Abstract
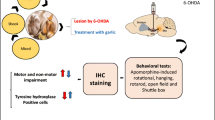
Hyoscyamus niger (L), of Solanaceae family, commonly known as henbane is used in the traditional Indian medical system of Ayurveda and Chinese system of medicine for the nervous system disorders. We have evaluated neuroprotective potential of methanol extract of Hyoscymus Niger (MHN) seeds in stereotaxically induced rotenone model of Parkinson’s disease in rats. MHN was characterized employing HPLC-UV and LCMS. The extract showed presence of L-dopa with significant inhibition in DPPH, ABTS in-vitro assay and monoamine oxidase activity. Male Wistar rats were pretreated with MHN (125, 250, 500 mg/kg body weight p.o.) once daily for 7 days and subjected to unilateral intrastriatal injection of rotenone (8 μg in 0.1 % ascorbic acid in normal saline). Three weeks after rotenone infusion, rats were tested for neurobehavioral activity and were sacrificed for estimation of lipid peroxidation (TBARS), total glutathione (GSH) content, and activity of antioxidant enzymes glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) in brain homogenates. Administration of the MHN (containing L-DOPA) significantly attenuated motor disabilities (actophotometer, rotarod and Morris water maze test). Rat treated with rotenone showed reduced levels of thiobarbituric acid reactive substance (TBARS) and increased level of GSH content and antioxidants enzymes activities (GPX, SOD and CAT) in the MHN treated PD rat. The findings suggest that MHN is a potential drug for treating oxidative damage, physiological abnormalities and is effective in neuroprotection in experimental models of PD.









Similar content being viewed by others
References
Ahmad M, Saleem S et al (2005) Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol 24(30):137–147
Alam M, Schmidt W (2000) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324
Begum S, Saxena B et al (2010) Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and isolation of a new coumarinolignan. Fitoterapia 81(3):178–184
Betarbet R, Sherer TB et al (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Chaturvedi R, Shukla S et al (2006) Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol Dis 22(2):421–434
Dhir A, Akula KK et al (2008) Tiagabine, a GABA uptake inhibitor, attenuates 3-nitropropionic acid-induced alterations in various behavioral and biochemical parameters in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 32(3):835–843
Emborg ME (2004) Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J Neurosci Methods 139(2):121–143
Eriksen JL, Petrucelli L (2004) Parkinson’s disease–molecular mechanisms of disease. Drug Discov Today Dis Mech 1(4):399–405
Gacche R, Shaikh R et al (2011) Kinetics of inhibition of monoamine oxidase using Cymbopogon martinii (Roxb.) Wats: a potential antidepressant herbal ingredient with antioxidant activity. Indian J Clin Biochem 26(3):303–308
Gaur V, Aggarwal A et al (2009) Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol 616(1–3):147–154
Gilani AH, Khan A et al (2008) Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fundam Clin Pharmacol 22(1):87–99
Green AL, Haughton TM (1961) A colorimetric method for the estimation of monoamine oxidase. Biochem J 78(1):172–175
Gülçın İ, Oktay M et al (2003) Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem 83(3):371–382
Hritcu L, Foyet HS et al (2011) Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J Ethnopharmacol 137(1):585–591
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Jollow DJ, Mitchell JR et al (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169
Katzung BG (2001) Basic and clinical pharmacology. 8th edn. International Edition. Long Medical Books, McGraw-Hill. New York.
Khatri DK, Juvekar P et al (2013) Phytochemical investigation and in vitro antioxidant activities indigofera cordifolia seed extracts. Int J Pharm Pharm Sci 5(2):71–75
Kirtikar K, Basu B (1988) Indian Medicinal Plants. vol. 2. International Book Distributors, Dehra Dun.
