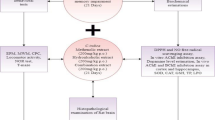
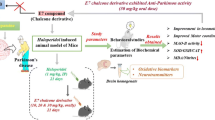
Abstract
Parkinsonism is a neurodegenerative disease, mainly imbalance in dopamine and acetylcholine neurotransimitter in mid brain, which manifestation of dysfunctions of extrapyramidal like akinesia, tremor, rigidity and catalepsy etc., even cognitive and memory loss. The current study is framed to evaluate the effect of Vitex negundo (VNL) leaf extract in Haloperidol induced PD in rats. In vitro studies of antioxidant capacity were checked via DPPH and NO assays and identified its Acetylcholinesterase (AChE) inhibitory activity. Secondly the In vivo study of anti-PD activity in Haloperidol induced in rats were evaluated by Rotarod, morris water maze (MWM), cooks pole climb (CPC), actophotometer, novel object recognition (NOR), and T-maze were utilized to assess extrapyramidal, cognitive and memory function. Thirdly, changes in biomarker level viz. (AChE), butyrylcholinesterase. (BChE) in hippocampus and cortex, reduced glutathione (GSH), malondialdehyde (MDA), total protein (TP), superoxide dismutase (SOD), catalase (CAT), and dopamine level in the whole brain were measured. Finally, histopathology of hippocampus and cortex was examined at 40x magnification to access restoring integrity and maintaining the architecture of neuronal cell in the treatment group compared to control group and L-DOPA as a standard treatment group. V. negundo showed potent antioxidant potency on scavenging of DPPH (IC50 84.81 μg/ml) and NO (IC50 133.20 μg/ml) and possess AChE inhibitory potency (IC50 114.35 μg/ml) by in vitro studies. The Rotarod, MWM, CPC, Actophotometer, NOR, T-maze demonstrated that Haloperidol group administration declines performance time, ELT, TL and decreases locomotion, cognitive and memory respectively. The treatment of VNL 100, 200, and 400 mg/kg p.o. significantly (p < 0.05 to p < 0.0001) reversed. Whole brain AChE, BChE, and MDA level were significantly raised and GSH, TP, SOD, CAT and Dopamine were significantly declined in Haloperidol treated group rats, especially V. negundo 400 mg/kg p.o. highly significantly ameliorate the Haloperidol group altered pathological changes through the restoration of the cholinergic function, enhancing the antioxidant defense and by increasing the dopaminergic function. The current study provides validation of V. negundo for its anti-PD activity and could be a valuable source for the treatment of PD in future.
Graphical abstract












Similar content being viewed by others
Data availability
The data are not publicly available due to their containing information that could compromise the privacy of research participants, but are available on request from the author.
Abbreviations
- V. negundo :
-
V. negundo
- VNL100 :
-
V. negundo 100 mg/kg Group
- VNL200 :
-
V. negundo 200 mg/kg Group
- VNL400 :
-
V. negundo 400 mg/kg Group
- ACh :
-
Acetylcholine
- AChE :
-
Acetylcholinesterase
- AChI :
-
Acetylthiocholine iodide
- BChI :
-
Butyrylcholines iodide
- AD :
-
Alzheimer’s Disease
- PD :
-
Parkinson’s Disease
- APP :
-
Amyloid Precursor Protein
- Aβ :
-
Amyloid Beta
- BCh :
-
Butyrylcholine
- BChE :
-
Butyrylcholinesterase
- DPPH :
-
2,2-diphenyl-1-picrylhydrazyl
- DTNB :
-
5,5′-dithio-bis(2-nitrobenzoic acid)
- ELT :
-
Escape Latency Time
- GSH :
-
Glutathione
- H2O2 :
-
Hydrogen Peroxide
- i.p:
-
Intraperitoneal
- IC50 :
-
Inhibition Concentration
- LPO :
-
Lipid Peroxidation
- MDA :
-
Malondialdehyde
- MWM :
-
Morris Water Maze
References
Aiyalu R, Govindarajan A, Ramasamy A (2015) Acute and sub-chronic toxicity study of methanol leaf extract of Cardiospermum halicacabum L and Vitex negundo L in rats. Pharmacog Commun 5(1):51–57
Alam MI, Gomes A (2003) Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol 86(1):75–80. https://doi.org/10.1016/s0378-8741(03)00049-7
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Biradar P, Patil V, Joshi H, Khanal P, Mallapur S et al (2020) Experimental validation and network pharmacology evaluation to decipher the mechanism of action of Erythrina variegata L. bark against scopolamine-induced memory impairment in rats. Adv Traditional Med:1–4
Brieger K, Schiavone S, Miller FJ, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659
Cacabelos R (2007) Donepezil in Alzheimer's disease: from conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat 3(3):303–333
