Abstract
Hepatocellular carcinoma (HCC) is an alarming epidemiological clinical problem worldwide. Pharmacological approaches currently available do not provide adequate responses due to poor effectiveness, high toxicity, and serious side effects. Our previous studies have shown that the wild edible plant Crithmum maritimum L. inhibits the growth of liver cancer cells and promotes liver cell differentiation by reducing lactic acid fermentation (Warburg effect). Here, we aimed to further characterise the effects of C. maritimum on lipid metabolism and markers of cellular metabolic health, such as AMP-activated protein kinase (AMPK), Sirtuin 1 (SIRT1), and Sirtuin 3 (SIRT3), as well as the insulin signalling pathway. To better mimic the biological spectrum of HCC, we employed four HCC cell lines with different degrees of tumorigenicity and lactic acid fermentation/Warburg phenotype. Lipid accumulation was assessed by Oil Red O (ORO) staining, while gene expression was measured by real-time quantitative PCR (RT-qPCR). The activation of AMPK and insulin signalling pathways was determined by Western blotting. Results indicate that C. maritimum prevents lipid accumulation, downregulates lipid and cholesterol biosynthesis, and modulates markers of metabolic health, such as AMPK, SIRT1 and SIRT3. This modulation is different amongst HCC cell lines, revealing an important functional versatility of C. maritimum. Taken together, our findings corroborate the importance of C. maritimum as a valuable nutraceutical, reinforcing its role for the improvement of metabolic health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alarming rise in metabolic abnormalities currently underlies many diseases, including cancer, cardiovascular disease, and neurodegenerative disorders [1,2,3,4]. Plant-derived products have gained renewed interest in several areas of medicine, including oncology [5], and plant-based diets and nutritional supplements are growing in importance [6]. Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer death worldwide and is projected to become the third leading cause of cancer death in Western countries by 2030 [7]. Metabolic dysregulation is now considered an important driver of HCC, as many HCC cases are the final stage of pathological progression from hepatic lipid accumulation (steatosis) [8] to inflammation, fibrosis and ultimately development of HCC [9]. In fact, it is estimated that approximately 35% of HCC cases may develop directly from simple steatosis [10]. The main strategy for HCC treatment is surgery, while pharmacological approaches rely on a combination of tyrosine kinase inhibitors and immunotherapy [11]. However, these interventions have not met expectations from the perspective of effectiveness and toxicity. In this context, there is an urgent need to scientifically demonstrate the effectiveness of new, less toxic, and more effective specific approaches to prevent and treat HCC. Based on this assumption, phytonutrients and nutritional supplements can provide valuable support. [12]. C maritimum has been known to people since ancient times. People living along the Mediterranean coast use seaweed as a diuretic, purifier, digestive aid, antiscorbutic, anti-cold and anti-inflammatory, as well as a wound healer and anthelmintic [13]. C. maritimum can also be used as a food: its leaves can be eaten in salads, cooked, or treated with vinegar like capers. This typical Apulian preparation is included in the list of traditional agricultural products of the Ministry of Agriculture [14]. Further information and references on these topics as well as on the botanical, phytochemical, and ethnopharmacological characteristics of C. maritimum can be found in the Supplementary Materials. Despite its long tradition as a food and medicinal plant, the scientific basis behind the efficacy of C. maritimum is missing, and its nutraceutical properties as an anti-tumour agent are lacking in evidence. Moreover, although in recent years several scientific works dealt with the antitumor action of plant extracts, we have been the first to focus on C. maritimum and to the characterisation of its “systemic-metabolic” effects (Additional References on this issue can be found in the Supplementary Material). We were the first to demonstrate