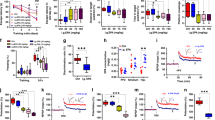
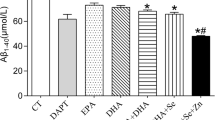
Abstract
Alpha-linolenic acid (ALA) is a major precursor of the essential n-3 polyunsaturated fatty acid (PUFA), whose deficiency alters the structure and function of membranes and induces cerebral dysfunctions. The major purpose of this study was to investigate the protective effect of prolonged ALA intake on cognitive function during natural aging. Female Sprague–Dawley rats aged 6 months were chronically treated with ALA and/or lard per day for 12 months. Regular diet-treated rats, both young and old (4 and 18 months old, respectively) served as controls. Rats fed on regular diet during aging showed memory deficits in Morris water maze, which were further exacerbated by lard intake. However, supplementation with ALA for 12 months dose-dependently improved the performance in spatial working memory tasks. Memory performance correlated well with the activation of cAMP response element-binding protein (CREB) and increases in both levels of brain-derived neurotrophic factor (BDNF) and its specific receptor tyrosine kinase B (TrkB) phosphorylation in the hippocampus. Further study identified that hippocampal extracellular signal-related kinase (ERK) and Akt rather than calcium calmodulin kinase IV (CaMKIV) and protein kinase A (PKA), the upstream signalings of CREB, were also activated by ALA supplement. Moreover, memory improvement was accompanied with alterations of hippocampal synaptic structure and number, suggestive of enhancement in synaptic plasticity. Together, these results suggest that long-term dietary intake of ALA enhances CREB/BDNF/TrkB pathway through the activation of ERK and Akt signalings in hippocampus, which contributes to its ameliorative effects on cognitive deficits in natural aging.






Similar content being viewed by others
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-Linolenic acid
- ANOVA:
-
Analyses of variance
- BDNF:
-
Brain-derived neurotrophic factor
- CaMKIV:
-
Calcium calmodulin kinase IV
- CNS:
-
Central nervous system
- CREB:
-
Cyclic AMP response element-binding protein
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- ERK:
-
Extracellular signal-regulated kinase
- FAME:
-
Fatty acid methyl esters
- FO:
-
Flaxseed oil
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HFD:
-
High-fat diet
- LSD:
-
Least significant difference
- LTM:
-
Long-term memory
- LTP:
-
Long-term potentiation
- MAPK:
-
Mitogenactivated protein kinase
- MUFA:
-
Monounsaturated fatty acid
- MWM:
-
Morris water maze
- PI-3K:
-
Phosphoinositide-3 kinase
- PKA:
-
Protein kinase A
- PKB:
-
Protein kinase B
- PSD:
-
Postsynaptic density
- PUFAs:
-
Polyunsaturated fatty acids
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- S:
-
Synapse
- SC:
-
Synaptic cleft
- SD:
-
Standard deviation
- SFA:
-
Saturated fatty acid
- SV:
-
Synaptic vesicle
- TrkB:
-
Tyrosine kinase B
- UI:
-
Unsaturated index
References
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217
Yeoman M, Scutt G, Faragher R (2012) Insights into CNS ageing from animal models of senescence. Nat Rev Neurosci 13(6):435–445
Kidd PM (2008) Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev 13(2):85–115
Izquierdo I, Bevilaqua LR, Rossato JI et al (2008) The molecular cascades of long-term potentiation underlie memory consolidation of one-trial avoidance in the CA1 region of the dorsal hippocampus, but not in the basolateral amygdala or the neocortex. Neurotox Res 14(2–3):273–294
Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84(1):87–136
VanGuilder HD, Farley JA, Yan H et al (2011) Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis 43(1):201–212
Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13(4):240–250
Carlezonjr W, Duman R, Nestler E (2005) The many faces of CREB. Trends Neurosci 28(8):436–445
Benito E, Barco A (2010) CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33(5):230–240
Josselyn SA, Shi C, Carlezon WJ et al (2001) Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 21(7):2404–2412
Brightwell JJ, Smith CA, Countryman RA et al (2005) Hippocampal overexpression of mutant creb blocks long-term, but not short-term memory for a socially transmitted food preference. Learn Mem 12(1):12–17
Yin JC, Del VM, Zhou H et al (1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81(1):107–115
Xu J, Rong S, Xie B et al (2010) Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J Gerontol A: Biol Med Sci 65A(9):933–940
Song J, Yu J, Tan L (2014) Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol: 1–17
Morris KA, Gold PE (2013) Epinephrine and glucose modulate training-related CREB phosphorylation in old rats: relationships to age-related memory impairments. Exp Gerontol 48(2):115–127