Lowry OH, Rosebrough NJ et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma CY, Liu WK et al (2002) Lignanamides and Nonalkaloidal Components of Hyoscyamus niger Seeds. J Nat Prod 65(2):206–209
Mandel SA, Amit T et al (2008) Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr 138(8):1578S–1583S
Manyam BV, Dhanasekaran M et al (2004) Effect of antiparkinson drug HP‐200 (Mucuna pruriens) on the central monoaminergic neurotransmitters. Phytother Res 18(2):97–101
Meissner W, Hill MP et al (2004) Neuroprotective strategies for Parkinson’s disease: conceptual limits of animal models and clinical trials. Trends Pharmacol Sci 25(5):249–253
Mohanasundari M, Srinivasan M et al (2006) Enhanced neuroprotective effect by combination of bromocriptine and Hypericum perforatum extract against MPTP-induced neurotoxicity in mice. J Neurol Sci 249(2):140–144
Nagashayana N, Sankarankutty P et al (2000) Association of L-DOPA with recovery following Ayurveda medication in Parkinson’s disease. J Neurol Sci 176(2):124–127
Oboh G, Rocha J (2008) Antioxidant and Neuroprotective Properties of Sour Tea (Hibiscus sabdariffa, calyx) and Green Tea (Camellia sinensis) on some Pro-oxidant-induced Lipid Peroxidation in Brain in vitro. Food Biophys 3(4):382–389
Pakkenberg B, Møller A et al (1991) The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry 54(1):30–33
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates, 4th edn. Academic, California
RajaSankar S, Manivasagam T et al (2009) Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol 125(3):369–373
Ramassamy C (2006) Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 545(1):51–64
Re R, Pellegrini N et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Rozas G, Garcia JL (1997) Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated rotarod test. Brain Res 749(2):188–99
Sankar SR, Manivasagam T et al (2007) The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: An analysis of behavioral and biochemical varibles. Cell Mol Biol Lett 12(4):473–81
Satav JG, Katyare SS (1982) Effect of experimental thyrotoxicosis on oxidative phosphorylation in rat liver, kidney and brain mitochondria. Mol Cell Endocrinol 28(2):173–89
Schuler F, Casida JE (2008) Functional coupling of PSST and ND1 subunits in NADH: ubiquinone oxidoreductase established by photoaffinity labeling. Biochim Biophys Acta 1506(1):79–87
Singer TP, Gutman M (1971) The DPNH dehydrogenase of the mitochondrial respiratory chain. Adv Enzymol Relat Areas Mol Biol 34:79–153
Singh N, Pillay V et al (2007) Advances in the treatment of Parkinson’s disease. Prog Neurobiol 81(1):29–44
Su J, Sripanidkulchai K et al (2010) Curcuma comosa improves learning and memory function on ovariectomized rats in a long-term Morris water maze test. J Ethnopharmacol 130(1):70–75
Swarnkar S, Singh S et al (2010) A study to correlate rotenone induced biochemical changes and cerebral damage in brain areas with neuromuscular coordination in rats. Toxicology 272(1-):17–22
Talpade DJ, Greene JG et al (2000) In vivo labeling of mitochondrial complex I (nadh: ubiquinone oxidoreductase) in rat brain using [3H] dihydrorotenone. J Neurochem 75(6):2611–2621
Turski W, Turska E (1973) Modification of spectrophotometric method of determination of monoamine-oxidase. Enzyme 14:211–20
Uttara B, Singh AV et al (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74
Uversky VN (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318(1):225–241
Yamaguchi S, Sakurada S et al (1994) Role of intracellular SOD in protecting human leukemic and cancer cells against superoxide and radiation. Free Radic Biol Med 17(5):389–95
Acknowledgments
Authors are thankful to the Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, India for providing all the necessary facilities to conduct this research. The authors are grateful to the University Grants Commission (UGC), New Delhi, India for financial support (Grant No. F. 38-4/2010 (SA-III)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
“All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
Committee for the Purpose of Control and Supervision on Experimentation on Animals (CPCSEA) “All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.”
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khatri, D.K., Juvekar, A.R. Propensity of Hyoscyamus niger seeds methanolic extract to allay stereotaxically rotenone-induced Parkinson’s disease symptoms in rats. Orient Pharm Exp Med 15, 327–339 (2015). https://doi.org/10.1007/s13596-015-0202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-015-0202-x