Abstract
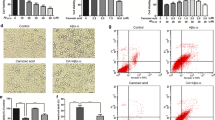
Alpha-linolenic acid (ALA), which is an omega-3 fatty acid from plant oils, has been reported to have beneficial effects on human brain health. However, the protective effect of ALA and its mechanism of action against amyloid beta (Aβ)-mediated neurotoxicity, neuronal apoptosis and amyloid precursor protein (APP) processing are unclear. To investigate the neuroprotective effect of ALA, we treated Aβ25-35-induced SH-SY5Y cells with ALA (1, 2.5, 5 and 25 μg/mL). In our results, Aβ25-35-induced neuronal cell loss was observed, whereas ALA significantly increased the cell viability and decreased lactate dehydrogenase release. In addition, over-production of reactive oxygen species caused by Aβ25-35 was attenuated by treatment with ALA, and these inhibitory activities were mediated by regulation of the mitogen-activated protein kinase signaling pathway. Furthermore, our data shows that Aβ25-35 cause an increase in protein expression of APP-C-terminal fragment β, β-site APP-cleaving enzyme and presenilin-1 in SH-SY5Y cells, while ALA significantly down-regulated the expression of those amyloidogenic APP processing-related proteins. In addition, we confirmed that ALA enhanced α-secretase activity by up-regulating the protein levels of A distintegrin and metalloprotease 10 and tumor necrosis factor-α-converting enzyme, indicating that ALA could promote non-amyloidogenic signaling pathways. ALA also significantly attenuated Aβ25-35-induced neuronal apoptosis by up-regulation of the Bcl-2/Bax ratio. These findings suggest that ALA may be a beneficial agent for promoting prevention of Alzheimer’s disease.







Similar content being viewed by others
References
Selkoe DJ (1994) Normal and abnormal biology of the β-amyloid precursor protein. Annu Rev Neurosci 17:489–517
Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258:126–129
De Strooper B, Annaert W (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 113:1857–1870
Zhang Y, Thompson R, Zhang H, Xu H (2011) APP processing in Alzheimer’s disease. Mol Brain 4:3
Torres M, Forman HJ (2003) Redox signaling and the MAP kinase pathways. BioFactors 17:287–296
McCubrey JA, Lahair MM, Franklin RA (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8:1775–1789
Zhu X, Lee HG, Raina AK, Perry G, Smith MA (2002) The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 11:270–281
Colombo A, Bastone A, Ploia C, Sclip A, Salmona M, Forloni G, Borsello T (2009) JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol Dis 33:518–525
Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M (2008) Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. J Neurochem 104:683–695
Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M (2000) Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 19:1784–1793
Laurin D, Verreault R, Lindsay J, Dewailly É, Holub BJ (2003) Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis 5:315–322
Ruxton CHS, Reed SC, Simpson MJA, Milingtor KJ (2004) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 17:449–459
Das UN (2006) Essential fatty acids—a review. Curr Pharm Biotechnol 7:467–482
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296:1885–1899
Asif M (2011) Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient Pharm Exp Med 11:51–59
Tvrzicka E, Kremmyda L, Stankova B, Zak A (2011) Fatty acids as biocompounds: their role in human metabolism, health and disease- a review. Part 1: classification, dietary sources and biological functions. Biomed Pap 155:117–130
Kim KY, Nam YA, Kim HS, Wallace Hayes A, Lee BM (2014) α-linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol 70:163–178
Gao H, Yan P, Zhang S, Huang H, Huang F, Sun T, Deng Q, Huang Q, Chen S, Ye K, Xu J, Liu L (2016) Long-term dietary alpha-linolenic acid supplement alleviates cognitive impairment correlate with activating hippocampal CREB signaling in natural aging rats. Mol Neurobiol 53:4772–4786
Pan H, Piermartiri TCB, Chen J, McDonough J, Oppel C, Driwech W, Winter K, McFarland E, Black K, Figueiredo T, Grunberg N, Marini AM (2015) Repeated systemic administration of the nutraceutical alpha-linolenic acid exerts neuroprotective efficacy, an antidepressant effect and improves cognitive performance when given after soman exposure. Neurotoxicology 51:38–50
Kim KB, Nam YA, Kim HS, Hayes AW, Lee BM (2014) α-linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol 70:163–178
Cunnane SC, Ganguli S, Menard C, Liede AC, Hamadeh MJ, Chen ZY, Wolever TMS, Jenkins DJA (1993) High α-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr 69:443–453
Lee AY, Choi JM, Lee J, Lee MH, Lee S, Cho EJ (2016) Effects of vegetable oils with different fatty acid compositions on cognition and memory ability in Aβ25-35-induced Alzheimer’s disease mouse model. J Med Food 19:912–921
Lee J, Rodriguez JP, Kim YJ, Lee MH, Cho EJ, Lee S (2016) Fatty acid content in perilla cultivars and commercial oils determined by GC analysis. Nat Prod Sci 22:259–262
Lee J, Lee MH, Cho EJ, Lee S (2016) High-yield methods for purification of α-linolenic acid from Perilla frutescens var. japonica oil. Appl Biol Chem 59:89–94
