Abstract
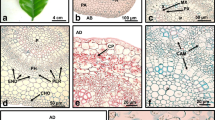
Plant tissue culture allows the maintenance of species of interest under controlled conditions. There have been few studies related to the morphological changes that take place during callus formation and redifferentiation processes. The aim of this work was to analyze the morphological changes during dedifferentiation and redifferentiation processes in leaf explants of marigold (Tagetes erecta L.) using transmission electron microscopy, digital image analysis and concepts of fractal dimension. Leaf explants were sown in MS medium supplemented with 2.0 mg L−1 of benzyladenine and 2.0 mg L−1 of 2,4-dichlorophenoxyacetic acid to induce dedifferentiation (callus formation). The redifferentiation process (bud formation) was obtained with explants sown in MS medium supplemented with 3.0 mg L−1 indoleacetic acid and 0.5 mg L−1 benzyladenine. During the first 8 days, the dedifferentiation process showed gradual changes in terms of the area and perimeter of the explants. The fractal dimension of the perimeter corresponding to the projected image of the calli changed from smooth to rough at day 20. These features indicate variations in tissue morphometry as a result of the callus formation process and further formation of cellular aggregates. Structural observations during the dedifferentiation process showed changes in shape and cellular organization as callus formation became evident. Round-shaped cells were observed in the callus, with few organelles and a low degree of differentiation. Indirect organogenesis was achieved over a period of 10 days; in addition, plantlets developed from the obtained buds in 20 days in growth regulator-free MS medium.





Similar content being viewed by others
References
Arenas-Ocampo M, Alamilla-Beltrán L, Vanegas-Espinoza PE, Camacho-Díaz BH, Campos-Mendiola R, Gutiérrez-López G, Jiménez-Aparicio A (2012) Fractal morphology of Beta vulgaris L. cell suspension culture permeabilized with triton X-100. Int Agrophys 26:1–6. https://doi.org/10.2478/v10247-012-0001-2
Ascough GD, Novák O, Pencik A, Rolcík J, Strnad M, Edwin JE, Van Staden J (2009) Hormonal and cell division analyses in Watsonia lepida seedlings. J Plant Physiol 166:1497–1507. https://doi.org/10.1016/j.jplph.2009.03.009
Belarmino MM, Abe T, Sasahara T (1992) Callus induction and plant regeneration in African marigold (Tagetes erecta L.). Jpn J Breed 42:835–841
Bespalhok JC, Hattori K (1998) Friable embryogenic callus and somatic embryogenic formation from cotyledon explants of African marigold (Tagetes erecta L.). Plant Cell Rep 17:870–875
Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13:171–201. https://doi.org/10.1146/annurev.cellbio.13.1.171
Cosgrove DJ (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38:109–124. https://doi.org/10.1016/S0981-9428(00)00164-9
Del Pozo JC, López-Matas A, Ramírez-Parra E, Gutiérrez C (2005) Hormonal control of the plant cell cycle. Physiol Plant 123:173–183. https://doi.org/10.1111/j.1399-3054.2004.00420.x
Del Villar-Martínez AA, Serrato-Cruz MA, Solano-Navarro A, Arenas-Ocampo ML, Quintero-Gutiérrez AG, Sánchez-Millán JL, Evangelista-Lozano S, Jiménez-Aparicio AR, García-Jiménez FA, Vanegas-Espinoza PE (2007) Carotenoides en Tagetes erecta L. La modificación genética como alternativa. Rev Fitotec Mex 30:109–118
Del Villar-Martínez AA, Vanegas-Espinoza PE, Paredes-López O (2010) Marigold regeneration and molecular analysis of carotenogenic genes. In: Ochatt SJ, Mohan Jain S (eds) Protocols for in vitro propagation of ornamental plants. Humana Press, Totowa, pp 213–219
Delgado-Vargas F, Jiménez AR, Paredes-López O (2000) Natural pigments: Carotenoids, anthocyanins and betalains. Characteristics biosynthesis, processing and stability. Crit Rev Food Sci 40:173–289. https://doi.org/10.1080/10408690091189257
Evert RF, Esau K (2008) Esau. Anatomía Vegetal. Ediciones Omega S. A., Barcelona
Ferreira S, Batista D, Serrazina S, Salomé PM (2009) Morphogenesis induction and organogenic nodule differentiation in Populus euphratica Oliv. leaf explants. Plant Cell Tiss Org 96:35–43. https://doi.org/10.1007/s11240-008-9457-y
Guzmán-Maldonado SH, Paredes-López O (1998) Functional products of plants indigenous to Latin America: Amaranth, quinoa, common beans, and botanicals. In: Mazza G (ed) Functional foods. Biochemical and processing aspects. Technomic Publishing Lancaster, Pennsylvania
Hedayat M, Abdi GH, Khosh-Khui M (2009) Regeneration via direct organogenesis from leaf and petiole segments of pyrethrum (Tanacetum cinerariifolium (Trevir.) Schultz-Bip.). Amer-Eurasian J Agric Environ Sci 6:81–87
Kothari SL, Chandra N (1984) In vitro propagation of African marigold. HortScience 19:703–705
Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N (2010) Chilli peppers—a review on tissue culture and transgenesis. Biotechnol Adv 28:35–48. https://doi.org/10.1016/j.biotechadv.2009.08.005
Kutschera U (2000) Review cell expansion in plant development. Rev Bras Fisiol Veg 12:65–95
Martínez-Pulido C, Harry IS, Thorpe TA (1992) Optimization of bud induction in cotyledonary explants of Pinus canariensis. Plant Cell Tiss Org 29:247–255
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599. https://doi.org/10.1105/tpc.11.4.587
Misra P, Datta SK (2001) Direct differentiation of shoots buds in leaf segments of white marigold (Tagetes erecta L.). In Vitro Cell Dev Biol-Plant 37:366–470. https://doi.org/10.1079/IVP2001195
Mohamed MA-H, Harris PJC, Henderson J (1999) An efficient in vitro regeneration protocol for Tagetes minuta. Plant Cell Tiss Org 55:211–215
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:472–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ntui VO, Thirukkumaran G, Iioka S, Mii M (2009) Efficient plant regeneration via organogenesis in ‘‘Egusi’’ melon (Colocynthis citrullus L.). Sci Hortic 119:397–402. https://doi.org/10.1016/j.scienta.2008.08.031
Olmos S, Luciano G, Galdeano E (2004) Micropropagación, biotecnología y mejoramiento vegetal. In: Echenique V, Rubinstein C, Mroginski L (eds) Biotecnología y Mejoramiento Vegetal. INTA Instituto Nacional de Tecnología Agropecuaria, Buenos Aires, pp 161–172
Orbán N, Boldizár I, Bóka K (2007) Structural and chemical study of callus formation from leaves of Rubia tinctorium. Biol Plant 51:421–429. https://doi.org/10.1007/s10535-007-0091-z
Radice S (2004) Morfogénesis in vitro. In: Echenique V, Rubinstein C, Mroginski L (eds) Biotecnología y Mejoramiento Vegetal. INTA Instituto Nacional de Tecnología Agropecuaria, Buenos Aires, pp 25–33
Rout GR, Mohapatra A, Mohan-Jain S (2006) Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol Adv 24:531–560. https://doi.org/10.1016/j.biotechadv.2006.05.001
Salisbury FB, Ross CW (1992) Plant physiology. Wadsworth Publishing Company, Belmont, pp 1–26
Sánchez-Hernández MA, Sánchez-Hernández C, Villanueva-Verduzco C, Gil-Vázquez I, Jiménez-Rojas MC, Sánchez-Cabrera I (2009) Multiplicación in vitro vía organogénesis en calabaza. Agron Mesoam 20:11–22
Segura J (2000) Introducción al desarrollo, concepto de hormona vegetal. In: Azcón-Bieto J, Talón M (eds) Fundamentos de Fisiología Vegetal. McGraw Hill Interamericana, Madrid, pp 285–303
Segura LS, Pérez JJC, Mendiola RC, Ocampo MLA, Jiménez-Aparicio AR (2011) Cell dynamics growth in Beta vulgaris L. culture in vitro using digital image analysis and fractal dimension. [Dinámica de crecimiento celular de Beta vulgaris L. cultivada in vitro mediante análisis digital de imágenes y de la dimensión fractal]. Interciencia 36:392–396
Sugiyama M (1999) Organogenesis in vitro. Plant Biol 2:61–64
Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19:800–811. https://doi.org/10.1016/j.cub.2009.07.056
Vanegas PE, Cruz-Hernández A, Valverde ME, Paredes-López O (2002) Plant regeneration via organogenesis in marigold. Plant Cell Tiss Org Cult 69:279–283
Vanegas-Espinoza PE, Ramos-Viveros V, Jiménez-Aparicio AR, López-Villegas O, Heredia-Mira FJ, Meléndez-Martínez AJ, Del Villar-Martínez AA (2011) Plastid analysis of pigmented undifferentiated cells of marigold Tagetes erecta L. by transmission electron microscopy. In Vitro Cell Dev Biol-Plant 47:596–603. https://doi.org/10.1007/s11627-011-9401-4
Victorio CP, Lage CLS (2009) In vitro flowering of Phyllanthus tenellus Roxb. cultured under different light qualities and growth regulators. Gen Appl Plant Physiol 35:44–50
Werner T, Schmüling TS (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12:527–538. https://doi.org/10.1016/j.pbi.2009.07.002
Yokota T, Tutumi N, Takahashi K (1999) Growth rate estimation of in vitro primarily induced carrot callus by a fractal based model. Biochem Eng J 3:231–234. https://doi.org/10.1016/S1369-703X(99)00003-0
Acknowledgements
The authors are thankful to the Secretaría de Investigación y Posgrado of the Instituto Politécnico Nacional (Grant No. 20180804) of México for the support granted for the accomplishment of this work.
Author information
Authors and Affiliations
Contributions
This work was designed and supervised by AAVM and OPL. Sample collection and laboratory work were conducted by LBRS and CB, and analysis of results was performed by PEVE and GACM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Tae-Ho Han, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanegas-Espinoza, P.E., Bazaldúa, C., Ríos-Salomé, L.B. et al. Cellular and morphological changes during leaf explant dedifferentiation and plant regeneration of Tagetes erecta. Hortic. Environ. Biotechnol. 61, 407–414 (2020). https://doi.org/10.1007/s13580-019-00210-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00210-z