Abstract
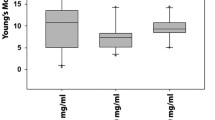
Recent investigations consider adipose-derived stem cells (ASCs) as a promising source of stem cells for clinical therapies. To obtain functional cells with enhanced cytoskeleton and aligned structure, mechanical stimuli are utilized during differentiation of stem cells to the target cells. Since function of muscle cells is associated with cytoskeleton, enhanced structure is especially essential for these cells when employed in tissue engineering. In this study by utilizing a custom-made device, effects of uniaxial tension (1Hz, 10% stretch) on cytoskeleton, cell alignment, cell elastic properties, and expression of smooth muscle cell (SMC) genes in ASCs are investigated. Due to proper availability of ASCs, results can be employed in cardiovascular engineering when production of functional SMCs in arterial reconstruction is required. Results demonstrated that cells were oriented after 24 hours of cyclic stretch with aligned pseudo-podia. Staining of actin filaments confirmed enhanced polymerization and alignment of stress fibers. Such phenomenon resulted in stiffening of cell body which was quantified by atomic force microscopy (AFM). Expression of SM α-actin and SM22 α-actin as SMC associated genes were increased after cyclic stretch while GAPDH was considered as internal control gene. Finally, it was concluded that application of cyclic stretch on ASCs assists differentiation to SMC and enhances functionality of cells.





Similar content being viewed by others
References
Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51–8.
Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem cells. 2012;30:804–10.
Colazzo F, Sarathchandra P, Smolenski RT, Chester AH, Tseng YT, Czernuszka JT, et al. Extracellular matrix production by adipose-derived stem cells: implications for heart valve tissue engineering. Biomaterials. 2011;32:119–27.
Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 2011;7:269–91.
Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb). 2012;4:1008–18.
Panadero J, Lanceros-Mendez S, Ribelles JG. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1–12.
Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–44.
Cardwell RD, Kluge JA, Thayer PS, Guelcher SA, Dahlgren LA, Kaplan DL, et al. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J Biomech Eng. 2015;137:071010.
Carlier MF, Pernier J, Montaville P, Shekhar S, Kühn S. Cytoskeleton dynamics and motility group. Control of polarized assembly of actin filaments in cell motility. Cell Mol Life Sci. 2015;72:3051–67.
Heng BC, Haider HKh, Sim EK, Cao T, Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42.
Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–60.
Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2011;10:939–53.
Tarbell JM, Shi ZD, Dunn J, Jo H. Fluid mechanics, arterial disease, and gene expression. Ann Rev Fluid Mech. 2014;46:591.
Firth AL, Yuan JX-J. Human models for smooth muscle cell differentiation. Focus on “A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells”. Am J Physiol Cell Physiol. 2013;304:C287–8.
Dan P, Velot É, Decot V, Menu P. The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. J Cell Sci. 2015;128:2415–22.
Kurpinski K, Park J, Thakar RG, Li S. Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. Mol Cell Biomech. 2006;3:21–34.
Koobatian MT, Liang MS, Swartz DD, Andreadis ST. Differential effects of culture senescence and mechanical stimulation on the proliferation and leiomyogenic differentiation of MSC from different sources: implications for engineering vascular grafts. Tissue Eng Part A. 2015;21:1364–75.
Yao R, Wong JY. The effects of mechanical stimulation on controlling and maintaining marrow stromal cell differentiation into vascular smooth muscle cells. J Biomech Eng. 2015;137:020907.
Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9.
Mizuno H, Hyakusoku H. Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J Nippon Med Sch. 2003;70:300–6.
Khorasani MT, Mirzadeh H, Kermani Z. Wettability of porous polydimethylsiloxane surface: morphology study. Appl Surf Sci. 2005;242:339–45.
Song G, Ju Y, Soyama H. Growth and proliferation of bone marrow mesenchymal stem cells affected by type I collagen, fibronectin and bFGF. Mater Sci Eng C. 2008;28:1467–71.
Palmer BM, Bizios R. Quantitative characterization of vascular endothelial cell morphology and orientation using Fourier transform analysis. J Biomech Eng. 1997;119:159–65.
Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265–73.
Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–77.
Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2011;168:306–14.
Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–68.
Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA. 2006;103:16095–100.
Li QS, Lee GY, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374:609–13.
Hutter JL, Chen J, Wan WK, Uniyal S, Leabu M, Chan BM. Atomic force microscopy investigation of the dependence of cellular elastic moduli on glutaraldehyde fixation. J Microsc. 2005;219:61–8.
Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72:021914.
Goli-Malekabadi Z, Tafazzoli-Shadpour M, Rabbani M, Janmaleki M. Effect of uniaxial stretch on morphology and cytoskeleton of human mesenchymal stem cells: static vs. dynamic loading. Biomed Tech (Berl). 2011;56:259–65.
Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–92.
Albinsson S, Bhattachariya A, Hellstrand P. Stretch-dependent smooth muscle differentiation in the portal vein—role of actin polymerization, calcium signaling, and microRNAs. Microcirculation. 2014;21:230–8.
Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr Biol (Camb). 2011;3:1063–70.
Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L958–65.
Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–90.
Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–9.
Azeloglu EU, Costa KD. Atomic force microscopy in mechanobiology: measuring microelastic heterogeneity of living cells. Atomic Force Microsc Biomed Res Methods Protoc. 2011;736:303–29.
Rodriguez ML, McGarry PJ, Sniadecki NJ. Review on cell mechanics: experimental and modeling approaches. Appl Mech Rev. 2013;65:060801.
Steward RL Jr, Rosner SR, Fredberg JJ. Emergent behaviors. Cell mechanics structure-based mechanics of tissues and organs. New York: Springer; 2016. p. 41–55.
Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32:344–52.
Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
Adipose tissue is excised from patients during orthopaedic knee surgery with informed consent considering ethical issues.
Rights and permissions
About this article
Cite this article
Rabbani, M., Tafazzoli-Shadpour, M., Shokrgozar, M.A. et al. Cyclic Stretch Effects on Adipose-Derived Stem Cell Stiffness, Morphology and Smooth Muscle Cell Gene Expression. Tissue Eng Regen Med 14, 279–286 (2017). https://doi.org/10.1007/s13770-017-0033-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0033-6