Claiborne A. et.al.,(1985) Catalase activity. In: Greenwald RA, editor. CRC hand book of methods for oxygen radical research. Boca Raton: CRC Press;p. 283–284
Cole E, Simundic A, Mossa FP et al (2019) Assessing object-recognition memory in rats: pitfalls of the existent tasks and the advantages of a new test. Learn Behav 47:141–155. https://doi.org/10.3758/s13420-018-0347-9
Dharmasiri M, Jayakody J, Galhena G, Liyanage S, Ratnasooriya W (2003) Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol 87(2):199–206
Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med Cell Longev 1245049. https://doi.org/10.1155/2016/1245049
Duyu T, Khanal P, Khatib NA, Patil BM (2020) Mimosa pudica modulates neuroactive ligand receptor interaction in Parkinson’s disease. Indian J Pharm Educ 54(3):732–9
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Finberg JPM, Rabey JM (2016) Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front Pharmacol 7:340
Gandhi KR, Saadabadi A, Levodopa (L-Dopa). (2021) StatPearls [internet]. Stat Pearls Publishing, Treasure Island
Gill BS, Mehra R, Navgeet KS (2018) Vitex negundo and its medicinal value. Mol Biol Rep 45(6):2925–2934. https://doi.org/10.1007/s11033-018-4421-3
Gourie-Devi M, Ramu MG, Venkataram BS (1991) Treatment of parkinson's disease in 'ayurveda' (ancient Indian system of medicine): discussion paper. J R Soc Med 84(8):491–492
Goverdhan P, Sravanthi A, Mamatha T (2012) Mar neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 22:2012
Greenland JC, Barker RA (2018) The differential diagnosis of Parkinson’s disease. In: Stoker TB, Greenland JC, editors. Parkinson’s disease: pathogenesis and clinical aspects [internet]. Brisbane (AU): Codon Publications; Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536715/. https://doi.org/10.15586/codonpublications.parkinsonsdisease.2018.ch6
Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW (1994) The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 11(2):187–96
Hu M, Li F, Wang W (2018) Vitexin protects dopaminergic neurons in MPTP-induced Parkinson’s disease through PI3K/Akt signaling pathway. Drug Design Develop Ther 12:565–573
Ishola IO, Ikuomola BO, Adeyemi OO (2018) Protective role of Spondias mombin leaf and Cola acuminata seed extracts against scopolamine- induced cognitive dysfunction. Alexandria J Med 54(1):27–39
Kamila S, Madhav NS, Sarkar CN (2014) Pharmacological evaluation of transcranially applied lignocaine gel as a novel amnesic agents on rodents. Int J Pharm Sci Invent 3(7):13–21
Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS, Choi DK (2012) The role of free radicals in the aging brain and Parkinson’s disease: convergence and parallelism. Int J Mol Sci 13(8):10478–10504
Lane RM, Potkin SG, Enz A (2006) Targeting acetylcholinesterase and butyrylcholinesterase indementia. Int J Neuropsychopharmacol 9(1):101–124
Li P, Snyder GL, Vanover KE (2016) Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem 16(29):3385–3403. https://doi.org/10.2174/1568026616666160608084834
Luo D, Reith M, Dutta AK (2020) Dopamine agonists in treatment of Parkinson’s disease: an overview. Diagnosis Manag Park Dis 445–60
Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a Mini review. Oxidative Med Cell Longev 2016:1–15
Mazzoni P, Shabbott B, Cortés JC (2012) Motor control abnormalities in Parkinson's disease. Cold Spring Harb Perspect Med 2(6):a009282. https://doi.org/10.1101/cshperspect.a009282
Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA (2012) Parkinson’s disease. Subcell Biochem 65:389
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a sample assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moisan F, Kab S, Mohamed F, Canonico M, Le Guern M, Quintin C et al (2016) Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J Neurol Neurosurg Psychiatry 87(9):952–957 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26701996
Naidu PS, Singh A, Kaur P, Sandhir R, Kulkarni SK (2003) Possible mechanism of action in melatonin attenuation of haloperidol-induced orofacial dyskinesia. Pharmacol Biochem Behav 74(3):641–648
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by Thiobarbituric acid reaction. Anal Biochem 358:351–358
Otari KV, Bichewar OG, Shete RV, Upasani CD (2012) Effect of hydroalcoholic extract of Vitex negundo Linn. Leaves on learning and memory in normal and cognitive deficit mice. Asian Pac J Trop Biomedicinevol 2(1):S104–S111