the cytostatic effect of the ethyl acetate extract of C. maritimum in HCC [15], linking it to a “rescheduling” of the tumour “metabolic signature,” with decreased levels of several metabolites, such as lactate, amino acids, cholesterol and polyunsaturated fatty acids (PUFA), as well as choline and phosphocholine, which fuel tumour growth. Additionally, we found an increase in healthy monounsaturated fatty acids (MUFAs) [16]. Our previous studies also showed that C. maritimum promoted oxidative phosphorylation (OXPHOS) in HCC cells [17] and could be used in combination with tyrosine kinase inhibitors to reduce their doses and thus reduce toxicity [18]. We later found that C. maritimum sensitises HCC cells to tyrosine kinase inhibitors by inhibiting lactate fermentation and rewires the cancerous metabolic profile of HCC, promoting a phenotype more similar to normal hepatocytes [19]. Here, we focused on further characterising the nutraceutical properties of C. maritimum on the regulation of the metabolic phenotype of HCC, using four cell lines with different degree of aggressiveness and differentiation state, and then with a different metabolic phenotype, ranging from prevalent oxidative phosphorylation (OXPHOS) to prevalent, lactic acid fermentation/Warburg effect [20]. This broad metabolic diversity was chosen to better characterise the impact of C. maritimum on the biological and metabolic changes found in liver metabolic diseases and HCC. Hence, the aim of this study was to provide preclinical evidence on the role of C. maritimum as a solid supportive nutraceutical tool in the clinical management of HCC, by the evaluation of the effect on key metabolic pathways which are dysregulated in HCC. Our results indicate that C. maritimum has potent homeostatic effects on the dysregulated metabolism of HCC cells by: i) decreasing the expression of the genes controlling lipogenesis and cholesterogenesis, ii) activating AMP-activated protein kinase (AMPK), Sirtuin 1 (SIRT1) and Sirtuin 3 (SIRT3), which are well-known metabolic health markers, iii) improving insulin response related signalling. As these metabolic pathways are dysregulated in HCC, C. maritimum has great potential as a nutraceutical tool for adjuvant therapy and prevention of HCC.
Materials and Methods
Preparation and Characterisation of C. Maritimum Ethyl Acetate Extract
C. maritimum ethyl acetate extract was prepared and characterised as we previously reported [15]. The ethyl acetate fraction was the most biologically active if compared to fractions obtained with polar solvents (ethanol) and with other non-polar solvents (Hexane, methanol) [15, 16]. Cytotoxicity of ethyl acetate extract and IC50 has been determined in our previous work [15].
Cell Lines and Culturing
A detailed description can be found in the Supplementary Materials section. Briefly, cells were treated with 0.5 mM C. maritimum ethyl acetate extract, as we previously showed that this represents the optimal dose in terms of effects and cytotoxicity in HCC cell lines [15,16,17, 19, 21].
Oil Red O (ORO) Staining for Evaluation of Lipid Accumulation
A detailed description can be found in the Supplementary Materials section.
Quantitative Real-Time PCR (RT q-PCR)
A detailed description can be found in the Supplementary Materials section.
Immunoblotting Analyses
A detailed description can be found in the Supplementary Materials section.
Statistical Analyses
A detailed description can be found in the Supplementary Materials section.
Results
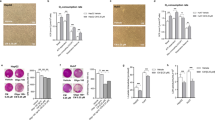
First, we explored the role of C. maritimum in regulating lipid and metabolic health homeostasis in HCC. Figure 1 shows that 0.5 mM ethyl acetate extract of C. maritimum prevented lipid accumulation in two HCC cell lines, HepG2 and HepaRG. These two cell lines were chosen because they have been shown to be good models of hepatic lipid accumulation in vitro [22]. To assess lipid accumulation, we used Oil Red O (ORO) staining, which stains neutral lipids [9]. In detail, Fig. 1a, b show that C. maritimum (CM) prevented the formation of intracellular lipid droplets induced by 24 h treatment with 100 μM oleic acid (OA) as determined by ORO staining. In fact, treatment with CM determined a significative inhibition of lipid accumulation (about 75%).
