies.
Results
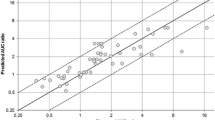
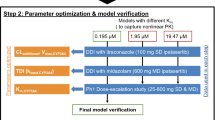
Forty-eight studies involving 5 substrates, 11 inhibitors and 18 inducers of CYP2B6 (overall 15 inhibition and 33 induction studies) were divided into test and validation sets and considered for estimation of the parameters. The proposed approach demonstrated a fair accuracy for predicting the extent of DDI related to CYP2B6 inhibition and induction, all predictions related to the validation test (N = 18) being 50–200% of the observed ratios.
Conclusions
This methodology can be used for proposing initial dose adaptations to be adopted, for example in clinical use or for designing DDI studies involving this enzyme.


Similar content being viewed by others
References
Desta Z, El-Boraie A, Gong L, Somogyi AA, Lauschke VM, Dandara C, et al. PharmVar GeneFocus: CYP2B6. Clin Pharmacol Ther. 2021;110(1):82–97.
Wang H, Tompkins L. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008;9(7):598–610.
Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 2008;82(10):667–715.
Mo S-L, Liu Y-H, Duan W, Wei M, Kanwar J, Zhou S-F. Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6. Curr Drug Metab. 2009;10(7):730–53.
Hedrich WD, Hassan HE, Wang H. Insights into CYP2B6-mediated drug-drug interactions. Acta Pharm Sin B. 2016;6(5):413–25.
Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(1–2):83–448.
Tornio A, Backman JT. Cytochrome P450 in pharmacogenetics: an update. Adv Pharmacol. 2018;83:3–32.
Desta Z, Gammal RS, Gong L, Whirl-Carrillo M, Gaur AH, Sukasem C, Hockings J, Myers A, Swart M, Tyndale RF, Masimirembwa C, Iwuchukwu OF, Chirwa S, Lennox J, Gaedigk A, Klein TE, Haas DW. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2B6 and efavirenz-containing antiretroviral therapy. Clin Pharmacol Ther. 2019;106(4):726–33.
Krayenbühl JC, Vozeh S, Kondo-Oestreicher M, Dayer P. Drug-drug interactions of new active substances: mibefradil example. Eur J Clin Pharmacol. 1999;55(8):559–65.
Peters SA, Dolgos H. Requirements to establishing confidence in physiologically based pharmacokinetic (PBPK) models and overcoming some of the challenges to meeting them. Clin Pharmacokinet. 2019;58(11):1355–71.
Frechen S, Rostami-Hodjegan A. Quality assurance of PBPK modeling platforms and guidance on building, evaluating, verifying and applying PBPK models prudently under the umbrella of qualification: why, when, what, how and by whom? Pharm Res. 2022. https://doi.org/10.1007/s11095-022-03250-w.
Reddy VP, Walker M, Sharma P, Ballard P, Vishwanathan K. Development, verification, and prediction of osimertinib drug-drug interactions using PBPK modeling approach to inform drug label. CPT Pharmacometrics Syst Pharmacol. 2018;7(5):321–30.
Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB, Tremaine LM. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J Pharmacol Exp Ther. 2006;316(1):336–48.
Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet. 2007;46(8):681–96.
Ohno Y, Hisaka A, Ueno M, Suzuki H. General framework for the prediction of oral drug interactions caused by CYP3A4 induction from in vivo information. Clin Pharmacokinet. 2008;47(10):669–80.
Tod M, Goutelle S, Clavel-Grabit F, Nicolas G, Charpiat B. Quantitative prediction of cytochrome P450 (CYP) 2D6-mediated drug interactions. Clin Pharmacokinet. 2011;50(8):519–30.
Goutelle S, Bourguignon L, Bleyzac N, Berry J, Clavel-Grabit F, Tod M. In vivo quantitative prediction of the effect of gene polymorphisms and drug interactions on drug exposure for CYP2C19 substrates. AAPS J. 2013;15(2):415–26.
Castellan AC, Tod M, Gueyffier F, Audars M, Cambriels F, Kassaï B, Nony P, Genophar Working Group. Quantitative prediction of the impact of drug interactions and genetic polymorphisms on cytochrome P450 2C9 substrate exposure. Clin Pharmacokinet. 2013;52(3):199–209.
