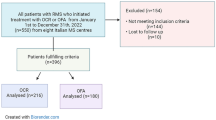
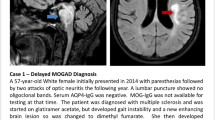
Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system. Although advances have been made in the treatment of MS, such as the use of IFN-β, glucocorticoids and stem cells, the therapeutic effects of these treatments are not sufficient. In the present study, we evaluated whether the combination of methylprednisolone (MP) and human bone marrow-derived mesenchymal stem cells (BM-MSCs) could enhance the therapeutic effectiveness in experimental autoimmune encephalomyelitis (EAE), a model for MS. EAE was induced by immunizing C57BL/6 mice with myelin oligodendrocyte glycoprotein 35-55 (MOG 35-55). The immunized mice received an intraperitoneal injection of MP (20 mg/kg), an intravenous injection of BM-MSCs (1 × 106 cells) or both on day 14 after immunization. Combination treatment significantly ameliorated the clinical symptoms, along with attenuating inflammatory infiltration and demyelination, compared to either treatment alone. Secretion of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-17) was significantly reduced, and anti-inflammatory cytokines (IL-4, IL-10) was significantly increased by the combination treatment as compared to either treatment alone. Flow cytometry analysis of MOG-reactivated T cells in spleen showed that combination treatment reduced the number of CD4+CD45+ and CD8+ T cells, and increased the number of CD4+CD25+Foxp3+ regulatory T cells. Furthermore, combination treatment enhanced apoptosis in MOG-reactivated CD4+ T cells, a key cellular subset in MS pathogenesis. Combination treatment with MP and BM-MSCs provides a novel treatment protocol for enhancing therapeutic effects in MS.







Similar content being viewed by others
References
El Behi M, Dubucquoi S, Lefranc D, Zéphir H, De Seze J, Vermersch P, et al. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Lett. 2005;96:11–26.
Reichardt HM, Gold R, Lühder F. Glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Expert Rev Neurother. 2006;6:1657–70.
Sospedra M, Martin R. Antigen-specific therapies in multiple sclerosis. Int Rev Immunol. 2005;24:393–413.
Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–9.
Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14.
Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–77.
Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245.
Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–25.
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Goodin DS, Frohman EM, Garmany GP Jr, Halper J, Likosky WH, Lublin FD, et al. Disease modifying therapies in multiple sclerosis: report of the therapeutics and technology assessment subcommittee of the american academy of neurology and the MS council for clinical practice guidelines. Neurology. 2002;58:169–78.
Gross-Aviv T, Vago R. The role of aragonite matrix surface chemistry on the chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2009;30:770–9.
Lee HK, Lee BH, Park SA, Kim CW. The proteomic analysis of an adipocyte differentiated from human mesenchymal stem cells using two-dimensional gel electrophoresis. Proteomics. 2006;6:1223–9.
Aleynik A, Gernavage KM, Mourad YSh, Sherman LS, Liu K, Gubenko YA, et al. Stem cell delivery of therapies for brain disorders. Clin Transl Med. 2014;3:24.
Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS). Int J Mol Sci. 2012;13:9298–331.
Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, et al. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917.
Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, et al. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177:1989–2001.
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–19.
Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a meta-analysis of randomized controlled clinical trials. J Neurol. 2000;247:435–42.
Ratzer R, Romme Christensen J, Romme Nielsen B, Sørensen PS, Börnsen L, Sellebjerg F. Immunological effects of methylprednisolone pulse treatment in progressive multiple sclerosis. J Neuroimmunol. 2014;276:195–201.
Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–74.
Pozzilli C, Marinelli F, Romano S, Bagnato F. Corticosteroids treatment. J Neurol Sci. 2004;223:47–51.
Tischner D, Reichardt HM. Glucocorticoids in the control of neuroinflammation. Mol Cell Endocrinol. 2007;275:62–70.