C. maritimum prevents lipid accumulation in HCC cell lines. Cells were treated for 24 h with 100 μM oleic acid (OA), C. maritimum 0.5 mM ethyl acetate extract (CM), and OA + CM. Lipid accumulation was evaluated by Oil Red O (ORO) staining. ORO pictures and quantification in HepG2 (a-b) and HepaRG cells (c-d). e Graphical summary of the key message described in the Figure. *** p < 0.001 compared to Vehicle; ** p < 0.01 compared to Vehicle ### p < 0.001 compared to OA
Next, we analysed the expression of two major genes involved in the lipid biosynthesis pathway, fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). Overexpression of these two genes has been associated to hepatocarcinogenesis [23, 24]. We also considered the expression of HMG-CoA reductase (HMG-CoA Red), a key gene involved in cholesterol biosynthesis, whose overexpression has been associated with the development of HCC [25]. Finally, we evaluated the expression of CD36, a fatty acid transporter whose overexpression has been associated with the development of hepatic steatosis and HCC [26]. Results show that C. maritimum effectively counteracts lipid gene dysregulation in HCC cell lines (Fig. 2), which promotes the development of HCC [23,24,25,26]. In fact, all of the above-mentioned genes were significantly down-regulated by a 24 h treatment with C. maritimum in HepG2 and HepaRG, while no significant effect was observed for HMG-CoA Red in Huh7 and HLE. Taken together, these results suggest that C. maritimum can contribute to normalise the transformed lipid phenotype in well-established HCC.
C. maritimum restores the expression of lipid metabolism genes in HCC cell lines. Cells were treated with C. maritimum 0.5 mM ethyl acetate extract (CM) for 24 h. The expression of four key genes in lipid and cholesterol metabolism in four HCC cell lines, HepG2 (a), HepaRG (b), Huh7 (c), and HLE (d), was measured by RT q-PCR. *** p < 0.001, ** p < 0.01, ns = not significant compared with vehicle
We then evaluated the nutritional potential of C. maritimum in modulating the expression of genes that are indicators of the metabolic state of the cell: lactate dehydrogenase A (LDHA), Lactate Dehydrogenase B (LDHB), AMP-activated protein kinase (AMPK), Sirtuin 1 (SIRT1), Sirtuin 3 (SIRT3). Each of these genes controls key steps in regulating metabolic homeostasis, and their expression is dysregulated in tumour cells and HCC, with LDHA typically upregulated [27] and LDHB downregulated [28]. All the other above-mentioned genes are downregulated [29]. Activation of AMPK, SIRT1, and SIRT3 is believed to be a promising option for the prevention and treatment of HCC [30,31,32]. The data reported in Fig. 3 demonstrate that C. maritimum reverses the pathological expression of these genes (Fig. 3a-d). Overall, C. maritimum was effective against all four cell lines used, which are models of different degrees of HCC transformation, differentiation, and invasiveness. Compared with Huh7, HLE are less differentiated and more dependent on lactic acid fermentation [20], while Huh7 is more invasive and fermentative compared with HepG2 and HepaRG, which are less invasive and tumorigenic. HepaRG are considered a good model of non-transformed hepatocytes. It is noteworthy that, although there are some differences, C. maritimum remains effective regardless of the characteristics of the cell line used: The effects on LDHA and AMPK were significant in all four cell lines. LDHB expression increased in Huh7 and HLE, the two more aggressive and fermentative cell lines, but did not significantly change in HepG2, and it is slightly but significantly decreased in HepaRG. SIRT1 expression was significantly increased in three cell lines: in HepG2, HepaRG, and HLE, but not in Huh7. SIRT3 was significantly upregulated in three cell lines (HepaRG, Huh7, HLE), whereas no changes were measured in HepG2. Western Blot analysis was employed to demonstrate the activation of AMPK signalling by phosphorylation at Thr172 (Fig. 3e). Interestingly, a stronger activation of AMPK was observed in less aggressive cell lines (HepG2, HepaRG). Figure 3f summarises the results.
