Abstract
Background:
Xanthine derivatives have been used to treat a variety of medical conditions including respiratory disease and neural degeneration. However, few studies have reported their effects on bone regeneration. Therefore, we investigated the effects of KPR-A148, a synthetic xanthine derivative on osteoblast differentiation in vitro and bone regeneration in vivo.
Methods:
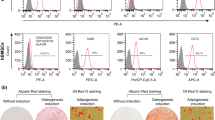
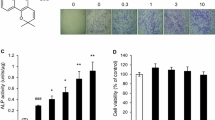
The cytotoxicity of KPR-A148 was evaluated using MC3T3-E1 cells by the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltertrazolium bromide assay. The effects of KPR-A148 on osteoblast differentiation were examined by alkaline phosphatase staining, Alizarin red S staining, and real-time PCR of osteoblast differentiation marker genes. To investigate the effects of KPR-A148 on in vivo bone regeneration, a KPR-A148-containing collagen sponge was implanted into a mouse calvarial defect and KPR-A148 was injected twice, weekly. Bone regeneration was evaluated quantitatively by micro-CT and qualitatively by hematoxylin and eosin, as well as Masson’s Trichrome staining.
Results:
KPR-A148 did not show toxicity in the MC3T3-E1 cells and promoted osteoblast differentiation in a concentration-dependent manner. 10 μM of KPR-A148 showed the most significant effect on alkaline phospatase staining and matrix mineralization. KPR-A148 increased the expression of osteoblast marker genes in both the early and late stages of differentiation. In addition, KPR-A148 significantly induced new bone formation in the calvarial defect model.
Conclusion:
These results demonstrate that KPR-A148 strongly induces osteoblast differentiation and new bone formation. Therefore, it could be used as a potential therapeutic agent for regenerating bone following its destruction by disease or trauma.




Similar content being viewed by others
References
Shivalkar S, Singh S. Solid freeform techniques application in bone tissue engineering for scaffold fabrication. Tissue Eng Regen Med. 2017;14:187–200.
Kashte S, Jaiswal AK, Kadam S. Artificial bone via bone tissue engineering: current scenario and challenges. Tissue Eng Regen Med. 2017;14:1–14.
Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat. 2015;3:95–104.
Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–62.
Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814.
Tollemar V, Collier ZJ, Mohammed MK, Lee MJ, Ameer GA, Reid RR. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016;3:56–71.
Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92:161–8.
Pountos I, Panteli M, Lampropoulos A, Jones E, Calori GM, Giannoudis PV. The role of peptides in bone healing and regeneration: a systematic review. BMC Med. 2016;14:103.
Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD, et al. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharm Des. 2013;19:3420–8.
Balmayor ER. Targeted delivery as key for the success of small osteoinductive molecules. Adv Drug Deliv Rev. 2015;94:13–27.
Kim JA, Yun HS, Choi YA, Kim JE, Choi SY, Kwon TG, et al. Magnesium phosphate ceramics incorporating a novel indene compound promote osteoblast differentiation in vitro and bone regeneration in vivo. Biomaterials. 2018;157:51–61.
Rosemeyer H. The chemodiversity of purine as a constituent of natural products. Chem Biodivers. 2004;1:361–401.
Müller CE, Jacobson KA. Xanthines as Adenosine Receptor Antagonists. In: Fredholm BB, editor. Methylxanthines. Handbook of Experimental Pharmacology, vol 200. Berlin: Springer; 2018. p. 151–99.
Cushley MJ, Holgate ST. Bronchodilator actions of xanthine derivatives administered by inhalation in asthma. Thorax. 1985;40:176–9.
Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–70.
Abdel-Hady H, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World J Clin Pediatr. 2015;4:81–93.
Barnes PJ. Theophylline. Am J Respir Crit Care Med. 2013;188:901–6.
Rother M, Erkinjuntti T, Roessner M, Kittner B, Marcusson J, Karlsson I. Propentofylline in the treatment of Alzheimer’s disease and vascular dementia: a review of phase III trials. Dement Geriatr Cogn Disord. 1998;9 Suppl 1:36–43.
Clough BH, Ylostalo J, Browder E, McNeill EP, Bartosh TJ, Rawls HR, et al. Theobromine upregulates osteogenesis by human mesenchymal stem cells in vitro and accelerates bone development in rats. Calcif Tissue Int. 2017;100:298–310.
Pal S, Porwal K, Khanna K, Gautam MK, Malik MY, Rashid M, et al. Oral dosing of pentoxifylline, a pan-phosphodiesterase inhibitor restores bone mass and quality in osteopenic rabbits by an osteogenic mechanism: a comparative study with human parathyroid hormone. Bone. 2019;123:28–38.
Vashghani Farahani MM, Ahadi R, Abdollahifar M, Bayat M. The effects of pentoxifylline adminstration on fracture healing in a postmenopausal osteoporotic rat model. Lab Anim Res. 2017;33:15–23.
Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, et al. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000;27:811–7.
Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–8.
Lee D, Lee S, Liu KH, Bae JS, Baek DJ, Lee T. Solid-phase synthesis of 1,3,7,8-tetrasubstituted xanthine derivatives on traceless solid support. ACS Comb Sci. 2016;18:70–4.
Beck GR Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–7.
Boswell-Smith V, Cazzola M, Page CP. Are phosphodiesterase 4 inhibitors just more theophylline? J Allergy Clin Immunol. 2006;117:1237–43.
Vignoli JA, Bassoli DG, Benassi MT. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–8.
Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309:689–94.
Liu T, Gao Y, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, et al. BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol. 2007;211:728–35.
Kim JA, Choi YA, Yun HS, Bae YC, Shin HI, Park EK. Extracellular calcium-binding peptide-modified ceramics stimulate regeneration of calvarial bone defects. Tissue Eng Regen Med. 2016;13:57–65.
Acknowledgements
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (MSIT) (NRF-2017M3A9E4047244).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All animal experiments were approved by the committee on the care and use of animals in research at Kyungpook National University, South Korea, and conducted in accordance with the guidelines for the Care and Use of Laboratory Animals (2017-0074).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, S., Kim, J.A., Lee, T. et al. Stimulatory Effects of KPR-A148 on Osteoblast Differentiation and Bone Regeneration. Tissue Eng Regen Med 16, 405–413 (2019). https://doi.org/10.1007/s13770-019-00200-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00200-3