Li XL, Zhang ZC, Zhang B, Jiang H, Yu CM, Zhang WJ, et al. Atorvastatin calcium in combination with methylprednisolone for the treatment of multiple sclerosis relapse. Int Immunopharmacol. 2014;23:546–9.
Ravnborg M, Sørensen PS, Andersson M, Celius EG, Jongen PJ, Elovaara I, et al. Methylprednisolone in combination with interferon beta-1a for relapsing-remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:672–80.
Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407.
Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40.
Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71.
Lv J, Du C, Wei W, Wu Z, Zhao G, Li Z, et al. The antiepileptic drug valproic acid restores T cell homeostasis and ameliorates pathogenesis of experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:28656–65.
Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248:156–69.
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8.
Ivanov VN, Nikolić-Zugić J. Biochemical and kinetic characterization of the glucocorticoid-induced apoptosis of immature CD4+ CD8+ thymocytes. Int Immunol. 1998;10:1807–17.
Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–84.
Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 2013;10:97–105.
Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1 clinical effects. J Neurol Neurosurg Psychiatry. 1987;50:511–6.
Shu YQ, Yang Y, Wang YG, Dai YQ, Xiao L, Qiu W, et al. Combined therapy with methylprednisolone and ulinastatin in experimental autoimmune encephalomyelitis. Chin Med J (Engl). 2013;126:3439–45.
Wei ZS, Hong MF, Su QX, Wang XH, Yu QY, Peng ZX, et al. Super-high-dose methylprednisolone does not improve efficacy or induce glucocorticoid resistance in experimental allergic encephalomyelitis. Neuroimmunomodulation. 2011;18:28–36.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23.
Paintlia AS, Paintlia MK, Singh I, Skoff RB, Singh AK. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2009;57:182–93.
Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65.
Rodríguez-Sáinz Mdel C, Sánchez-Ramón S, de Andrés C, Rodríguez-Mahou M, Muñoz-Fernández MA. Th1/Th2 cytokine balance and nitric oxide in cerebrospinal fluid and serum from patients with multiple sclerosis. Eur Cytokine Netw. 2002;13:110–4.
Nagelkerken L. Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz J Med Biol Res. 1998;31:55–60.
Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11:41–4.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517.
Schmidt-Weber CB, Alexander SI, Henault LE, James L, Lichtman AH. IL-4 enhances IL-10 gene expression in murine Th2 cells in the absence of TCR engagement. J Immunol. 1999;162:238–44.
Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, et al. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol. 2007;190:139–45.
Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7.
Abdul-Majid KB, Wefer J, Stadelmann C, Stefferl A, Lassmann H, Olsson T, et al. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4-/- and CD8-/- DBA/1 mice defines qualitative roles of different T cell subsets. J Neuroimmunol. 2003;141:10–9.
Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–41.
Valencia X, Lipsky PE. CD4+ CD25+ FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–26.
Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J Immunol. 2008;181:4638–47.
Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun. 2014;2:163.
Galligan CL, Pennell LM, Murooka TT, Baig E, Majchrzak-Kita B, Rahbar R, et al. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult Scler. 2010;16:1458–73.
Makar TK, Trisler D, Bever CT, Goolsby JE, Sura KT, Balasubramanian S, et al. Stem cell based delivery of IFN-beta reduces relapses in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;196:67–81.
Tabi Z, McCombe PA, Pender MP. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitis: further evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int Immunol. 1995;7:967–73.
Nguyen KB, McCombe PA, Pender MP. Increased apoptosis of T lymphocytes and macrophages in the central and peripheral nervous systems of Lewis rats with experimental autoimmune encephalomyelitis treated with dexamethasone. J Neuropathol Exp Neurol. 1997;56:58–69.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2054772, 2016R1D1A1B03931146) and by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01004525).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC), the Catholic University of Korea (Permit Number; 2015-0147-01).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, M.J., Ryu, C.H., Kim, S.M. et al. Combined Treatment with Methylprednisolone and Human Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Experimental Autoimmune Encephalomyelitis. Tissue Eng Regen Med 15, 183–194 (2018). https://doi.org/10.1007/s13770-017-0101-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0101-y