Gabriel L, Tod M, Goutelle S. Quantitative prediction of drug interactions caused by CYP1A2 inhibitors and inducers. Clin Pharmacokinet. 2016;55(8):977–90.
Di Paolo V, Ferrari FM, Poggesi I, Quintieri L. A quantitative approach to the prediction of drug-drug interactions mediated by cytochrome P450 2C8 inhibition. Expert Opin Drug Metab Toxicol. 2021;17(11):1345–52.
Fan L, Wang JC, Jiang F, Tan ZR, Chen Y, Li Q, Zhang W, Wang G, Lei HP, Hu DL, Wang D, Zhou HH. Induction of cytochrome P450 2B6 activity by the herbal medicine baicalin as measured by bupropion hydroxylation. Eur J Clin Pharmacol. 2009;65(4):403–9.
Kustra R, Corrigan B, Dunn J, Duncan B, Hsyu P-H. Lack of effect of cimetidine on the pharmacokinetics of sustained-release bupropion. J Clin Pharmacol. 1999;39(11):1184–8.
Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005;77(6):553–9.
Jiang F, Desta Z, Shon JH, Yeo CW, Kim HS, Liu KH, Bae SK, Lee SS, Flockhart DA, Shin JG. Effects of clopidogrel and itraconazole on the disposition of efavirenz and its hydroxyl metabolites: exploration of a novel CYP2B6 phenotyping index. Br J Clin Pharmacol. 2013;75(1):244–53.
McCance-Katz EF, Gruber VA, Beatty G, Lum P, Ma Q, Difrancesco R, Hochreiter J, Wallace PK, Faiman MD, Morse GD. Interaction of disulfiram with antiretroviral medications: efavirenz increases while atazanavir decreases disulfiram effect on enzymes of alcohol metabolism. Am J Addict. 2014;23(2):137–44.
Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K. Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2003;74(4):326–33.
Schmid Y, Rickli A, Schaffner A, Duthaler U, Grouzmann E, Hysek CM, Liechti ME. Interactions between bupropion and 3,4-methylenedioxymethamphetamine in healthy subjects. J Pharmacol Exp Ther. 2015;353(1):102–11.
Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 2: in vivo induction and in vitro to in vivo correlation of induction of cytochrome P450 1A2, 2B6, and 2C9 by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39(12):2329–37.
Evaluation D, Documents A. Center for drug evaluation and application number: 2010;(2004):21–3.
Wang J, Zhang ZY, Lu S, Powers D, Kansra V, Wang X. Effects of rolapitant administered orally on the pharmacokinetics of dextromethorphan (CYP2D6), tolbutamide (CYP2C9), omeprazole (CYP2C19), efavirenz (CYP2B6), and repaglinide (CYP2C8) in healthy subjects. Support Care Cancer. 2019;27(3):819–27.
Peltoniemi MA, Saari TI, Hagelberg NM, Reponen P, Turpeinen M, Laine K, Neuvonen PJ, Olkkola KT. Exposure to oral S-ketamine is unaffected by itraconazole but greatly increased by ticlopidine. Clin Pharmacol Ther. 2011;90(2):296–302.
Kharasch ED, Stubbert K. Role of cytochrome P4502B6 in methadone metabolism and clearance. J Clin Pharmacol. 2013;53(3):305–13.
Desta Z, Metzger IF, Thong N, Lu JBL, Callaghan JT, Skaar TC, Flockhart DA, Galinsky RE. Inhibition of cytochrome P450 2B6 activity by voriconazole profiled using efavirenz disposition in healthy volunteers. Antimicrob Agents Chemother. 2016;60(11):6813–22.
Ji P, Damle B, Xie J, Unger SE, Grasela DM, Kaul S. Pharmacokinetic interaction between efavirenz and carbamazepine after multiple-dose administration in healthy subjects. J Clin Pharmacol. 2008;48(8):948–56.
Fda. CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 212839Orig1s000 OTHER REVIEW(S).
Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49(5):513–9.
Kharasch ED, Whittington D, Ensign D, Hoffer C, Bedynek PS, Campbell S, Stubbert K, Crafford A, London A, Kim T. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2012;91(4):673–84.