Pandey MM, Rastogi S, Rawat AKS (2013) Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evidence-Based Complement Altern Med 2013:1–12 Available from: http://www.hindawi.com/journals/ecam/2013/376327/
Pfeiffer EF, Laube H (1974) Obesity and diabetes mellitus. Adv Metab Disord 7:243–255 Available from: http://www.ncbi.nlm.nih.gov/pubmed/4606857
Rahiman RA, Rajan N, Sreekumaran E (2015) Neuroprotective effect of vitex negundo against scopolamine induced cognitive impairment and oxidative stress in Wistar albino rats. Biosci Biotechnol Res Asia 12:301–307
Rahman S, Marwaha R (2021) Haloperidol. [Updated 2021 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560892/
Ramakrishnan P, Chandrasekhar T, Muralidharan P (2015) Cognitive enhancing, anti acetylcholinesterase, and antioxidant properties of Tagetes patula on scopolamine induced amnesia in mice. Int J Green Pharm 167–74
Rascol O (1999) Dopamine agonists: what is the place of the newer compounds in the treatment of Parkinson's disease? J Neural Transm Suppl 55:33–45. https://doi.org/10.1007/978-3-7091-6369-6_4
Rizek P, Kumar N, Jog MS (2016) An update on the diagnosis and treatment of Parkinson disease. CMAJ 188(16):1157–1165
Roberts HC, Syddall HE, Butchart JW, Stack EL, Cooper C, Sayer AA (2015) The Association of Grip Strength with Severity and Duration of Parkinson's: a cross-sectional study. Neurorehabil Neural Repair 29(9):889–896. https://doi.org/10.1177/1545968315570324
Schwarting RK, Borta A (2005) Analysis of behavioral asymmetries in the elevated plus-maze and in the T-maze. J Neurosci Methods 141(2):251–260. https://doi.org/10.1016/j.jneumeth.2004.06.013
Sedlak J, Lindsay RH et al (1968) Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25:192–205
Shalavadi MH, Chandrashekhar VM, Avinash SP, Sowmya C, Ramkishan A (2012) Neuroprotective activity of Stereospermum suaveolens DC against 6-OHDA induced Parkinson's disease model. Indian J Pharmacol 44(6):737
Singh RC. Prasad M, Dadhich OP (2018) Efficacy of kampavatari rasa in kampavata w.s.r. to parkinson’s disease. Journal of Ayurveda XII(4): 4–10
Sonne J, Reddy V, Beato MR (2021) Neuroanatomy, Substantia Nigra. [Updated 2020 Nov 8]. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536995/
Tandon V, Gupta RK (2004) Histomorphological changes induced by Vitex negundo in albino rats. Indian J Pharmacol 36(3):176
Tønjum T, Bukholm G, Bøvre K (1989) Differentiation of some species of Neisseriaceae and other bacterial groups by DNA-DNA hybridization. APMIS 97(5):395–405. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2730785
Triarhou LC (2013) Dopamine and Parkinson's disease. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6271
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. https://doi.org/10.1038/nprot.2006.116
Wenk GL (1998) Assessment of spatial memory using the T maze. Current protocols in neuroscience 4(1):8–5
Acknowledgements
Thanks to the Prof.(Dr) Sunil S Jalapure, Principal and Prof (Dr.) N A Khatib, HOD, Department of Pharmacology and Toxicology, KLE College of Pharmacy, KAHER, Belagavi, India for providing necessary facilities to conduct the work. The authors thank the Vamsi Labs Ltd. Solapur, India for providing Haloperidol and Micro Labs Ltd. Bangalore for providing L-DOPA as a gift sample.
Funding
This study has not having Funding from any Agencies.
Author information
Authors and Affiliations
Contributions
Prakash R. Biradar supervised the experiments, confirmed the findings, helped in data analysis, helped in the manuscript writing. Aishwarya Vannur design the study, conducted the experiment, collected the data, drafted the manuscript. Vishal S. Patil statistical analysis and helped in manuscript drafting.
Corresponding author
Ethics declarations
Ethical statement
The study protocol was reviewed and approved by the Institutional Animal Ethical Committee, KLE College of Pharmacy, KAHER, Belagavi, and Resolution No. KLECOP/CPCSEAReg. No. 221/Po/Re/S/2000/CPCSEA, Res. 25–13/10/2020. The animal experiments were carried out in accordance with the CPCSEA guidelines.
Conflict of interest
All the authors of this manuscript confirm that they do not have any conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Vannur, A., Biradar, P.R. & Patil, V. Experimental validation of Vitex negundo leaves hydroalcoholic extract for neuroprotection in haloperidol induced parkinson’s disease in rat. Metab Brain Dis 37, 411–426 (2022). https://doi.org/10.1007/s11011-021-00878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00878-2