C. maritimum improves the expression of metabolic health marker genes in HCC cell lines. Cells were treated for 24 h with C. maritimum 0.5 mM ethyl acetate extract (CM). The expression of key genes involved in the control of metabolic health status was evaluated by RT q-PCR in four HCC cell lines: HepG2 (a), HepaRG (b), Huh7 (c), HLE (d). The activation of AMPK has been analysed by Western Blot in HepG2 and Huh7 cells (e). f Graphical summary of the key message described in the Figure. *** p < 0.001, ** p < 0.01, * p < 0.05, ns = not significant compared with vehicle
Finally, we investigated the role of C. maritimum in regulating the insulin signalling pathway, as its dysregulation has been implicated in the development and progression of HCC. In particular, we have measured the expression of the three main genes involved in the control of the pathway: Insulin Receptor (IR), Insulin Receptor Substrate 1 (IRS-1) and Insulin Receptor Substrate 2 (IRS-2). In addition, we evaluated the phosphorylation of Akt at Ser473, a well-established marker of activation of the insulin signalling pathway. The results reported in Fig. 4 paint an interesting scientific picture: indeed, C. maritimum showed different differential effects that varied according to the degree of invasiveness and Warburg phenotype of the cell lines used. In fact, in HepG2 and HepaRG, with a low tumorigenic/ “normal phenotype”, gene expression and Western blot analyses show an activation of insulin signalling: slight variations of IR, IRS-1 and IRS-2 gene expression (Fig. 4 a, e), while the phosphorylation of Akt at Ser473 was increased (Fig. 4b). In the more tumorigenic and invasive Huh7 and HLE cells, C. maritimum instead significantly downregulated the expression of the analysed insulin signalling genes, and no effect was observed on Akt phosphorylation at Ser473 (Fig. 4 c-e). Notably, IRS-1 and IRS-2 are upregulated in HCC and actively support the tumour phenotype [33]. A graphical summary is showed in Fig. 4f.
C. maritimum differentially regulates insulin signalling depending on the degree of tumorigenicity of HCC cells. For gene expression experiments, cells were treated for 24 h with C. maritimum 0.5 mM ethyl acetate extract (CM). The expression of three genes involved in the control of insulin signalling was evaluated by RT q-PCR in four HCC cell lines HepG2 (a), HepaRG (b), Huh7 (c), HLE (d). For Western Blot experiments, cells were treated for 45 min with C. maritimum 0.5 mM ethyl acetate extract (CM). Activation of insulin signalling has been verified by Western Blot by evaluating Akt phosphorylation at Ser473 in HepG2 and Huh7 cells (e). f Graphical summary of the information presented in the Figure. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05, ns = not significant compared with vehicle
Discussion
Hepatocellular carcinoma (HCC) is now an epidemiologic emergency, which can be explained by the increase in metabolic diseases. We previously reported that C. maritimum inhibits HCC cell growth [15] and modulates metabolic [16] and bioenergetic characteristics of HCC cells by inhibiting lactic acid fermentation and activating OXPHOS [17]. We also demonstrated that C. maritimum reduced the expression of HCC markers α-fetoprotein (α-FP) and α1-antitrypsin (α1-AT) and improved the sensitivity of HCC cell to anti-neoplastic drugs by determining a shift in the HCC bioenergetic profile from lactic acid fermentation to OXPHOS [19]. Interestingly, in line with this, we recently showed that OXPHOS impairment is associated with drug resistance in HCC cell lines [34]. This supports the possible applicability of C. maritimum as a concrete nutraceutical option in the clinical management of HCC. Our current findings provide evidence that C. maritimum interacts with HCC cells to improve their lipid and metabolic profiles and to orient them towards the phenotype of non-transformed cells. Moreover, here, we have demonstrated the “normalisation” of three well-known “cell metabolic health promoters,” such as AMPK, SIRT1 and SIRT3, as well as the selective inhibition of insulin signalling only in the more aggressive cell lines. This reinforces the importance of C. maritimum as a valuable nutritional option supporting a lifestyle aimed at preventing and treating metabolic disorders, which are becoming a serious health problem worldwide, especially in Western countries [1,2,3,4]. Overall, C. maritimum shows a strong multifaceted bioactive action, which can be an important resource in the management of HCC and possibly of other types of tumours. It is worth to underline here the systemic and multi-targeted action exerted by the blend of compounds found in C. maritimum (Fig. S1), which guarantees a full-spectrum action on several of the central bioenergetic traits characteristic of transformed hepatocytes. It is also important to emphasize that the effects of C. maritimum appear to be regulated according to the cellular metabolic phenotype. In fact, C. maritimum stimulated insulin signalling in low tumorigenic cells (mainly relying on OXPHOS), whereas in high tumorigenic cells (Warburg phenotype) an inhibition of insulin signalling was observed (Fig. 5). This important characteristic gives to C. maritimum specificity and selectivity towards normal or HCC cells.