Fahmi OA, Shebley M, Palamanda J, Sinz MW, Ramsden D, Einolf HJ, Chen L, Wang H. Evaluation of CYP2B6 induction and prediction of clinical drug-drug interactions: considerations from the IQ Consortium induction working group—an industry perspective. Drug Metab Dispos. 2016;44(10):1720–30.
Gao L, He Y, Tang J, Yin J, Huang Z, Liu F, Ouyang D, Chen X, Zhang W, Liu Z, Zhou H. Genetic variants of pregnane X receptor (PXR) and CYP2B6 affect the induction of bupropion hydroxylation by sodium ferulate. PLoS ONE. 2013;8(6):e62489.
Yamazaki T, Desai A, Goldwater R, Han D, Howieson C, Akhtar S, Kowalski D, Lademacher C, Pearlman H, Rammelsberg D, Townsend R. Pharmacokinetic effects of isavuconazole coadministration with the cytochrome P450 enzyme substrates bupropion, repaglinide, caffeine, dextromethorphan, and methadone in healthy subjects. Clin Pharmacol drug Dev. 2017;6(1):54–65.
Pharmacology C. Center for Drug Evaluation and Clinical Pharmacology and Biopharmaceutics Review ( S ). Food Drug Adm. 2009;1–5.
Hogeland GW, Swindells S, McNabb JC, Kashuba ADM, Yee GC, Lindley CM. Lopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjects. Clin Pharmacol Ther. 2007;81(1):69–75.
Qin WJ, Zhang W, Liu ZQ, Chen XP, Tan ZR, Hu DL, Wang D, Fan L, Zhou HH. Rapid clinical induction of bupropion hydroxylation by metamizole in healthy Chinese men. Br J Clin Pharmacol. 2012;74(6):999–1004.
Veldkamp AI, Harris M, Montaner JSG, Moyle G, Gazzard B, Youle M, Johnson M, Kwakkelstein MO, Carlier H, van Leeuwen R, Beijnen JH, Lange JM, Reiss P, Hoetelmans RM. The steady-state pharmacokinetics of efavirenz and nevirapine when used in combination in human immunodeficiency virus type 1-infected persons. J Infect Dis. 2001;184(1):37–42.
Chung JY, Cho JY, Lim HS, Kim JR, Yu KS, Lim KS, Shin SG, Jang IJ. Effects of pregnane X receptor (NR1I2) and CYP2B6 genetic polymorphisms on the induction of bupropion hydroxylation by rifampin. Drug Metab Dispos. 2011;39(1):92–7.
Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther. 2006;80(1):75–84.
Cho DY, Shen JHQ, Lemler SM, Skaar TC, Li L, Blievernicht J, Zanger UM, Kim KB, Shin JG, Flockhart DA, Desta Z. Rifampin enhances cytochrome P450 (CYP) 2B6-mediated efavirenz 8-hydroxylation in healthy volunteers. Drug Metab Pharmacokinet. 2016;31(2):107–16.
Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52(5):1663–9.
Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84(4):497–505.
Park J, Vousden M, Brittain C, McConn DJ, Iavarone L, Ascher J, Sutherland SM, Muir KT. Dose-related reduction in bupropion plasma concentrations by ritonavir. J Clin Pharmacol. 2010;50(10):1180–7.
Peltoniemi MA, Saari TI, Hagelberg NM, Laine K, Neuvonen PJ, Olkkola KT. St John’s wort greatly decreases the plasma concentrations of oral S-ketamine. Fundam Clin Pharmacol. 2012;26(6):743–50.
Lei HP, Yu XY, Xie HT, Li HH, Fan L, Dai LL, Chen Y, Zhou HH. Effect of St. John’s wort supplementation on the pharmacokinetics of bupropion in healthy male Chinese volunteers. Xenobiotica. 2010;40(4):275–81.
Younis IR, Lakota EA, Volpe DA, Patel V, Xu Y, Sahajwalla CG. Drug-drug interaction studies of methadone and antiviral drugs: lessons learned. J Clin Pharmacol. 2019;59(8):1035–43.
Richter T, Mürdter TE, Heinkele G, Pleiss J, Tatzel S, Schwab M, Eichelbaum M, Zanger UM. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J Pharmacol Exp Ther. 2004;308(1):189–97.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the two-fold measure of prediction success for ratios: application for the assessment of drug-drug interactions. Drug Metab Dispos. 2011;39(2):170–3.