Cory AH, Owen TC, Barltrop JA, Cory JG (1991) Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 3:207–212
Racher AJ, Looby D, Griffiths JB (1990) Use of lactate dehydrogenase release to assess changes in culture viability. Cytotechnology 3:301–307
Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134:111–116
Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW (1993) Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A 90:7951–7955
Chui DH, Dobo E, Makifuchi T, Akiyama H, Kawakatsu S, Petit A, Checler F, Araki W, Takahashi K, Tabira T (2001) Apoptotic neurons in Alzheimer’s disease frequently show intracellular Aβ42 labeling. J Alzheimers Dis 3:231–239
Behl C (2005) Oxidative stress in Alzheimer’s disease: implications for prevention and therapy. Subcell Biochem 38:65–78
Mattson MP (1995) Free radicals and disruption of neuronal ion homeostasis in AD: a role for amyloid beta-peptide? Neurobiol Aging 16:679–682
Yan SD, Yan SF, Chen X, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, Zweier JL, Stern D (1995) Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid β-peptide. Nat Med 1:693–699
Reddy PH (2006) Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem 96:1–13
Baptista F, Henriques AG, Silva AMS, Wiltfang J, da Cruz e Silva OAB (2014) Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease.ACS Chem. Neurosci. 5:83–92
Choi DY, Lee YJ, Hong JT, Lee HJ (2012) Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res Bull 87:144–153
Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78:431–441
Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lütjohann D, Heerschap A, Tanila H, Kiliaan AJ (2007) Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol Dis 28:16–29
Kim JH, Choi S, Jung JE, Roh EJ, Kim HJ (2006) Capacitative Ca2 + entry is involved in regulating soluble amyloid precursor protein (sAPPαAPPry is involved in regulating soluble amyloid precursor protein (sAPneuroblastoma SH-SY5Y cells. J Neurochem 97:245–254
Zheng L, Roberg F, Jerhammar F, Marcusson J, Terman A (2006) Autophage of amyloid beta- protein in differentiated neuroblastoma cells exposed to oxidative stress. Neurosci Lett 394:184–189
Li YP, Bushnell AF, Lee CM, Perlmutter LS, Wong SKF (1996) β-amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain Res 738:196–204
Shibata N, Kobayashi M (2008) The role for oxidative stress in neurodegenerative disease. Brain Nerve 60:157–170
Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris LP, Smith MA (1999) Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. NeuroReport 10:2411–2415
Ren J, Chung SH (2007) Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J Agric Food Chem 55:5073–5080
Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, Oh KW, Hong JY (2009) Green tea (-)-epigallocatechin-3-gallate inhibits β-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κB pathways in mice. J Nutr 139:1987–1993
Mazzitelli S, Xu P, Ferrer I, Davis RJ, Tournier C (2011) The loss of c-jun N-terminal protein kinase activity prevents the amlyoidogenic cleavage of amyloid precursor protein and the formation of amyloid plaques in vivo. J Neurosci 31:16969–16976
Zhang Y, Xu H (2007) Molecular and cellular mechanisms for Alzheimer’s disease: understanding APP metabolism. Curr Mol Med 7:687–696
Amin FU, Shah SA, Badshah H, Khan M, Kim MO (2017) Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1-42-induced oxidative stress. J Nanobiotechnol 15:12
Marshall AJ, Rattray M, Vaughan PFT (2006) Chronic hypoxia in the human neuroblastoma SH-SY5Y causes reduced expression of the putative α-secretases, ADAM10 and TACE, without altering their mRNA levels. Brain Res 1099:18–24
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Lee K, Lee DH, Jung YJ, Shin SY, Lee YH (2016) The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl Biol Chem 59:193–199
Barcelo-Coblijn G, Murphy EJ (2009) Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res 48:355–374
Simon JA, Fong J, Bernert JT Jr, Browner WS (1995) Serum fatty acids and the risk of stroke. Stroke 26:778–782
Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gorelick PB, Marini AM (2015) Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed Res Int 2015: Article ID: 519830
Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C (2003) Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 38:564–575
Ide T, Kobayashi H, Ashakumary L, Rouyer IA, Takahashi Y, Aoyama T, Hashimoto T, Mizugaki M (2000) Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. BBA-Mol Cell Biol Lipids 1485:23–35
Lee AY, Lee MH, Lee S, Cho EJ (2017) Alpha-linolon of fatty acid oxidation enzymes in rat liver. BBA-Mol Agric Food Chem. https://doi.org/10.1021/acs.jafc.7b03941
Acknowledgment
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (PJ01015603),” Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, A.Y., Lee, MH., Lee, S. et al. Alpha-linolenic acid regulates amyloid precursor protein processing by mitogen-activated protein kinase pathway and neuronal apoptosis in amyloid beta-induced SH-SY5Y neuronal cells. Appl Biol Chem 61, 61–71 (2018). https://doi.org/10.1007/s13765-017-0334-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0334-4