Schematic representation of the effects of C. maritimum on HCC cell lines with different degrees of tumorigenicity and Warburg phenotype. As described in the text, C. maritimum prevents lipid accumulation, reduces the expression of key genes that control lipogenesis, and selectively promotes the normalisation of metabolic markers of cellular health. Specifically, insulin signalling was inhibited in the highly tumorigenic/Warburg-dependent cell lines Huh7 and HLE, whereas it was promoted in the low tumorigenic/OXPHOS-dependent cell lines HepaRG and HepG2
All these versatile characteristics make C. maritimum particularly important in the context of HCC therapy and prevention of the predisposing dysmetabolic liver conditions, even if compared with other efficacious plant-based approaches, such as Chinese herbal formulation YIV-906, which have been recently tested in clinical trials as a supplement to improve conventional HCC drug treatments [35]. In the next step of our study, we will continue to explore the OXPHOS-mediated promotion of drug sensitivity and the mechanism behind the drug sensitization of C. maritimum. This may disclose additional translational applications for the nutraceutical properties of C. maritimum.
Future research activities will also be directed towards the preparation of formulations for use in human clinical trials, case–control studies in HCC patients, and combining appropriate extract preparations with standard therapy. We are also about to begin a clinical study to test the effects of a C. maritimum formulation as a nutritional supplement to improve metabolic health, with a focus on triglyceride and cholesterol levels and insulin sensitivity.
Conclusions
Based on our previous findings and those presented here, we propose C. maritimum as a specific nutraceutical tool for developing strategies to support conventional HCC drug treatment and reduce side effects. Additionally, supplementation with C. maritimum may be used to prevent metabolic diseases that cause or contribute to abnormal liver metabolism and ultimately hepatocellular carcinoma. Our study demonstrated that C. maritimum has broad-spectrum and multi-target effects, which may provide its application in the treatment of other types of cancer.
Data Availability
All data presented in this study are available upon request.
References
Zhang Y, Ding Y, Zhu N, Mi M, Lu Y, Zheng J, Weng S, Yuan Y (2023) Emerging patterns and trends in global cancer burden attributable to metabolic factors, based on the Global Burden of Disease Study 2019. Front Oncol 13:1032749. https://doi.org/10.3389/fonc.2023.1032749
Hu W, Zhai C, Sun H, Gong X, Cui L, Cai L, Zong Q, Yu G, Wang F, Zou Y (2023) The global burden of disease attributable to metabolic risks in 204 countries and territories from 1990 to 2019. Diabetes Res Clin Pract 196:110260. https://doi.org/10.1016/j.diabres.2023.110260
Collaborators GBDD (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402(10397):203–234. https://doi.org/10.1016/S0140-6736(23)01301-6
Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, Lim WH, Huang DQ, Quek J, Fu CE, Xiao J, Syn N, Foo R, Khoo CM, Wang JW, Dimitriadis GK, Young DY, Siddiqui MS, Lam CSP, Wang Y, Figtree GA, Chan MY, Cummings DE, Noureddin M, Wong VW, Ma RCW, Mantzoros CS, Sanyal A, Muthiah MD (2023) The global burden of metabolic disease: data from 2000 to 2019. Cell Metab 35(3):414–428.e413. https://doi.org/10.1016/j.cmet.2023.02.003
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Pena-Jorquera H, Cid-Jofre V, Landaeta-Diaz L, Petermann-Rocha F, Martorell M, Zbinden-Foncea H, Ferrari G, Jorquera-Aguilera C, Cristi-Montero C (2023) Plant-based nutrition: exploring health benefits for atherosclerosis, chronic diseases, and metabolic syndrome-a comprehensive review. Nutrients 15(14). https://doi.org/10.3390/nu15143244
de Lope CR, Tremosini S, Forner A, Reig M, Bruix J (2012) Management of HCC. J Hepatol 56(Suppl 1):S75–87. https://doi.org/10.1016/S0168-8278(12)60009-9