Thomas A, Best N, Way R. WinBUGS User Manual. 2003;(January).
Ekins S, VandenBranden M, Ring BJ, Wrighton SA. Examination of purported probes of human CYP2B6. Pharmacogenetics. 1997;7(3):165–79.
Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108(3):363–74.
Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321(1):389–99.
Marok FZ, Fuhr LM, Hanke N, Selzer D, Lehr T. Physiologically based pharmacokinetic modeling of bupropion and its metabolites in a CYP2B6 drug-drug-gene interaction network. Pharmaceutics. 2021;13(3):1–23.
Parkinson A, Ogilvie BW, Buckley DB, Kazmi F, Czerwinski M, Parkinson O. Biotransformation of Xenobiotics. In: Klaassen CD. eds. Casarett and Doull's Toxicology: The Basic Science of Poisons, Eighth Edition. McGraw Hill; 2013. Accessed July 09, 2022. https://accesspharmacy.mhmedical.com/Content.aspx?bookid=958§ionid=53483726.
Xie HJ, Yasar Ü, Lundgren S, Griskevicius L, Terelius Y, Hassan M, Rane A. Role of polymorphic human CYP2B6 in cyclophosphamide bioactivation. Pharmacogenomics J. 2003;3(1):53–61.
Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, Zanger UM. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2008;325(1):284–92.
Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Décosterd L, Blievernicht J, Saussele T, Günthard HF, Schwab M, Eichelbaum M, Telenti A, Zanger UM. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81(4):557–66.
Ayuso P, Neary M, Chiong J, Owen A. Meta-analysis of the effect of CYP2B6, CYP2A6, UGT2B7 and CAR polymorphisms on efavirenz plasma concentrations. J Antimicrob Chemother. 2019;74(11):3281–90.
Ariyoshi N, Ohara M, Kaneko M, Afuso S, Kumamoto T, Nakamura H, et al. Q172H replacement overcomes effects on the metabolism of cyclophosphamide and efavirenz caused by CYP2B6 variant with Arg262. Drug Metab Dispos. 2011;39(11):2045–8.
Radloff R, Gras A, Zanger UM, Masquelier C, Arumugam K, Karasi JC, Arendt V, Seguin-Devaux C, Klein K. Novel CYP2B6 enzyme variants in a Rwandese population: functional characterization and assessment of in silico prediction tools. Hum Mutat. 2013;34(5):725–34.
Watanabe T, Saito T, Rico EMG, Hishinuma E, Kumondai M, Maekawa M, Oda A, Saigusa D, Saito S, Yasuda J, Nagasaki M, Minegishi N, Yamamoto M, Yamaguchi H, Mano N, Hirasawa N, Hiratsuka M. Functional characterization of 40 CYP2B6 allelic variants by assessing efavirenz 8-hydroxylation. Biochem Pharmacol. 2018;156:420–30.
Wang PF, Neiner A, Kharasch ED. Efavirenz metabolism: influence of polymorphic CYP2B6 variants and stereochemistry. Drug Metab Dispos. 2019;47(10):1195–205.
Nakajima M, Komagata S, Fujiki Y, Kanada Y, Ebi H, Itoh K, et al. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics. 2007;17(6):431–45.
Honda M, Muroi Y, Tamaki Y, Saigusa D, Suzuki N, Tomioka Y, Matsubara Y, Oda A, Hirasawa N, Hiratsuka M. Functional characterization of CYP2B6 allelic variants in demethylation of antimalarial artemether. Drug Metab Dispos. 2011;39(10):1860–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Veronica Di Paolo, Francesco Maria Ferrari, Italo Poggesi and Luigi Quintieri declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data are available as tables in the manuscript.
Code availability
Not applicable.
Author contributions
All authors were involved in the study design, data collection and analysis, interpretation of findings, and writing of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di Paolo, V., Ferrari, F.M., Poggesi, I. et al. Quantitative Prediction of Drug Interactions Caused by Cytochrome P450 2B6 Inhibition or Induction. Clin Pharmacokinet 61, 1297–1306 (2022). https://doi.org/10.1007/s40262-022-01153-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01153-y