Abstract
Objectives
Type 2 diabetes mellitus (DMT2) is contributed to dual interactions between environmental factors and certain genetic factors. This impressed a great need for novel treatment strategy. Nevertheless, Hyssopus officinalis (H. officinalis) as a terrestrial herb is considered to be an important source of natural antioxidants, it could be assessed as an anti-hyperglycemic agent.
Methods
In the current study, HPLC identified the active constitutes of H. officinalis, including total polyphenols, and flavonoids. Type 2 diabetes mellitus was induced in male Wistar albino rats via a single ip dose of streptozotocin (STZ) (35 mg/kg BW). One week post diabetes induction, rats were administrated H. officinalis (500 mg/ kg BW) orally for one month. Molecular analysis was assessed to investigate the efficiency of H. officinalis on modulating ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1) genes, in addition to apoptotic biomarkers, glycogen synthase kinase-3β (GSK-3β) and cellular oncogene-fos (C-fos) genes. Furthermore, inflammatory biomarkers, nuclear factor kappa-B (NF-κB) and tumor necrosis factor-α (TNF-α) gene expression were also assessed.
Results
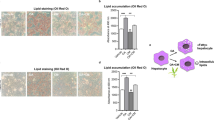
H. officinalis alcoholic extract declared the presence of polyphenols as gallic acid and flavonoids as quercetin in addition to many active constituents. Apigenin-7-glucoside and Chlorgenic acid were the most common constituents in the extract. RT-PCR results declared a significant up-regulation in mRNA gene expression of ABCA1 and ABCG1 upon H. officinalis treatment. Meanwhile, C-fos gene expression recorded a slight down-regulation. Gene expression of apoptotic biomarker GSK-3β demonstrated a significant down regulation as well as inflammatory biomarkers NF-κB and TNF-α.
Conclusion
From the data recorded, it could be concluded that H. officinalis exerts a great hypoglycemic potential via modulating C-fos, GSK-3β, NF-κB, TNF-α, ABCA1 and ABCG1 gene expression and signaling pathways and could be considered as an effective candidate for DMT2 treatment.




Similar content being viewed by others
References
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Kizil S, Toncer O, Ipek A, Arslan N, Saglam S, Mahmood Khawar K. Blooming stages of Turkish hyssop (Hyssopus officinalis L.) affect essential oil composition. Acta Agric Scand Sect B Soil Plant Sci. 2008;58(3):273–9.
Miyazaki H, Matsuura H, Yanagiya C, Mizutani J, Tsuji M, Ishihara C. Inhibitory effects of hyssop (Hyssopus officinalis) extract on intestinal alpha-glucosidase activity and postprandial hyperglycemia. J Nutr Sci Vitaminol (Tokyo). 2003;49(5):346–9.
Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Frahangi A, Alla, et al. induction of diabetes by streptozotocin in rats. IJCB. 2007;22:60–4.
Patti ME. Gene expression in the pathophysiology of type 2 diabetes mellitus. Curr Diab Rep. 2004;4(3):176–81.
Tang C, Kanter JE, Bornfeldt KE, Leboeuf RC, Oram JF. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J Lipid Res. 2010;51(7):1719–28.
Alharbi KK, Khan IA, Al-Daghri NM, Munshi A, Sharma V, Mohammed AK, et al. ABCA1 C69T gene polymorphism and risk of type 2 diabetes mellitus in a Saudi population. J Biosci. 2013;38(5):893–7.
MacAulay K, Woodgett JR. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin Ther Targets. 2008;12(10):1265–74.
Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, et al. CNS Inflammation and Bone Marrow Neuropathy in Type 1 Diabetes CNS Inflammation and Bone Marrow Neuropathy in Type 1 Diabetes. Am J Pathol. 2014;183(5):1608–20.
Saiedullah M. Diabetes case reports insulin sensitivity or resistance in type 2 diabetes mellitus with obesity 2016; 1(2): 10000.
Sigala I, Zacharatos P, Toumpanakis D et al (2011) MAPKs and NF-κB differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 300(5):1152–62
Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: Temporal control and selective gene activation. Science. 2002;298(5596):1241–5.
Reyna SM, Ghosh S, Tantiwong P, Meka CSR, Eagan P, Jenkinson CP, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57(10):2595–602.
Gorinstein S, Zachwieja Z, Katrich E, Pawelzik E, Haruenkit R, Trakhtenberg S. Comparison of the contents of the main antioxidant compounds and the antioxidant activity of white grape fruit and his new hybrid. Leb Wiss Technol. 2004;37:337–43.
Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–7.
Kim KH, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–73.
Bruce RD. An up-and-down procedure for acute toxicity testing. Fund Appl Tox. 1985;5:151–7.
Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64(1):129–39.
Salama MF, Bayele HK, Srai SSK. Tumour necrosis factor alpha downregulates human hemojuvelin expression via a novel response element within its promoter. J Biomed Sci. 2012;19(1):83.
Yokoyama K, Hiyama A, Arai F, Nukaga T, Sakai D, Mochida J. C-Fos regulation by the MAPK and PKC pathways in intervertebral disc cells. PLoS One. 2013;8(9):1–14.
Nazarian H, Ghaffari Novin M, Jalili MR, Mirfakhraie R, Heidari MH, Hosseini SJ, et al. Expression of Glycogen synthase kinase 3-β (GSK3-β) gene in azoospermic men. Iran J Reprod Med. 2014;12(5):313–20.