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P (2018) Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 75(18):3313–3327. https://doi.org/10.1007/s00018-018-2860-6
Gnocchi D, Afonso MB, Cavalluzzi MM, Lentini G, Ingravallo G, Sabba C, Rodrigues CMP, Mazzocca A (2023) Inhibition of lysophosphatidic acid receptor 6 upregulated by the choline-deficient l-amino acid-defined diet prevents hepatocarcinogenesis in mice. Mol Carcinog 62(5):577–582. https://doi.org/10.1002/mc.23516
Grgurevic I, Bozin T, Mikus M, Kukla M, O'Beirne J (2021) Hepatocellular carcinoma in non-alcoholic fatty liver disease: from epidemiology to diagnostic approach. Cancers (Basel) 13(22). https://doi.org/10.3390/cancers13225844
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet. https://doi.org/10.1016/S0140-6736(18)30010-2
Tran Q, Lee H, Kim C, Kong G, Gong N, Kwon SH, Park J, Kim SH, Park J (2020) Revisiting the warburg effect: diet-based strategies for cancer prevention. Biomed Res Int 2020:8105735. https://doi.org/10.1155/2020/8105735
Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A (2011) Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J Med Plants Res 5(16):3564–3571
Renna M, Gonnella M, Caretto S, Mita G, Serio F (2017) Sea fennel (Crithmum maritimum L.): from underutilized crop to new dried product for food use. Genet Resour Crop Ev 64(1):205–216. https://doi.org/10.1007/s10722-016-0472-2
Gnocchi D, Cesari G, Calabrese GJ, Capone R, Sabba C, Mazzocca A (2020) Inhibition of hepatocellular carcinoma growth by ethyl acetate extracts of apulian brassica oleracea L. and Crithmum maritimum L. Plant Foods Hum Nutr 75(1):33–40. https://doi.org/10.1007/s11130-019-00781-3
Gnocchi D, Del Coco L, Girelli CR, Castellaneta F, Cesari G, Sabba C, Fanizzi FP, Mazzocca A (2021) (1)H-NMR metabolomics reveals a multitarget action of Crithmum maritimum ethyl acetate extract in inhibiting hepatocellular carcinoma cell growth. Sci Rep 11(1):1259. https://doi.org/10.1038/s41598-020-78867-1
Gnocchi D, Sabba C, Mazzocca A (2022) The edible plant crithmum maritimum shows nutraceutical properties by targeting energy metabolism in hepatic cancer. Plant Foods Hum Nutr 77(3):481–483. https://doi.org/10.1007/s11130-022-00986-z
Gnocchi D, Castellaneta F, Cesari G, Fiore G, Sabba C, Mazzocca A (2021) Treatment of liver cancer cells with ethyl acetate extract of Crithmum maritimum permits reducing sorafenib dose and toxicity maintaining its efficacy. J Pharm Pharmacol. https://doi.org/10.1093/jpp/rgab070
Gnocchi D, Sabba C, Mazzocca A (2023) Crithmum maritimum improves sorafenib sensitivity by decreasing lactic acid fermentation and inducing a pro-hepatocyte marker profile in hepatocellular carcinoma. Plant Foods Hum Nutr 78(1):230–232. https://doi.org/10.1007/s11130-022-01037-3
Nwosu ZC, Battello N, Rothley M, Pioronska W, Sitek B, Ebert MP, Hofmann U, Sleeman J, Wolfl S, Meyer C, Megger DA, Dooley S (2018) Liver cancer cell lines distinctly mimic the metabolic gene expression pattern of the corresponding human tumours. J Exp Clin Cancer Res 37(1):211. https://doi.org/10.1186/s13046-018-0872-6
Gnocchi D, Castellaneta F, Cesari G, Fiore G, Sabba C, Mazzocca A (2021) Treatment of liver cancer cells with ethyl acetate extract of Crithmum maritimum permits reducing sorafenib dose and toxicity maintaining its efficacy. J Pharm Pharmacol 73(10):1369–1376. https://doi.org/10.1093/jpp/rgab070
Ramos MJ, Bandiera L, Menolascina F, Fallowfield JA (2022) In vitro models for non-alcoholic fatty liver disease: Emerging platforms and their applications. iScience 25(1):103549. https://doi.org/10.1016/j.isci.2021.103549
Shen Y, Wang X, Ni Z, Xu S, Qiu S, Zheng W, Zhang J (2023) Identification of acetyl-CoA carboxylase alpha as a prognostic and targeted candidate for hepatocellular carcinoma. Clin Transl Oncol 25(8):2499–2513. https://doi.org/10.1007/s12094-023-03137-1