Leite MR, Marcondes SM, de Freitas ML et al (2014) Caffeine and diphenyl diselenide improve long-term memory impaired in middle-aged rats. Exp Gerontol 53:67–73
Zhao H, Li Q, Pei X et al (2009) Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav Brain Res 201(2):311–317
Wu A, Ying Z, Gomez-Pinilla F (2004) The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 19(7):1699–1707
Molteni R, Barnard RJ, Ying Z et al (2002) A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112(4):803–814
Porquet D, Casadesús G, Bayod S et al (2013) Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 35(5):1851–1865
Blondeau N, Nguemeni C, Debruyne DN et al (2009) Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology 34(12):2548–2559
Feng Z, Zou X, Jia H et al (2012) Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: possible role of neuronal mitochondria metabolism. Antioxid Redox Signal 16(3):275–289
Zhang W, Hu X, Yang W et al (2010) Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke 41(10):2341–2347
Kidd PM (2007) Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev 12(3):207–227
Coblijn GB (2008) Flaxseed oil and fish-oil capsule consumption alters human red blood cell n – 3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr 88(3):801–809
Su H (2010) Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J Nutr Biochem 21(5):364–373
Rao JS, Ertley RN, Lee HJ et al (2007) n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry 12(1):36–46
Williams CM, El MM, Vauzour D et al (2008) Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med 45(3):295–305
Xu J (2009) Procyanidins extracted from the lotus seedpod ameliorate scopolamine-induced memory impairment in mice. Phytother Res 23(12):1742–1747
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25(4):402–408
Yehuda S, Rabinovitz S, Carasso RL et al (2002) The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 23(5):843–853
Logan AC (2003) Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev 8(4):410–425
Hashimoto M, Tanabe Y, Fujii Y et al (2005) Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr 135(3):549–555
Kim KB, Nam YA, Kim HS et al (2014) Alpha-linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol 70:163–178
Pepe M, Recchia FA (2010) Omega-3 fatty acids for the prevention of myocardial infarction and arrhythmias. Cardiovasc Ther 28(4):e1–e4
Barcelo-Coblijn G, Murphy EJ (2009) Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res 48(6):355–374
Ledesma MD, Martin MG, Dotti CG (2012) Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Prog Lipid Res 51(1):23–35
Petursdottir AL, Farr SA, Morley JE et al (2008) Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J Gerontol A Biol Sci Med Sci 63(11):1153–1160
Bekinschtein P, Cammarota M, Izquierdo I et al (2007) Reviews: BDNF and memory formation and storage. Neuroscientist 14(2):147–156
Simon W, Hapfelmeier G, Kochs E et al (2001) Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology 94(6):1058–1065
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35(4):605–623
Eckert GP, Franke C, Noldner M et al (2010) Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Pharmacol Res 61(3):234–241
Stackman RW, Blasberg ME, Langan CJ et al (1997) Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem 67(2):167–171
Acknowledgments
We thank Mengjing Xu (research fellow), Yilin Jin (research fellow), and Shuke Nie (doctoral student) for helping raise the animals.
Role of Funding Source
This work was supported by the National Natural Science Foundation of China (NSFC-31171681 and NSFC-81472978) and the earmarked fund for Modern Agro-industry Technology Research System (CARS-17), China. The funding source had no further role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Contributors
Author Hui Gao designed and wrote a first draft of the paper. Peipei Yan and Hao Huang carried out all the experiments. Shun Zhang and Fenghong Huang undertook the statistical analysis and created the figures. Taoping Sun, Sijing Chen, and Qianchun Deng performed dissection procedures. Qingde Huang managed the literature searches. Keqiang Ye assisted with the preparation and proof-reading of the manuscript. Jiqu Xu and Liegang Liu contributed to the design of the study, reviewed the manuscript, and contributed to the final version. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors can identify no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, H., Yan, P., Zhang, S. et al. Long-Term Dietary Alpha-Linolenic Acid Supplement Alleviates Cognitive Impairment Correlate with Activating Hippocampal CREB Signaling in Natural Aging Rats. Mol Neurobiol 53, 4772–4786 (2016). https://doi.org/10.1007/s12035-015-9393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9393-x