Lou J, Zhou H, Li C, Hu L, Lu X, Li J, et al. ABCA1 and ABCG1 Expression in the Small Intestine of Type 2 Diabetic Rats. Lab Med. 2014;45(1):17–24.
Montesanto A, Bonfigli AR, Crocco P, Garagnani P, De Luca M, Boemi M, Marasco E, Pirazzini C, Giuliani C, Franceschi C, Passarino G, Testa R, Olivieri F, Rose G. Genes associated with Type 2 Diabetes and vascular complications. Aging. 2018;10:178–96.
Kim Y, Keogh JB, Clifton PM. Polyphenols glycemic control nutrients. 2016;8:17; 1–27. https://doi.org/10.3390/nu8010017.
Hossain C, Ghosh M, Satapathy BS, Dey NS, Mukherjee B. Apigenin causes biochemical modulation, GLUT4, and CD38 alterations to improve diabetes and to protect damages of some vital organs in experimental diabetes 2014; 9(2): 39–52.
Bumke-Vogt C, Osterhoff MA, Borchert A, Guzman-Perez V, Sarem Z, Birkenfeld AL, et al. The flavones apigenin and luteolin induce FOXO1 translocation but inhibit gluconeogenic and lipogenic gene expression in human cells. PLoS One. 2014;9:e104321.
Ohno M, Shibata C, Kishikawa T, Yoshikawa T, Takata A, Kojima K, et al. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci Rep. 2013;3:2553.
Chen BC, Chang YS, Kang JC, Hsu MJ, Sheu JR, Chen TL, et al. Peptidoglycan induces nuclear factor-kappaB activation and cyclooxygenase-2 expression via Ras, Raf-1, and ERK in RAW 264.7 macrophages. J Biol Chem. 2004;279(4):20889–97.
Tuorkey MJ. Molecular targets of luteolin in cancer. Eur J Cancer Prev. 2016;25(1):65–76.
Maalik A, Bukhari SM, Zaidi A, Shah KH, Khan FA. Chlorogenic acid: a pharmacologically potent molecule. Acta Pol Pharm. 2016;73(4):851–4.
Vinayagam R, Jayachandran M, Xu B. Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother Res. 2016;30(2):184–99.
Tousch D, Lajoix AD, Hosy E, Azay-Milhau J, Ferrare K, Jahannault C, et al. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem Biophys Res Commun. 2008;377(1):131–5.
Ong KW, Hsu A, Tan BKH. Chlorogenic acid simulates glucose transport in skeletal muscle via AMPK Activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7(3):e32718.
Subhasree N, Kamella A, Kaliappan I, Agrawal A, Dubey GP. Antidiabetic and antihyperlipidemic activities of a novel polyherbal formulation in a high-fat dietstreptozotocin-induced diabetic rat model. Indian J Pharmacol. 2015;47(5):509–13.
Ramar M, Manikandan B, Raman T, Priyadarsini A, Palanisamy S, Velayudam M, et al. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur J Pharmacol. 2012;690(1–3):226–35.
Han M, Wen JK, Zheng B, Zhang DQ. Acetylbritannilatone suppresses NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci. 2004;75:675–84. https://doi.org/10.1016/j.lfs.2003.12.022.
Chang WC, Kuo PL, Chen CW, Wu J, Shen SC. Caffeic acid improves memory impairment and brain glucose metabolism via ameliorating cerebral insulin and leptin signaling pathways in high-fat-diet-induced hyperinsulinemic rats. Food Res Int. 2015;77(1):24–33.
Yoon S-A, Kang S-I, Shin H-S, Kang S-W, Kim J-H, Ko H-C, et al. p-Coumaric acid modulates glucose and lipid metabolism via AMP-activated protein kinase in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2013;432(4):553–7.
Eizirik DL, Miani M, Cardozo AK. Signaling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56(2):234–41.
Olivier M, Bott GR, Frisdal E, Nowick M, Plengpanich W, Desmarchelier C, et al. ABCG1 is involved in vitamin E efflux. Biochim Biophys Acta. 2014;1841(12):1741–51.
Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A. The beta-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32(1):131–9.
Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis, and inflammation. Atherosclerosis. 2010;211(2):361–70.
Pagler TA, Wang M, Mondal M, Murphy AJ, Westerterp M, Moore KJ, et al. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ Res. 2011;108(2):194–200.
Lou J, Zhou H, Li C, Hu L, Lu X, Li J, et al. ABCA1 and ABCG1 Expression in the Small Intestine of Type 2 Diabetic Rats. Lab Med. 2014;45(1):17–24.
Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, Yang GM, Choi HY, Cho SG. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016 Apr 15; 17(4):569.
Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–7.
Mccue P, Shetty K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac J Clin Nutr. 2004;13:101–6.
Akram M. Diabetes mellitus type-II: treatment strategies and options: a review. J Diabetes Metab. 2013;4:1–9.
Tahir M, Khushtar M, Fahad M, Rahman A. Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.). J App Pharm Sci. 2018;8(07):132–40.
Acknowledgements
The authors are grateful to the National Research Centre for it’ssupport to complete this research.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Megeed, R.M., El Newary, S.A., Kadry, M.O. et al. Hyssopus officinalis exerts hypoglycemic effects on streptozotocin-induced diabetic rats via modulating GSK-3β, C-fos, NF-κB, ABCA1 and ABGA1 gene expression. J Diabetes Metab Disord 19, 483–491 (2020). https://doi.org/10.1007/s40200-020-00535-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00535-y