Hao Q, Li T, Zhang X, Gao P, Qiao P, Li S, Geng Z (2014) Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncol Rep 32(6):2471–2476. https://doi.org/10.3892/or.2014.3484
Sutter AP, Maaser K, Hopfner M, Huether A, Schuppan D, Scherubl H (2005) Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J Hepatol 43(5):808–816. https://doi.org/10.1016/j.jhep.2005.04.010
Zeng H, Qin H, Liao M, Zheng E, Luo X, Xiao A, Li Y, Chen L, Wei L, Zhao L, Ruan XZ, Yang P, Chen Y (2022) CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol Metab 57:101428. https://doi.org/10.1016/j.molmet.2021.101428
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G (2012) Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J 279(20):3898–3910. https://doi.org/10.1111/j.1742-4658.2012.08748.x
Chen R, Zhou X, Yu Z, Liu J, Huang G (2015) Low expression of LDHB correlates with unfavorable survival in hepatocellular carcinoma: strobe-compliant article. Medicine (Baltimore) 94(39):e1583. https://doi.org/10.1097/MD.0000000000001583
Gnocchi D, Sabba C, Massimi M, Mazzocca A (2023) Metabolism as a new avenue for hepatocellular carcinoma therapy. Int J Mol Sci 24(4). https://doi.org/10.3390/ijms24043710
Zeng X, Wang N, Zhai H, Wang R, Wu J, Pu W (2017) SIRT3 functions as a tumor suppressor in hepatocellular carcinoma. Tumour Biol 39(3):1010428317691178. https://doi.org/10.1177/1010428317691178
Varghese B, Chianese U, Capasso L, Sian V, Bontempo P, Conte M, Benedetti R, Altucci L, Carafa V, Nebbioso A (2023) SIRT1 activation promotes energy homeostasis and reprograms liver cancer metabolism. J Transl Med 21(1):627. https://doi.org/10.1186/s12967-023-04440-9
Tseng HI, Zeng YS, Lin YJ, Huang JW, Lin CL, Lee MH, Yang FW, Fang TP, Mar AC, Su JC (2022) A novel AMPK activator shows therapeutic potential in hepatocellular carcinoma by suppressing HIF1alpha-mediated aerobic glycolysis. Mol Oncol 16(11):2274–2294. https://doi.org/10.1002/1878-0261.13211
Sakurai Y, Kubota N, Takamoto I, Obata A, Iwamoto M, Hayashi T, Aihara M, Kubota T, Nishihara H, Kadowaki T (2017) Role of insulin receptor substrates in the progression of hepatocellular carcinoma. Sci Rep 7(1):5387. https://doi.org/10.1038/s41598-017-03299-3
Gnocchi D, Kurzyk A, Mintrone A, Lentini G, Sabba C, Mazzocca A (2022) Inhibition of LPAR6 overcomes sorafenib resistance by switching glycolysis into oxidative phosphorylation in hepatocellular carcinoma. Biochimie. https://doi.org/10.1016/j.biochi.2022.07.016
Yang X, Lam W, Jiang Z, Guan F, Han X, Hu R, Cai W, Cheng W, Liu SH, Cheng P, Cai Y, Rattray NJW, Johnson CH, Chen L, Cheng YC (2021) YIV-906 potentiated anti-PD1 action against hepatocellular carcinoma by enhancing adaptive and innate immunity in the tumor microenvironment. Sci Rep 11(1):13482. https://doi.org/10.1038/s41598-021-91623-3
Acknowledgements
We are grateful to Ms. Loredana Angela Acquaro for the valuable technical support. We also thank “Naturawas srl” for producing the freeze-dried C. maritimum used for the preparation of the extract.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. This work was supported by the “Tecnonidi” project promoted by Regione Puglia.
Author information
Authors and Affiliations
Contributions
D.G. conceived the article, performed the experiments and wrote the manuscript, D.N. performed the experiments, R.R.P. performed the experiments, C.S. revised the manuscript, and A.M. conceived the article, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
This article has been approved by all authors.
Consent for Publication
This manuscript has not been copyrighted or published elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gnocchi, D., Nikolic, D., Paparella, R.R. et al. Crithmum maritimum Extract Restores Lipid Homeostasis and Metabolic Profile of Liver Cancer Cells to a Normal Phenotype. Plant Foods Hum Nutr 79, 417–424 (2024). https://doi.org/10.1007/s11130-024-01188-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-024-01188-5